Polymer Free Amphilimus Drug Eluting Stent for Infrapopliteal Arterial Disease in Patients with Critical Limb Ischemia: A New Device in the Armamentarium
Abstract
1. Introduction
2. Mechanism of Polymer-DES Restenosis
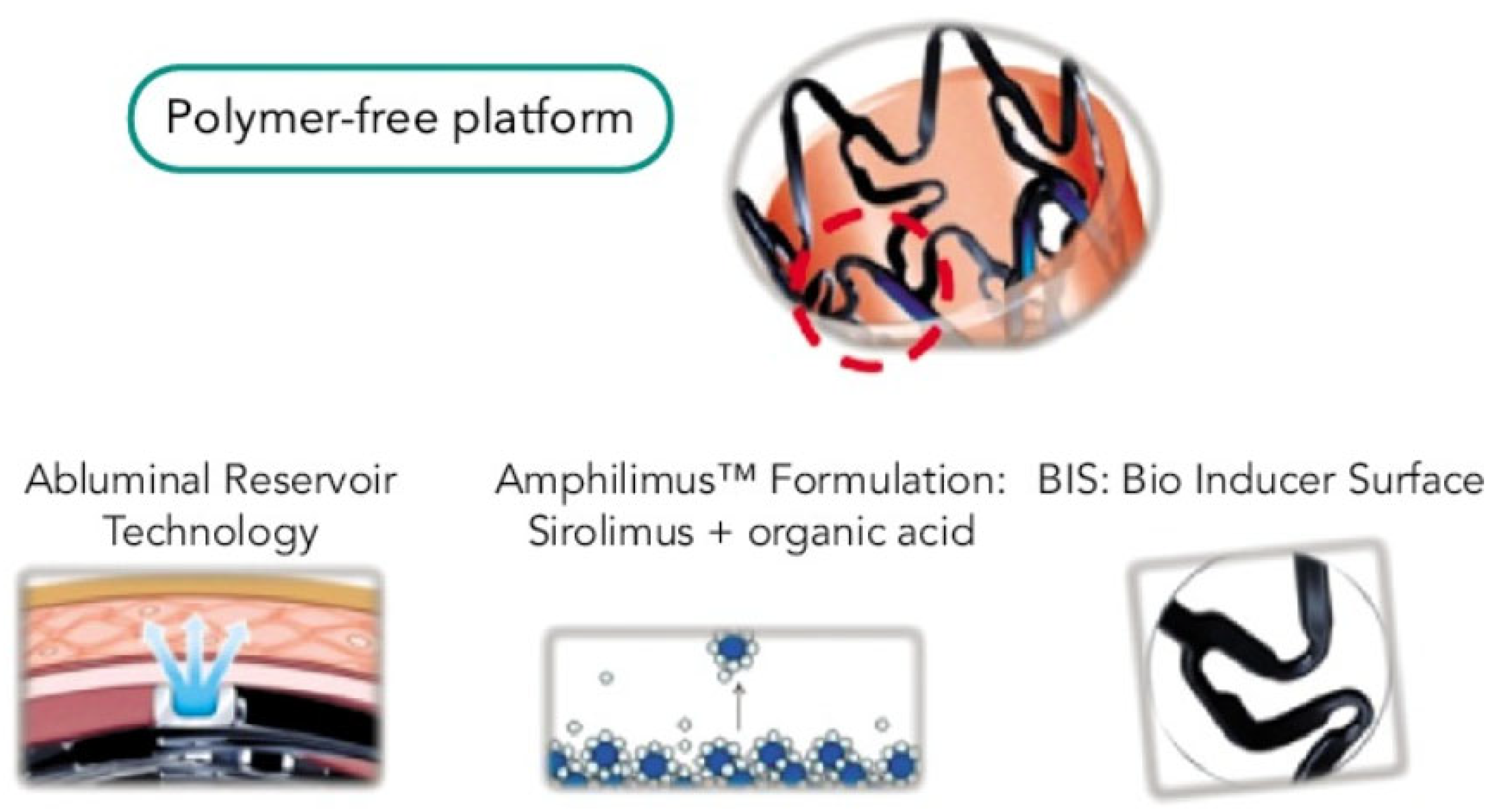
3. Device Characteristics
4. Clinical Application in CLI Patients
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morley, R.L.; Sharma, A.; Horsch, A.D.; Hinchliffe, R.J. Peripheral artery disease. BMJ 2018, 360, j5842. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.H.; Grant, A.A.; Kalbaugh, C.A.; Blackhurst, D.W.; Langan, E.M., 3rd; Taylor, S.A.; Cull, D.L. The Impact of Isolated Tibial Disease on Outcomes in the Critical Limb Ischemic Population. Ann. Vasc. Surg. 2010, 24, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Malyar, N.; Fürstenberg, T.; Wellmann, J.; Meyborg, M.; Lüders, F.; Gebauer, K.; Bunzemeier, H.; Roeder, N.; Reinecke, H. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: A nationwide population-based analysis. Eur. Heart J. 2013, 34, 2706–2714. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—Summary of recommendations. J. Vasc. Interv. Radiol. 2006, 17, 1383–1397, quiz1398. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulos, S.; Theodosiadou, V.; Katsanos, K.; Kitrou, P.; Kagadis, G.C.; Siablis, D.; Karnabatidis, D. Long-Term clinical outcomes of infrapopliteal drug-Eluting stent placement for critical limb ischemia in diabetic patients. J. Vasc. Interv. Radiol. 2015, 26, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Mohler, E., 3rd; Giri, J.; American College of Cardiology/ American Heart Association (ACC/AHA). Management of peripheral arterial disease patients: Comparing the ACC/AHA and TASC-II guidelines. Curr. Med. Res. Opin. 2008, 24, 2509–2522. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.-B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109.e33. [Google Scholar] [CrossRef]
- Spreen, M.I.; Martens, J.M.; Hansen, B.E.; Knippenberg, B.; Verhey, E.; van Dijk, L.C.; de Vries, J.-P.P.; Vos, J.-A.; de Borst, G.J.; Vonken, E.-J.P.; et al. Percutaneous Transluminal Angioplasty and Drug-Eluting Stents for Infrapopliteal Lesions in Critical Limb Ischemia (PADI) Trial. Circ. Cardiovasc. Interv. 2016, 9, e002376. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, A.; Poncyljusz, W.; Wilk, G.; Szczerbo-Trojanowska, M. The evaluation of primary stenting of sirolimus-eluting versus bare-metal stents in the treatment of atherosclerotic lesions of crural arteries. Eur. Radiol. 2009, 19, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Tepe, G.; Schmehl, J.; Heller, S.; Brechtel, K.; Heuschmid, M.; Fenchel, M.; Kramer, U.; Miller, S.; Claussen, C.D. Drug eluting stents versus PTA with GP IIb/IIIa blockade below the knee in patients with current ulcers--The BELOW Study. J. Cardiovasc. Surg. 2010, 51, 203–212. [Google Scholar] [PubMed]
