Safety and Efficacy of the Transaxillary Access for Minimally Invasive Aortic Valve Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Study Design and Ethical Statement
2.3. Patients and Groups
2.4. Statistical Analysis
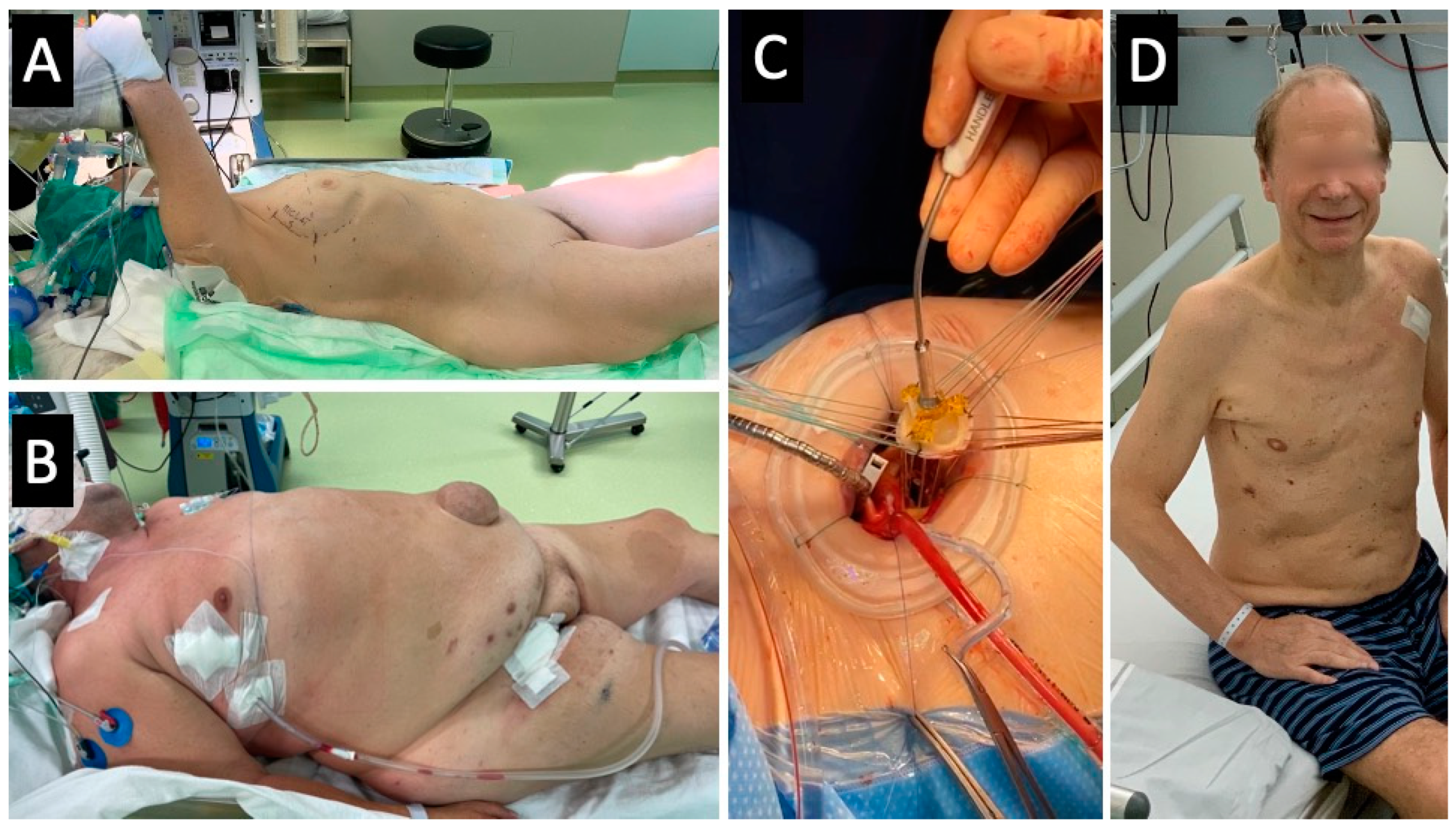
2.5. Surgical Techniques
2.5.1. Treatment Group—Transaxillary Minimally Invasive Aortic Valve Surgery
2.5.2. Control Group—Full Sternotomy Aortic Valve Surgery
2.5.3. Prosthesis Choice
2.5.4. Intensive Care Unit Setup
3. Results
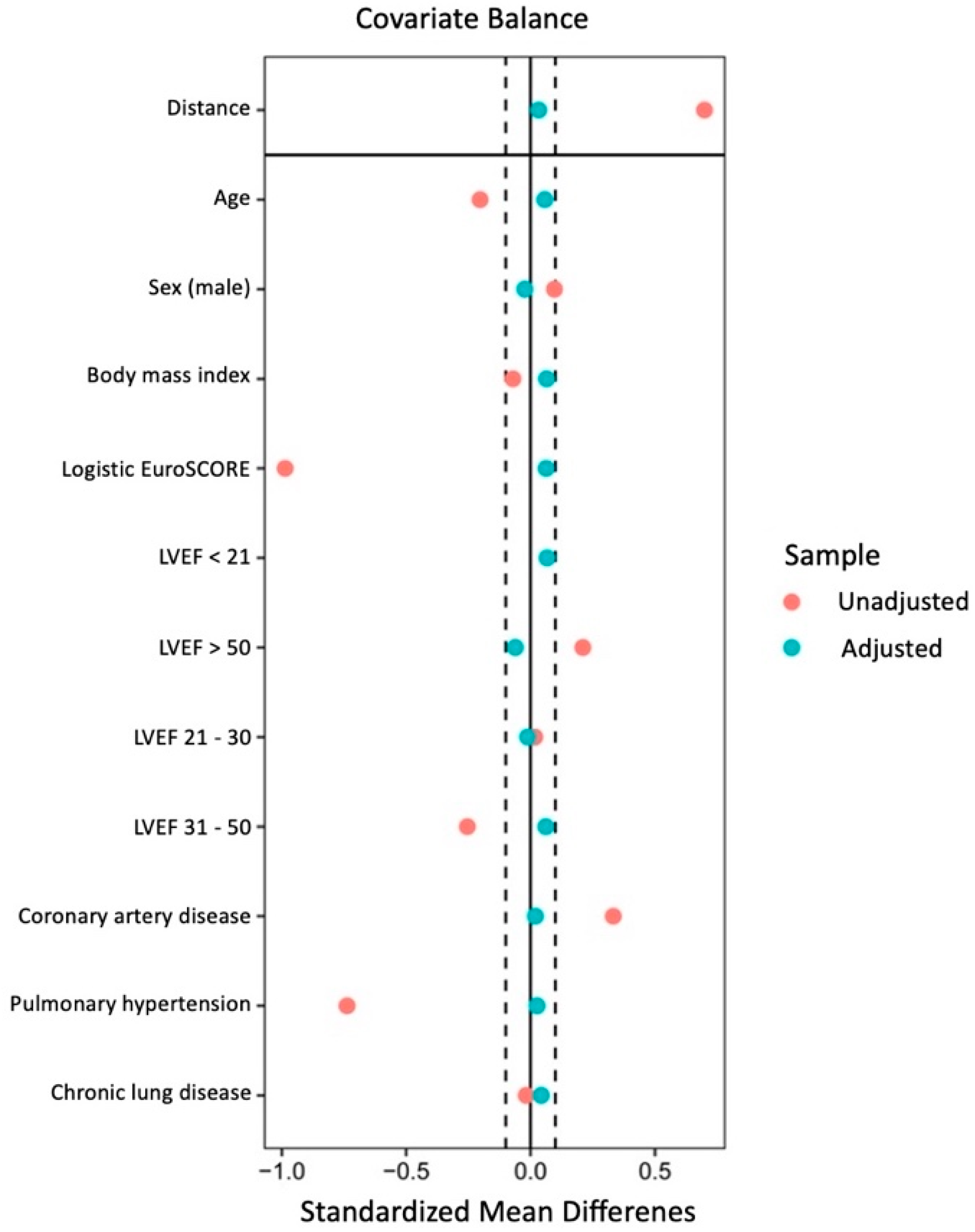
3.1. Propensity Matching Results
3.2. Clinical Baseline Parameters
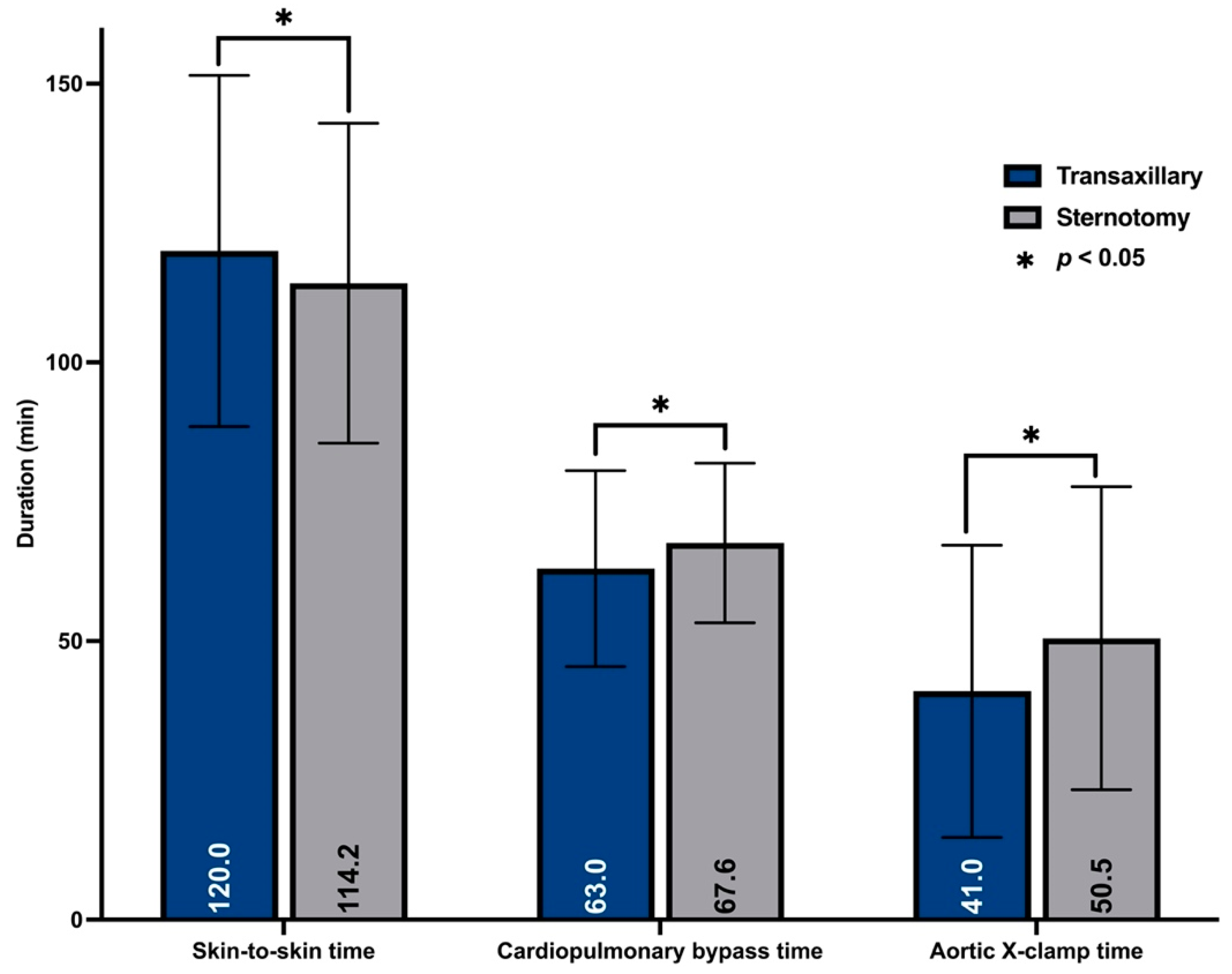
3.3. Procedural Outcomes
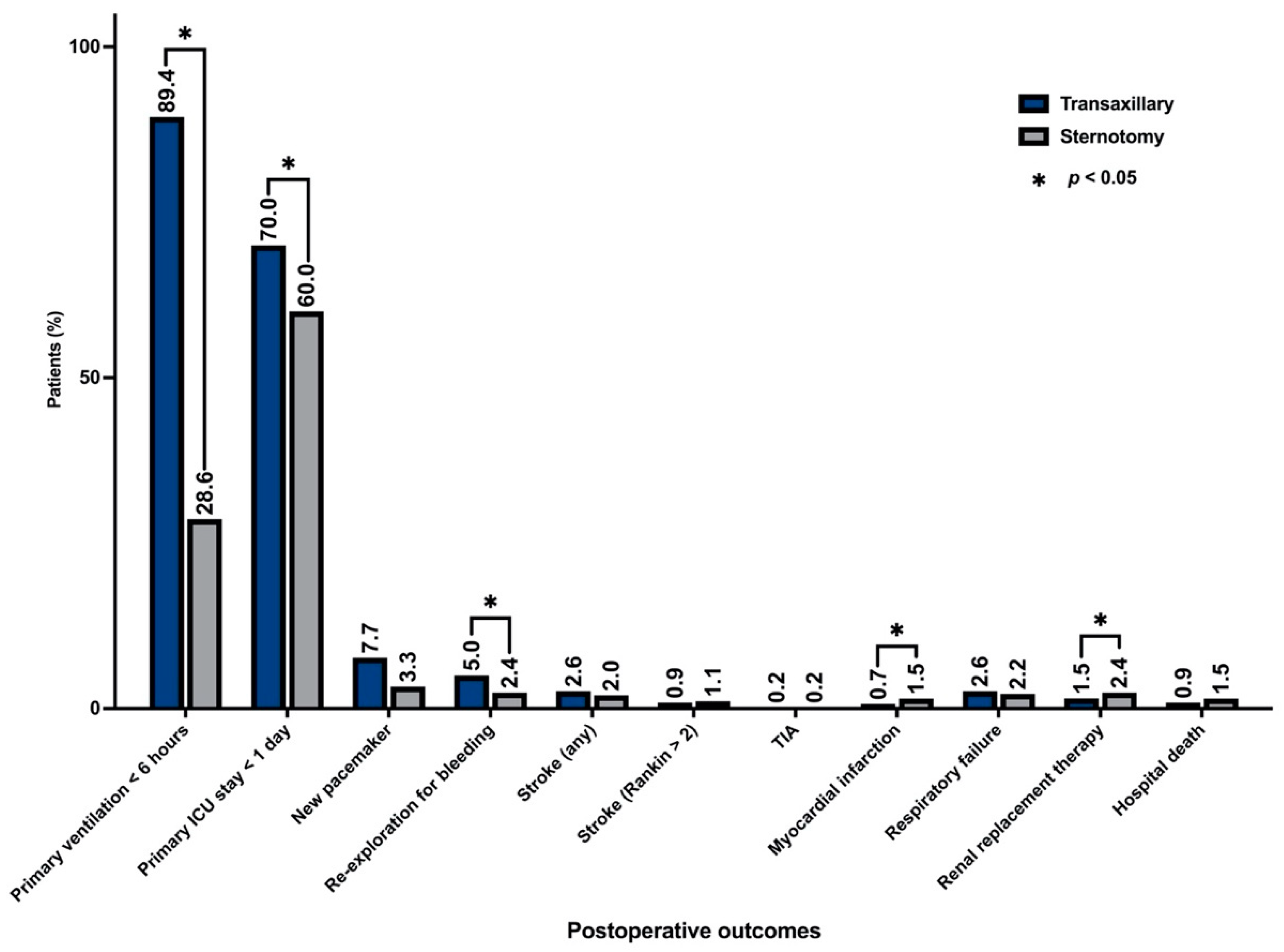
3.4. Postoperative Outcomes
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, P.N.; Kumar, A.S. Aortic Valve Replacement through Right Thoracotomy. Tex. Heart Inst. J. 1993, 20, 307–308. [Google Scholar] [PubMed]
- Cosgrove, D.M., 3rd; Sabik, J.F. Minimally Invasive Approach for Aortic Valve Operations. Ann. Thorac. Surg. 1996, 62, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.G.; D’Agostino, R.S. Minimal-Access Aortic and Valvular Operations, Including the “J/J” Incision. Ann. Thorac. Surg. 1998, 66, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.G.; D’Agostino, R.S. “J” Incision Minimal-Access Valve Operations. Ann. Thorac. Surg. 1998, 66, 1110–1112. [Google Scholar] [CrossRef]
- Chang, Y.S.; Lin, P.J.; Chang, C.H.; Chu, J.J.; Tan, P.P. “I” Ministernotomy for Aortic Valve Replacement. Ann. Thorac. Surg. 1999, 68, 40–45. [Google Scholar] [CrossRef]
- Aris, A. Reversed “C” Ministernotomy for Aortic Valve Replacement. Ann. Thorac. Surg. 1999, 67, 1806–1807. [Google Scholar] [CrossRef]
- Loures, D.R.; Mulinari, L.A.; Tyszka, A.L.; Ribeiro, E.; Carvalho, R.G.; Almeida, R. Partial Median Sternotomy in H. A New Approach for Cardiac Surgery. Arq. Bras. Cardiol. 1998, 70, 71–73. [Google Scholar] [CrossRef]
- Lamelas, J. Minimally Invasive Aortic Valve Replacement: The “Miami Method”. Ann. Cardiothorac. Surg. 2015, 4, 71–77. [Google Scholar]
- Van Praet, K.M.; Van Kampen, A.; Kofler, M.; Unbehaun, A.; Hommel, M.; Jacobs, S.; Falk, V.; Kempfert, J. Minimally Invasive Surgical Aortic Valve Replacement through a Right Anterolateral Thoracotomy. Multimed. Man. Cardiothorac. Surg. 2020. [Google Scholar] [CrossRef]
