Abdominal Stent-Graft Treatment of Ascending Aortic Pseudoaneurysm Following Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
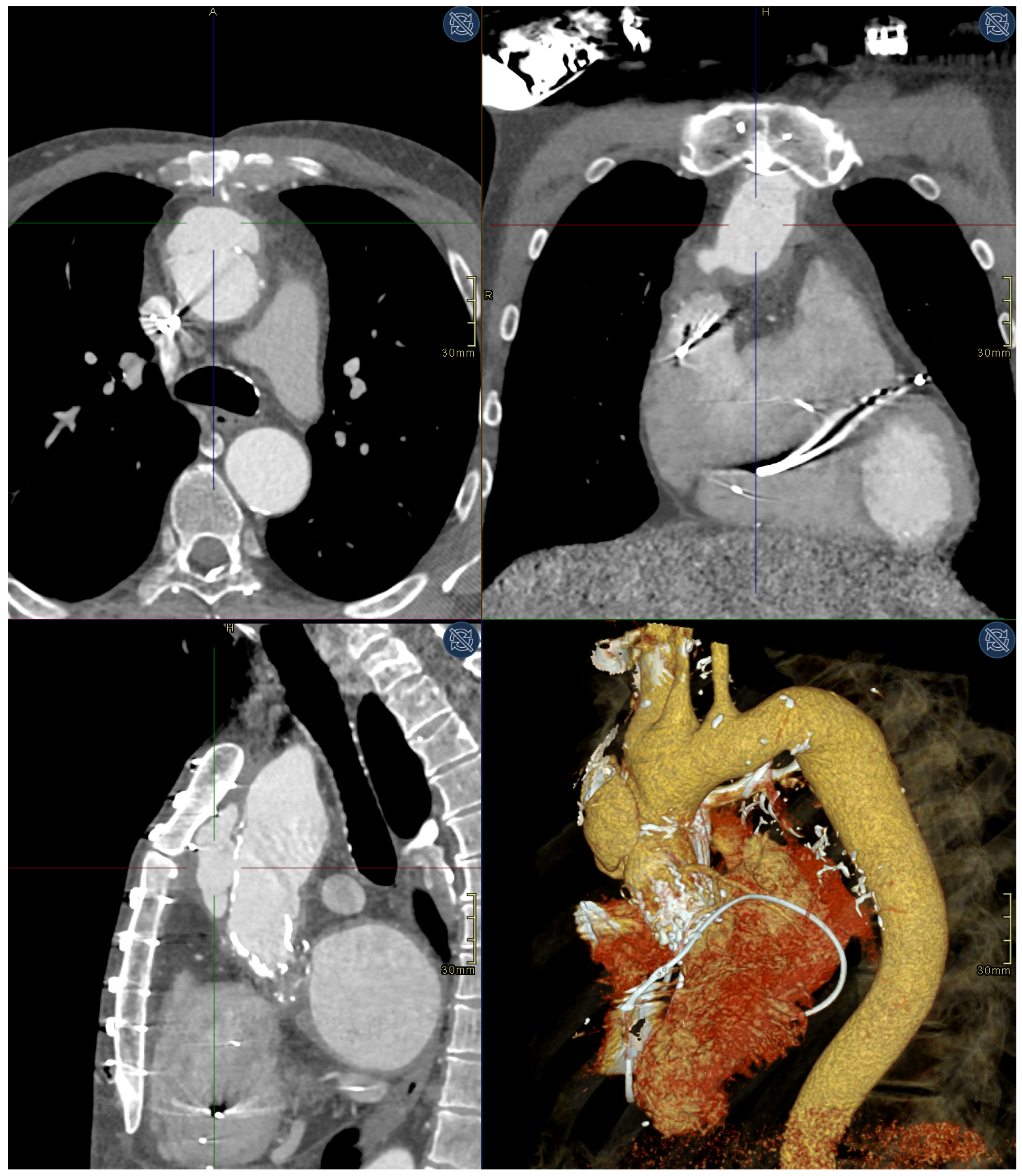
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aminian, A.; Lalmand, J.; El Nakadi, B. Perforation of the descending thoracic aorta during transcatheter aortic valve implantation (TAVI): An unexpected and dramatic procedural complication. Catheter. Cardiovasc. Interv. 2011, 77, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Roselli, E.E.; Idrees, J.; Greenberg, R.K.; Johnston, D.R.; Lytle, B.W. Endovascular stent grafting for ascending aorta repair in high-risk patients. J. Thorac. Cardiovasc. Surg. 2015, 149, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.; Oliveira-Pinto, J.; Soares, T.; Lachat, M.; Teixeira, J. Symptomatic Distal Anastomotic Pseudo-aneurysm after the Bentall Procedure Successfully Treated by Supra-aortic Trunk Debranching and Zone 0 Thoracic Endovascular Aneurysm Repair. EJVES Vasc. Forum 2020, 47, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Bonnet, N.; Leprince, P.; Kolsi, M.; Rama, A.; Pavie, A.; Gandjbakhch, I. Reoperation for false aneurysm of the ascending aorta after its prosthetic replacement: Surgical strategy. Ann. Thorac. Surg. 2005, 79, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, M.A.; Orszulak, T.A.; Sundt, T.M., III; Daly, R.C.; Dearani, J.A.; McGregor, C.G.; Mullany, C.J.; Puga, F.J.; Zehr, K.J.; Schaff, H.V. Thoracic aorta false aneurysm: What surgical strategy should be recommended? Ann. Thorac. Surg. 2006, 82, 81–89; discussion 89. [Google Scholar] [CrossRef]