- Scheinert, D.; Katsanos, K.; Zeller, T.; Koppensteiner, R.; Commeau, P.; Bosiers, M.; Krankenberg, H.; Baumgartner, I.; Siablis, D.; Lammer, J.; et al. ACHILLES Investigators. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J. Am. Coll. Cardiol. 2012, 60, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Rastan, A.; Brechtel, K.; Krankenberg, H.; Zahorsky, R.; Tepe, G.; Noory, E.; Schwarzwälder, U.; Macharzina, R.; Schwarz, T.; Bürgelin, K.; et al. Sirolimus-eluting stents for treatment of infrapopliteal arteries reduce clinical event rate compared to bare-metal stents: Long-term results from a randomized trial. J. Am. Coll. Cardiol. 2012, 60, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Bosiers, M.; Scheinert, D.; Peeters, P.; Torsello, G.; Zeller, T.; Deloose, K.; Schmidt, A.; Tessarek, J.; Vinck, E.; Schwartz, L.B. Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J. Vasc. Surg. 2011, 55, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Liistro, F.; Porto, I.; Angioli, P.; Grotti, S.; Ricci, L.; Ducci, K.; Falsini, G.; Ventoruzzo, G.; Turini, F.; Bellandi, G.; et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): A randomized trial in diabetic patients with critical limb ischemia. Circulation 2013, 128, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Cassese, S.; Ndrepepa, G.; Tepe, G.; King, L.; Ott, I.; Nerad, M.; Schunkert, H.; Kastrati, A. Drug-eluting stents for revascularization of infrapopliteal arteries: Updated meta-analysis of randomized trials. JACC Cardiovasc. Interv. 2013, 6, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Widimský, P. Resolute zotarolimus-eluting coronary stent system for the treatment of coronary artery disease. Expert Rev. Med. Devices 2014, 11, 247–257. [Google Scholar] [CrossRef]
- Piccolo, R.; Nicolino, A.; Danzi, G.B. The Nobori biolimus-eluting stent: Update of available evidence. Expert Rev. Med. Devices 2014, 11, 275–282. [Google Scholar] [CrossRef]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Caputo, R.P.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 2003, 349, 1315–1323. [Google Scholar] [CrossRef]
- Windecker, S.; Remondino, A.; Eberli, F.R.; Jüni, P.; Räber, L.; Wenaweser, P.; Togni, M.; Billinger, M.; Tüller, D.; Seiler, C.; et al. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N. Engl. J. Med. 2005, 353, 653–662. [Google Scholar] [CrossRef]
- Rizas, K.D.; Mehilli, J. Stent Polymers: Do They Make a Difference? Circ. Cardiovasc. Interv. 2016, 9, e002943. [Google Scholar] [CrossRef] [PubMed]
- A Byrne, R.; Joner, M.; Kastrati, A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009, 57, 567–584. [Google Scholar] [PubMed]