- Wilbring, M.; Matschke, K.E.; Alexiou, K.; Di Eusanio, M.; Kappert, U. Surgery without Scars: Right Lateral Access for Minimally Invasive Aortic Valve Replacement. Thorac. Cardiovasc. Surg. 2021, 69, 461–465. [Google Scholar] [CrossRef]
- Wilbring, M.; Arzt, S.; Alexiou, K.; Matschke, K.; Kappert, U. Surgery without Visible Scars-Double Valve Surgery Using the Right Lateral Access. Ann. Cardiothorac. Surg. 2020, 9, 424–926. [Google Scholar] [CrossRef] [PubMed]
- Szecel, D.; Eurlings, R.; Rega, F.; Verbrugghe, P.; Meuris, B. Perceval Sutureless Aortic Valve Implantation: Midterm Outcomes. Ann. Thorac. Surg. 2021, 111, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Meuris, B.; Flameng, W.J.; Laborde, F.; Folliguet, T.A.; Haverich, A.; Shrestha, M. Five-year results of the pilot trial of a sutureless valve. J. Thorac. Cardiovasc. Surg. 2015, 150, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Meuris, B.; Shrestha, M.; Carrel, T.; Fischlein, T.; Madonna, F.; Flameng, W.; Laborde, F.; Misfeld, M.; Haverich, A.; Folliguet, T. European multicentre experience with the sutureless Perceval valve: Clinical and haemodynamic outcomes up to 5 years in over 700 patients. Eur. J. Cardiothorac. Surg. 2016, 49, 234–241. [Google Scholar]
- Gummert, J.F.; Funkat, A.; Beckmann, A.; Schiller, W.; Hekmat, K.; Ernst, M.; Haverich, A. Thoracic German Society for, and Surgery Cardiovascular. Cardiac Surgery in Germany During 2007: A Report on Behalf of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2008, 56, 328–336. [Google Scholar] [CrossRef]
- Beckmann, A.; Meyer, R.; Lewandowski, J.; Markewitz, A.; Blassfeld, D.; Boning, A. German Heart Surgery Report 2021: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2022, 70, 362–376. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 Esc/Eacts Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Yun, J.J.; OSaleh, A.; Chung, J.W.; Bakaeen, F.G.; Unai, S.; Tong, M.Z.; Roselli, E.E.; Johnston, D.R.; Soltesz, E.G.; Rajeswaran, J.; et al. Cardiac Operations after Transcatheter Aortic Valve Replacement. Ann. Thorac. Surg. 2022, 114, 52–59. [Google Scholar] [CrossRef]