- Parihar, B.; Choudhary, L.S.; Madhu, A.P.; Alpha, M.K.; Thankachen, R.; Shukla, V. Pseudoaneurysm of ascending aorta after aortic valve replacement. Ann. Thorac. Surg. 2005, 79, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Kouchoukos, N.T.; Wareing, T.H.; Murphy, S.F.; Perrillo, J.B. Sixteen-year experience with aortic root replacement. Results of 172 operations. Ann. Surg. 1991, 214, 308–320. [Google Scholar] [CrossRef]
- Bingley, J.A.; Gardner, M.A.; Stafford, E.G.; Mau, T.K.; Pohlner, P.G.; Tam, R.K.; Jalali, H.; Tesar, P.J.; O’Brien, M.F. Late complications of tissue glues in aortic surgery. Ann. Thorac. Surg. 2000, 69, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; Ginat, M.; Lecerf, L.; Houël, R.; Loisance, D. Aortic wall alterations after use of gelatin-resorcinol-formalin glue. Ann. Thorac. Surg. 2002, 73, 642–644. [Google Scholar] [CrossRef]
- Vejtasova, V.; Bonaventura, J.; Topalo, R.; Veselka, J. Ascending Aorta Pseudoaneurysm as a Rare, Late Complication after Valve-in-Valve Transcatheter Aortic Valve Implantation Procedure. Acta Cardiol. Sin. 2022, 38, 642–645. [Google Scholar] [CrossRef]
- Ruparelia, N.; Prendergast, B.D. Technical aspects of transcatheter aortic valve implantation (TAVI). In E-Journal of Cardiology Practice; European Society of Cardiology: Brussels, Belgium, 2016; Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-14/technical-aspects-of-transcatheter-aortic-valve-implantation-tavi (accessed on 5 November 2021).
- Thangam, M.; Balan, P. Chapter 2: Standard Wires and Balloones Used in TAVR. In TAVR Handbook; American Colledge of Cardiology: Washington, DC, USA, 2018; Available online: https://www.acc.org/-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/TAVR-Handbook/Chapter-2-Standard-wire-and-balloons-used-in-TAVR-March-2-2018.pdf (accessed on 28 November 2022).