- Virmani, R.; Guagliumi, G.; Farb, A.; Musumeci, G.; Grieco, N.; Motta, T.; Orazio, V.; Frank, D.K. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: Should we be cautious? Circulation 2004, 109, 701–705. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, S.J.; Carpinone, P.L.; Vock, D.M.; Batich, C.D.; Pepine, C.J. Changes to polymer surface of drug-eluting stents during balloon expansion. JAMA 2012, 307, 2148–2150. [Google Scholar] [CrossRef] [PubMed]
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of drug-eluting stents in humans: Delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 2006, 48, 193–202. [Google Scholar] [CrossRef]
- Aoki, J.; Tanabe, K. Mechanisms of drug-eluting stent restenosis. Cardiovasc. Interv. Ther. 2021, 36, 23–29. [Google Scholar] [CrossRef]
- Fedel, M.; Motta, A.; Maniglio, D.; Migliaresi, C. Carbon coatings for cardiovascular applications: Physico-chemical properties and blood compatibility. J. Biomater. Appl. 2010, 25, 57–74. [Google Scholar] [CrossRef]
- Pivato, C.A.; Leone, P.P.; Petriello, G.; Sanz-Sanchez, J.; Chiarito, M.; Stefanini, G.G. The Cre8 Amphilimus-Eluting Stent for the treatment of coronary artery disease: Safety and efficacy profile. Expert Rev. Med. Devices 2020, 17, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Mountfort, K.; Antoniucci, D.; Mehran, R.; DeLuca, G.; Nef, H.; Vrolix, M.; Khashaba, A. Cre8™ Unique Technology in Challenging Daily Practice: Proceedings of a satellite symposium held at EuroPCR on 20–23 May 2014 in Paris. Interv. Cardiol. 2014, 9, 180–183. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef]
- Romaguera, R.; Gómez-Lara, J.; Jacobi, F.; Cequier, A. Polymer-free amphilimus-eluting stents in patients with diabetes mellitus. Minerva Cardioangiol. 2014, 62, 421–426. [Google Scholar] [PubMed]
- Moretti, C.; Lolli, V.; Perona, G.; Vignolini, M.-C.; Cabiale, K.; Falzone, M.; Galloni, M. Cre8™ coronary stent: Preclinical in vivo assessment of a new generation polymer-free DES with Amphilimus™ formulation. EuroInterv. J. Eur. Collab. Work. Group. Interv. Cardiol. Eur. Soc. Cardiol. 2012, 7, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Tigkiropoulos, K.; Lazaridis, I.; Nikas, S.; Abatzis-Papadopoulos, M.; Sidiropoulou, K.; Stavridis, K.; Karamanos, D.; Saratzis, A.; Saratzis, N. One-year outcomes following primary stenting of infrapopliteal steno-occlusive arterial disease using a non-polymer sirolimus-eluting stent: Results from a prospective single-centre cohort study. Front. Surg. Oct. 2022, 9, 955211. [Google Scholar] [CrossRef]
- Santos, D.; Valdivia, A.R.; Olmos, C.G.; Guaita, J.O.; Zúñiga, C.G. LEA 29. Initial experience with cre8 stent for below-knee disease in patients with critical limb ischemia. J. Vasc. Surg. 2019, 70, e126. [Google Scholar] [CrossRef]
- Cre8 BTK clinical results in below the knee complex disease. Recorded on-Site at LINC 2022, Leipzig. Marc Sirvent. Available online: http:www.leipzig-interventional-course.com (accessed on 19 September 2022).