- Ogami, T.; Ridgley, J.; Serna-Gallegos, D.; Kliner, D.E.; Toma, C.; Sanon, S.; Brown, J.A.; Yousef, S.; Sultan, I. Outcomes of Surgical Aortic Valve Replacement after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2022, 182, 63–68. [Google Scholar] [CrossRef]
- Fukuhara, S.; Nguyen, C.T.N.; Kim, K.M.; Yang, B.; Ailawadi, G.; Patel, H.J.; Deeb, G.M. Aortic Valve Reintervention after Transcatheter Aortic Valve Replacement. J. Thorac. Cardiovasc. Surg. 2021. [Google Scholar] [CrossRef]
- Fukuhara, S.; Nguyen, C.T.N.; Yang, B.; Bolling, S.F.; Romano, M.A.; Kim, K.M.; Patel, H.J.; Deeb, G.M. Cardiac Reoperations in Patients with Transcatheter Aortic Bioprosthesis. Ann. Thorac. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jawitz, O.K.; Gulack, B.C.; Grau-Sepulveda, M.V.; Matsouaka, R.A.; Mack, M.J.; Holmes, D.R., Jr.; Carroll, J.D.; Thourani, V.H.; Brennan, J.M. Reoperation after Transcatheter Aortic Valve Replacement: An Analysis of the Society of Thoracic Surgeons Database. JACC Cardiovasc. Interv. 2020, 13, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Salenger, R.; Gammie, J.S.; Collins, J.A. Minimally Invasive Aortic Valve Replacement. J. Card. Surg. 2016, 31, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.R.; Atik, F.A.; Rajeswaran, J.; Blackstone, E.H.; Nowicki, E.R.; Sabik, J.F., 3rd; Mihaljevic, T.; Gillinov, A.M.; Lytle, B.W.; Svensson, L.G. Outcomes of Less Invasive J-Incision Approach to Aortic Valve Surgery. J. Thorac. Cardiovasc. Surg. 2012, 144, 852–858.e3. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.; Umakanthan, R.; Cohn, L.H.; Bolman, R.M., 3rd; PShekar, S.; Chen, F.Y.; Couper, G.S.; Aranki, S.F. Early and Late Outcomes of 1000 Minimally Invasive Aortic Valve Operations. Eur. J. Cardiothorac. Surg. 2008, 33, 537–541. [Google Scholar] [CrossRef]
- Gilmanov, D.; Bevilacqua, S.; Murzi, M.; Cerillo, A.G.; Gasbarri, T.; Kallushi, E.; Miceli, A.; Glauber, M. Minimally Invasive and Conventional Aortic Valve Replacement: A Propensity Score Analysis. Ann. Thorac. Surg. 2013, 96, 837–843. [Google Scholar] [CrossRef]