- Mulder, E.J.; van Bockel, J.H.; Maas, J.; van den Akker, P.J.; Hermans, J. Morbidity and mortality of reconstructive surgery of noninfected false aneurysms detected long after aortic prosthetic reconstruction. Arch. Surg. 1998, 133, 45–49. [Google Scholar] [CrossRef]
- Katsumata, T.; Moorjani, N.; Vaccari, G.; Westaby, S. Mediastinal false aneurysm after thoracic aortic surgery. Ann. Thorac. Surg. 2000, 70, 547–552. [Google Scholar] [CrossRef]
- Murakami, M.; Morikage, N.; Samura, M.; Yamashita, O.; Suehiro, K.; Hamano, K. Fluorine-18-fluorodeoxyglucose positron emission tomography-computed tomography for diagnosis of infected aortic aneurysms. Ann. Thorac. Surg. 2014, 28, 575–578. [Google Scholar] [CrossRef]
- Quevedo, H.C.; Santiago-Trinidad, R.; Castellanos, J.; Atianzar, K.; Anwar, A.; Abi Rafeh, N. Systematic review of interventions to repair ascending aortic pseudoaneurysms. Ochsner J. 2014, 14, 576–585. [Google Scholar]
- Cohn, L.H.; Rizzo, R.J.; Adams, D.H.; Aranki, S.F.; Couper, G.S.; Beckel, N.; Collins, J.J. Reduced mortality and morbidity for ascending aortic aneurysm resection regardless of cause. Ann. Thorac. Surg. 1996, 62, 463–468. [Google Scholar] [CrossRef]
- Lakew, F.; Pasek, P.; Zacher, M.; Diegeler, A.; Urbanski, P.P. Femoral versus aortic cannulation for surgery of chronic ascending aortic aneurysm. Ann. Thorac. Surg. 2005, 80, 84–88. [Google Scholar] [CrossRef]
- Khoynezhad, A.; Donayre, C.E.; Walot, I.; Koopmann, M.C.; Kopchok, G.E.; White, R.A. Feasibility of endovascular repair of ascending aortic pathologies as part of an FDA-approved physician-sponsored investigational device exemption. J. Vasc. Surg. 2016, 63, 1483–1495. [Google Scholar] [CrossRef]
- Wang, C.; Regar, E.; Lachat, M.; von Segesser, L.K.; Maisano, F.; Ferrari, E. Endovascular treatment of non-dissected ascending aorta disease: A systematic review. Eur. J. Cardio-Thorac. Surg. 2018, 53, 317–324. [Google Scholar] [CrossRef]
- Fernández-Alonso, L.; Fernández Alonso, S.; Martínez Aguilar, E.; Santamarta Fariña, E.; Alegret Solé, J.; Atienza Pascual, M.; Martín, M.L.S.; Rodríguez, J.M.S.; Alvarez, A.; Vallepuga, R.C. Fenestrated and Scalloped Endovascular Grafts in Zone 0 and Zone 1 for Aortic Arch Disease. Ann. Thorac. Surg. 2020, 69, 360–365. [Google Scholar] [CrossRef]
- Khanji, M.Y.; Kumar, P.; Ionescu, A. Ascending aortic pseudo-aneurysm treated with ‘coil and plug’. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 1299. [Google Scholar] [CrossRef] [PubMed]
- Felipe Gaia, D.; Bernal, O.; Castilho, E.; Baeta Neves Duarte Ferreira, C.; Dvir, D.; Simonato, M.; Palma, J.H. First-in-Human Endo-Bentall Procedure for Simultaneous Treatment of the Ascending Aorta and Aortic Valve. JACC Case Rep. 2020, 2, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Parmer, S.S.; Carpenter, J.P.; Stavropoulos, S.W.; Fairman, R.M.; Pochettino, A.; Woo, E.Y.; Moser, W.; Bavaria, J.E. Endoleaks after endovascular repair of thoracic aortic aneurysms. J. Vasc. Surg. 2006, 44, 447–452. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhelj, D.; Langel, Č.; Bunc, M.; Kšela, J. Abdominal Stent-Graft Treatment of Ascending Aortic Pseudoaneurysm Following Transcatheter Aortic Valve Implantation. Medicina 2023, 59, 16. https://doi.org/10.3390/medicina59010016
Kuhelj D, Langel Č, Bunc M, Kšela J. Abdominal Stent-Graft Treatment of Ascending Aortic Pseudoaneurysm Following Transcatheter Aortic Valve Implantation. Medicina. 2023; 59(1):16. https://doi.org/10.3390/medicina59010016
Chicago/Turabian StyleKuhelj, Dimitrij, Črt Langel, Matjaž Bunc, and Juš Kšela. 2023. "Abdominal Stent-Graft Treatment of Ascending Aortic Pseudoaneurysm Following Transcatheter Aortic Valve Implantation" Medicina 59, no. 1: 16. https://doi.org/10.3390/medicina59010016
APA StyleKuhelj, D., Langel, Č., Bunc, M., & Kšela, J. (2023). Abdominal Stent-Graft Treatment of Ascending Aortic Pseudoaneurysm Following Transcatheter Aortic Valve Implantation. Medicina, 59(1), 16. https://doi.org/10.3390/medicina59010016