- Colombo, A.; Godino, C.; Donahue, M.; Testa, L.; Chiarito, M.; Pavon, A.G.; Colantonio, R.; Cappelletti, A.; Monello, A.; Magni, V.; et al. One-year clinical outcome of amphilimus polymer-free drug-eluting stent in diabetes mellitus patients: Insight from the ASTUTE registry (AmphilimuS iTalian mUlticenTre rEgistry). Int. J. Cardiol. 2016, 214, 113–120. [Google Scholar] [CrossRef]
- Sardella, G.; Stefanini, G.G.; Briguori, C.; Tamburino, C.; Fabbiocchi, F.; Rotolo, F.; Tomai, F.; Paggi, A.; Lombardi, M.; Gioffrè, G.; et al. Safety and efficacy of polymer-free biolimus-eluting stents in all-comer patients: The RUDI-FREE study. EuroIntervention 2018, 14, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, R.; Gómez-Hospital, J.A.; Gomez-Lara, J.; Brugaletta, S.; Pinar, E.; Jiménez-Quevedo, P.; Gracida, M.; Roura, G.; Ferreiro, J.L.; Teruel, L.; et al. A Randomized Comparison of Reservoir-Based Polymer-Free Amphilimus-Eluting Stents Versus Everolimus-Eluting Stents with Durable Polymer in Patients with Diabetes Mellitus the RESERVOIR Clinical Trial. JACC Cardiovasc. Interv. 2016, 9, 42–50. [Google Scholar] [CrossRef] [PubMed]
- van Hemert, N.D.; Voskuil, M.; Rozemeijer, R.; Stein, M.; Frambach, P.; Pereira, B.; Rittersma, S.Z.; Kraaijeveld, A.O.; Leenders, G.E.; Timmers, L.; et al. 3-Year Clinical Outcomes After Implantation of Permanent-Polymer Versus Polymer-Free Stent: ReCre8 Landmark Analysis. JACC Cardiovasc. Interv. 2021, 14, 2477–2486. [Google Scholar] [CrossRef]
- Prati, F.; Romagnoli, E.; Valgimigli, M.; Burzotta, F.; De Benedictis, M.; Ramondo, A.; Mehran, R.; Stella, P.R. Randomized comparison between 3-month Cre8 DES vs. 1-month Vision/Multilink8 BMS neointimal coverage assessed by OCT evaluation: The DEMONSTRATE study. Int. J. Cardiol. 2014, 176, 904–909. [Google Scholar] [CrossRef]
- Romaguera, R.; Salinas, P.; Gomez-Lara, J.; Brugaletta, S.; Gómez-Menchero, A.; A Romero, M.; García-Blas, S.; Ocaranza, R.; Bordes, P.; Kockar, M.J.; et al. Amphilimus- vs. zotarolimus-eluting stents in patients with diabetes mellitus and coronary artery disease: The SUGAR trial. Eur. Hear J. 2022, 43, 1320–1330. [Google Scholar] [CrossRef]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Paraskevopoulos, I.; Karnabatidis, D. Risk of Death and Amputation with Use of Paclitaxel-Coated Balloons in the Infrapopliteal Arteries for Treatment of Critical Limb Ischemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Vasc. Interv. Radiol. 2020, 31, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.Y.; Liu, X.; Josiah, N.G.; Stanley, E.L.; Jun, J.N.; Andrew, C. A Systematic Review and Meta-Analysis of Sirolimus-Eluting Stents for Treatment of Below-the-Knee Arterial Disease. J. Vasc. Surg. 2022, S0741–5214, 02320-5. [Google Scholar] [CrossRef] [PubMed]
- Giaquinta, A.; Vincenzo, A.; De Marco, E.; Veroux, M.; Veroux, P. Everolimus-Eluting Stent for Patients with Critical Limb Ischemia and Infrapopliteal Arterial Occlusive Disease. Vasc. Endovasc. Surg. 2017, 51, 60–66. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Beasley, M.; Davis, M.; Adams, E.; Lee, A.; Shapiro, J.; Dean, L.S.; Davis, E. Use of drug-eluting stents in patients with critical limb ischemia and infrapopliteal arterial disease: A real-world single-center experience. J. Vasc. Surg. 2021, 74, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Taeymans, K.; Bosiers, M.; Deloose, K.; Callaert, J.; Keirse, K.; Verbist, J.; Eynde, W.V.D.; Torsello, G.; Wauters, J. One-year outcome of the everolimus-eluting, balloon-expandable Promus Element and Promus Element Plus stent in the treatment of below-the-knee lesions in CLI patients. J. Cardiovasc. Surg. 2020, 61, 445–450. [Google Scholar] [CrossRef]