- Glauber, M.; Miceli, A.; Gilmanov, D.; Ferrarini, M.; Bevilacqua, S.; Farneti, P.A.; Solinas, M. Right Anterior Minithoracotomy Versus Conventional Aortic Valve Replacement: A Propensity Score Matched Study. J. Thorac. Cardiovasc. Surg. 2013, 145, 1222–1226. [Google Scholar] [CrossRef]
- Sharony, R.; Grossi, E.; Saunders, P.C.; Schwartz, C.F.; Ribakove, G.H.; Baumann, F.G.; Galoway, A.; Colvin, S.B. Propensity Score Analysis of a Six-Year Experience with Minimally Invasive Isolated Aortic Valve Replacement. J. Heart Valve Dis. 2004, 13, 887–893. [Google Scholar]
- Ghanta, R.K.; Lapar, D.J.; Kern, J.A.; Kron, I.L.; Speir, A.M.; Fonner, E.; Quader, M.; Ailawadi, G. Minimally Invasive Aortic Valve Replacement Provides Equivalent Outcomes at Reduced Cost Compared with Conventional Aortic Valve Replacement: A Real-World Multi-Institutional Analysis. J. Thorac. Cardiovasc. Surg. 2015, 149, 1060–1065. [Google Scholar] [CrossRef]
- Borger, M.A.; Moustafine, V.; Conradi, L.; Knosalla, C.; Richter, M.; Merk, D.R.; Doenst, T.; Hammerschmidt, R.; Treede, H.; Dohmen, P.; et al. A Randomized Multicenter Trial of Minimally Invasive Rapid Deployment Versus Conventional Full Sternotomy Aortic Valve Replacement. Ann. Thorac. Surg. 2015, 99, 17–25. [Google Scholar] [CrossRef]
- Doll, N.; Borger, M.A.; Hain, J.; Bucerius, J.; Walther, T.; Gummert, J.F.; Mohr, F.W. Minimal Access Aortic Valve Replacement: Effects on Morbidity and Resource Utilization. Ann. Thorac. Surg. 2002, 74, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Farhat, F.; Lu, Z.; Lefevre, M.; Montagna, P.; Mikaeloff, P.; Jegaden, O. Prospective Comparison between Total Sternotomy and Ministernotomy for Aortic Valve Replacement. J. Card. Surg. 2003, 18, 396–401. [Google Scholar] [CrossRef]
- Masiello, P.; Coscioni, E.; Panza, A.; Triumbari, F.; Preziosi, G.; Di Benedetto, G. Surgical Results of Aortic Valve Replacement Via Partial Upper Sternotomy: Comparison with Median Sternotomy. Cardiovasc. Surg. 2002, 10, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Borger, M.A.; Ivanov, J.; Armstrong, S.; Christie-Hrybinsky, D.; Feindel, C.M.; David, T.E. Twenty-Year Results of the Hancock Ii Bioprosthesis. J. Heart Valve Dis. 2006, 15, 49–55. [Google Scholar]
- Christiansen, S.; Stypmann, J.; Tjan, T.D.; Wichter, T.; Van Aken, H.; Scheld, H.H.; Hammel, D. Minimally-Invasive Versus Conventional Aortic Valve Replacement--Perioperative Course and Mid-Term Results. Eur. J. Cardiothorac. Surg. 1999, 16, 647–652. [Google Scholar] [CrossRef]
- Bakir, I.; Casselman, F.P.; Wellens, F.; Jeanmart, H.; De Geest, R.; Degrieck, I.; Van Praet, F.; Vermeulen, Y.; Vanermen, H. Minimally Invasive Versus Standard Approach Aortic Valve Replacement: A Study in 506 Patients. Ann. Thorac. Surg. 2006, 81, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Korach, A.; Shemin, R.J.; Hunter, C.T.; Bao, Y.; Shapira, O.M. Minimally Invasive Versus Conventional Aortic Valve Replacement: A 10-Year Experience. J. Cardiovasc. Surg. 2010, 51, 417–421. [Google Scholar]
- De Smet, J.M.; Rondelet, B.; Jansens, J.L.; Antoine, M.; De Canniere, D.; Le Clerc, J.L. Assessment Based on Euroscore of Ministernotomy for Aortic Valve Replacement. Asian Cardiovasc. Thorac. Ann. 2004, 12, 53–57. [Google Scholar] [CrossRef]
- Corbi, P.; Rahmati, M.; Donal, E.; Lanquetot, H.; Jayle, C.; Menu, P.; Allal, J. Prospective Comparison of Minimally Invasive and Standard Techniques for Aortic Valve Replacement: Initial Experience in the First Hundred Patients. J. Card. Surg. 2003, 18, 133–139. [Google Scholar] [CrossRef]
- Brinkman, W.T.; Hoffman, W.; Dewey, T.M.; Culica, D.; Prince, S.L.; Herbert, M.A.; Mack, M.J.; Ryan, W.H. Aortic Valve Replacement Surgery: Comparison of Outcomes in Matched Sternotomy and Port Access Groups. Ann. Thorac. Surg. 2010, 90, 131–135. [Google Scholar] [CrossRef]
- de Vaumas, C.; Philip, I.; Daccache, G.; Depoix, J.P.; Lecharny, J.B.; Enguerand, D.; Desmonts, J.M. Comparison of Minithoracotomy and Conventional Sternotomy Approaches for Valve Surgery. J. Cardiothorac. Vasc. Anesth 2003, 17, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Foghsgaard, S.; Schmidt, T.A.; Kjaergard, H.K. Minimally Invasive Aortic Valve Replacement: Late Conversion to Full Sternotomy Doubles Operative Time. Tex. Heart Inst. J. 2009, 36, 293–297. [Google Scholar] [PubMed]
- Sansone, F.; Punta, G.; Parisi, F.; Dato, G.M.; Zingarelli, E.; Flocco, R.; Forsennati, P.G.; Bardi, G.L.; del Ponte, S.; Casabona, R. Right Minithoracotomy Versus Full Sternotomy for the Aortic Valve Replacement: Preliminary Results. Heart Lung Circ. 2012, 21, 169–173. [Google Scholar] [CrossRef] [PubMed]