- Bosiers, M.J.; Deloose, K.; Peeters, P.; Torsello, G.; Zeller, T. Schneinert DOutcome of a drug-eluting stent in longer below-the-knee lesions in patients with critical limb ischemia. J. Cardiovasc. Surg. 2017, 58, 49–54. [Google Scholar] [CrossRef]


| Nominal Diameter (mm) | Number of Cells per Circumference | Strut Thickness (Microns) |
|---|---|---|
| 2.25 | 4 cells | 70 |
| 2.5–2.75 | 4 cells | 80 |
| 3.00–3.50 | 5 cells | 80 |
| 4.00–4.50 | 6 cells | 80 |
| Studies | Patients (n = 79) | Mean Age | R.C 5–6 | Male | Hypertension | D.M | ESRD | DSL | CAD |
|---|---|---|---|---|---|---|---|---|---|
| Tigkiropoulos [33] | 27 | 79 | 88.8% | 74% | 100% | 100% | 30% | 11% | 33% |
| Santos [34] | 10 | 69 | 60% | 40% | 80% | 80% | 11% | N/R | N/R |
| Sirvent [35] | 42 | 76 | 88.1% | 78.6% | 91% | 73.8% | N/R | 74% | N/R |
| Studies | Lesions Treated (n = 107) | Occlusive Lesions (%) | Stent Length (mm) | Primary Patency | Secondary Patency | Limb Salvage | Mortality | Freedom from CD-TLR |
|---|---|---|---|---|---|---|---|---|
| Tigkiropoulos [33] | 31 | 55% | 20 | 81% | 96% | 85% | 15% | 96% |
| Santos [34] | 10 | NR | NR | 80% | NR | 90% | NR | NR |
| Sirvent [35] | 66 | 52.4% | 35 | 83.8% | 97.5% | 95.6% | 23.8% | 83.8% |
| Author | Study | Patients | Mean Lesion Length | Primary Patency at 1 Year | Freedom from TLR at 1 Year | Limb Salvage (No Major Amputation) at 1 Year |
|---|---|---|---|---|---|---|
| Giaquinta [44] | Prospective | 122 | 52.7 mm | 88.9% | 91.5% | 93% |
| AbuRahma [45] | Retrospective | 98 | 41 mm | 57% | 92% | 87% |
| Taeymans [46] | Prospective | 70 | 22.83 mm | 86.2% | 93% | 100% |
| Bosiers [47] | Prospective | 60 | 47.4 mm | 75.4% | 84.9% | 94.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tigkiropoulos, K.; Abatzis-Papadopoulos, M.; Sidiropoulou, K.; Stavridis, K.; Karamanos, D.; Lazaridis, I.; Saratzis, N. Polymer Free Amphilimus Drug Eluting Stent for Infrapopliteal Arterial Disease in Patients with Critical Limb Ischemia: A New Device in the Armamentarium. Medicina 2023, 59, 39. https://doi.org/10.3390/medicina59010039
Tigkiropoulos K, Abatzis-Papadopoulos M, Sidiropoulou K, Stavridis K, Karamanos D, Lazaridis I, Saratzis N. Polymer Free Amphilimus Drug Eluting Stent for Infrapopliteal Arterial Disease in Patients with Critical Limb Ischemia: A New Device in the Armamentarium. Medicina. 2023; 59(1):39. https://doi.org/10.3390/medicina59010039
Chicago/Turabian StyleTigkiropoulos, Konstantinos, Manolis Abatzis-Papadopoulos, Katerina Sidiropoulou, Kyriakos Stavridis, Dimitrios Karamanos, Ioannis Lazaridis, and Nikolaos Saratzis. 2023. "Polymer Free Amphilimus Drug Eluting Stent for Infrapopliteal Arterial Disease in Patients with Critical Limb Ischemia: A New Device in the Armamentarium" Medicina 59, no. 1: 39. https://doi.org/10.3390/medicina59010039
APA StyleTigkiropoulos, K., Abatzis-Papadopoulos, M., Sidiropoulou, K., Stavridis, K., Karamanos, D., Lazaridis, I., & Saratzis, N. (2023). Polymer Free Amphilimus Drug Eluting Stent for Infrapopliteal Arterial Disease in Patients with Critical Limb Ischemia: A New Device in the Armamentarium. Medicina, 59(1), 39. https://doi.org/10.3390/medicina59010039