| Propensity Matched Cohort (n = 908) | |||
|---|---|---|---|
| Transaxillary (n = 454) | Sternotomy (n = 454) | p | |
| Age (years); mean ± SD | 69.5 ± 18.3 | 69.2 ± 17.3 | 0.5293 |
| BMI (kg/m2); mean ± SD | 27.1 ± 8.3 | 27.4 ± 4.6 | 0.2994 |
| LVEF by group: n (%) | |||
| >50% | 365 (80.4) | 376 (82.8) | 0.2904 |
| 31–50% | 69 (15.2) | 59 (13.0) | |
| 21–30% | 18 (4.0) | 19 (4.2) | |
| <21% | 2 (0.4) | 0 (0.0) | |
| Pulmonary hypertension *, n (%) | 13 (2.9) | 11 (2.4) | 0.837 |
| Coronary artery disease, n (%) | 126 (27.8) | 122 (26.9) | 0.8232 |
| Chronic lung disease | 32 (7.1) | 27 (6.0) | 0.5905 |
| Logistic EuroSCORE (%); mean ± SD | 4.1 ± 4.0 | 3.9 ± 3.8 | 0.3312 |
| Propensity Matched Cohort (n = 908) | |||
|---|---|---|---|
| Transaxillary (n = 454) | Sternotomy (n = 454) | p | |
| Age (years); mean ± SD | 69.5 ± 18.3 | 69.2 ± 17.3 | 0.5293 |
| Sex (male): n (%) | 281 (61.9) | 286 (63.0) | 0.7840 |
| BMI (kg/m2); mean ± SD | 27.1 ± 8.3 | 27.4 ± 4.6 | 0.2994 |
| Diabetes mellitus: n (%) | 125 (27.5) | 132 (29.1) | 0.9795 |
| Coronary artery disease: n (%) | 126 (27.8) | 122 (26.9) | 0.8232 |
| LVEF (%): n (%) | |||
| >50% | 365 (80.4) | 376 (82.8) | 0.2904 |
| 31%–50% | 69 (15.2) | 59 (13.0) | |
| 21%–30% | 18 (4.0) | 19 (4.2) | |
| <21% | 2 (0.4) | 0 (0.0) | |
| Pulmonary arterial hypertension: n (%) | 13 (2.9) | 11 (2.4) | 0.837 |
| Hemodialysis: n (%) | 3 (0.7) | 0 (0.0) | 0.2492 |
| Creatinine clearance (mL/min): mean ± SD | 75.5 ± 28.0 | 75.9 ± 24.5 | 0.7792 |
| Peripheral artery disease: n (%) | 27 (6.0) | 39 (8.5) | 0.1000 |
| Carotid artery stenosis >50%: n (%) | 20 (4.4) | 19 (4.2) | 0.957 |
| h/o TIA: n (%) | 5 (1.1) | 0 (0.0) | 0.0618 |
| h/o ischemic stroke: n (%) | 24 (5.3) | 21 (4.6) | 0.7601 |
| Atrial fibrillation: n (%) | 88 (19.4) | 58 (12.7) | 0.0621 |
| Smoker status: n (%) | 52 (11.5) | 49 (10.8) | 0.795 |
| Chronic lung disease | 32 (7.1) | 27 (6.0) | 0.5905 |
| NYHA class III or IV: n (%) | 297 (65.4) | 280 (61.7) | 0.472 |
| Logistic EuroSCORE (%); mean ± SD | 4.1 ± 4.0 | 3.9 ± 3.8 | 0.3312 |
| EuroSCORE II (%); mean ± SD | 1.63 ± 1.1 | 1.49 ± 1.0 | 0.4953 |
| Propensity-Matched Cohort (n = 908) | |||
|---|---|---|---|
| Transaxillary (n = 454) | Sternotomy (n = 454) | p | |
| Skin-to-skin time (min) mean ± SD | 120.0 ± 31.5 | 114.2 ± 28.7 | <0.001 |
| Aortic x-clamp time (min) mean ± SD | 41.0 ± 26.2 | 50.5 ± 27.2 | <0.001 |
| pts. with sutured prosthesis (min) mean ± SD | 51.7 ± 14.4 | 50.5 ± 27.2 | 0.967 |
| pts. with rapid deployment valve (min) mean ± SD | 35.9 ± 25.9 | n/a | n/a |
| CPB time (min): mean ± SD | 63.0 ± 17.6 | 67.6 ± 14.3 | <0.001 |
| pts. with sutured prosthesis (min) mean ± SD | 79.2 ± 20.9 | 67.6 ± 14.3 | 0.869 |
| pts. with rapid deployment valve (min) mean ± SD | 47.9 ± 14.8 | n/a | n/a |
| Repeated x-clamping: n (%) | 5 (1.1) | 6 (1.3) | 1.0000 |
| Intraoperative death: n (%) | 0 (0) | 0 (0) | 1.000 |
| Conversion to sternotomy: n (%) | 4 (0.9) | n/a | n/a |
| Major intraoperative complications †: n (%) | 0 (0) | 0 (0) | 1.000 |
| Arterial cannulation: n (%) | <0.001 | ||
| aorta | 2 (0.4) | 454 (100) | |
| femoral artery | 450 (99.1) | 0 (0) | |
| axillary artery | 2 (0.4) | 0 (0) | |
| Prosthesis type: n (%) | |||
| biologic (sutured) | 59 (13.0) | 336 (74.0) | <0.001 |
| biologic (rapid deployment) | 374 (82.4) | 0 (0.0) | |
| mechanic | 21 (4.6) | 118 (26.0) | |
| Labeled valve size (mm): mean ± SD | 24.5 ± 2.8 | 23.4 ± 1.7 | <0.001 |
| Hemodynamic outcome (6th day TTE) * | |||
| Pmax (mmHg); mean ± SD | 24.1 ± 8.9 | 28.0 ± 6.7 | <0.001 |
| Pmean (mmHg); mean ± SD | 12.9 ± 4.8 | 16.3 ± 5.3 | <0.001 |
| Paravalvular leakage (more than trace) ** | 3 (0.6) | 2 (0.4) | 1.000 |
| Propensity-Matched Cohort (n = 908) | |||
|---|---|---|---|
| Transaxillary (n = 454) | Sternotomy (n = 454) | p | |
| Hospital death: n (%) | 4 (0.88) | 7 (1.54) | 0.5463 |
| Hospital stay (days); mean ± SD | 7.0 ± 5.1 | 11.1 ± 6.5 | <0.001 |
| ICU stay (days): n (%) | |||
| <1 day | 318 (70.0) | 272 (60.0) | <0.001 |
| ≤2 days | 41 (9.0) | 86 (18.9) | |
| ≤3 days | 47 (10.4) | 45 (9.9) | |
| >3 days | 48 (10.6) | 51 (11.2) | |
| Ventilation time (hours): n (%) | |||
| <6 h | 406 (89.4) | 130 (28.6) | <0.001 |
| <12 h | 35 (7.7) | 302 (66.5) | |
| >12 h | 13 (2.9) | 22 (4.8) | |
| Respiratory failure †: n (%) | 12 (2.6) | 10 (2.2) | 0.8297 |
| Postoperative transfusions: | |||
| PRBC (pcs); mean ± SD | 0.57 ± 1.6 | 0.82 ± 1.6 | 0.0401 |
| Patients; n (%) | 127 (27.9) | 161 (35.5) | 0.0423 |
| Re-exploration for bleeding: n (%) | 23 (5.0) | 11 (2.4) | <0.001 |
| Skin emphysema: n (%) | 33 (7.3) | 0 (0) | <0.001 |
| Renal replacement therapy: n (%) | 7 (1.54) | 11 (2.4) | 0.4762 |
| Delirium: n (%) | 83 (18.3) | 72 (15.9) | 0.8550 |
| TIA: n (%) | 1 (0.23) | 1 (0.23) | 1.000 |
| Stroke: n (%) | |||
| any | 12 (2.6) | 9 (2.0) | 0.0891 |
| Rankin >2 | 4 (0.9) | 5 (1.1) | 0.7910 |
| Postoperative myocardial infarction: n (%) | 3 (0.7) | 7 (1.5) | 0.0473 |
| Impaired wound healing: n (%) | 18 (3.9) | 17 (3.7) | 0.7341 |
| surgical access site | 7 (1.5) | 17 (3.7) | 0.0379 |
| cannulation site (groin) | 11 (2.4) * | 0 (0) | <0.001 |
| treatable in ambulatory care | 14 (3.1) | 1 (0.2) | <0.001 |
| Mesenteric ischemia: n (%) | 0 (0) | 0 (0) | 1.000 |
| Permanent pacemaker implantation: n (%) | 35 (7.7) | 15 (3.3) | 0.2203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilbring, M.; Alexiou, K.; Schmidt, T.; Petrov, A.; Taghizadeh-Waghefi, A.; Charitos, E.; Matschke, K.; Arzt, S.; Kappert, U. Safety and Efficacy of the Transaxillary Access for Minimally Invasive Aortic Valve Surgery. Medicina 2023, 59, 160. https://doi.org/10.3390/medicina59010160
Wilbring M, Alexiou K, Schmidt T, Petrov A, Taghizadeh-Waghefi A, Charitos E, Matschke K, Arzt S, Kappert U. Safety and Efficacy of the Transaxillary Access for Minimally Invasive Aortic Valve Surgery. Medicina. 2023; 59(1):160. https://doi.org/10.3390/medicina59010160
Chicago/Turabian StyleWilbring, Manuel, Konstantin Alexiou, Torsten Schmidt, Asen Petrov, Ali Taghizadeh-Waghefi, Efstratios Charitos, Klaus Matschke, Sebastian Arzt, and Utz Kappert. 2023. "Safety and Efficacy of the Transaxillary Access for Minimally Invasive Aortic Valve Surgery" Medicina 59, no. 1: 160. https://doi.org/10.3390/medicina59010160
APA StyleWilbring, M., Alexiou, K., Schmidt, T., Petrov, A., Taghizadeh-Waghefi, A., Charitos, E., Matschke, K., Arzt, S., & Kappert, U. (2023). Safety and Efficacy of the Transaxillary Access for Minimally Invasive Aortic Valve Surgery. Medicina, 59(1), 160. https://doi.org/10.3390/medicina59010160






