Benefit of Uracil–Tegafur Used as a Postoperative Adjuvant Chemotherapy for Stage IIA Colon Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population and Definition of Statin Exposure
2.3. Outcome and Comorbidities Measurement
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
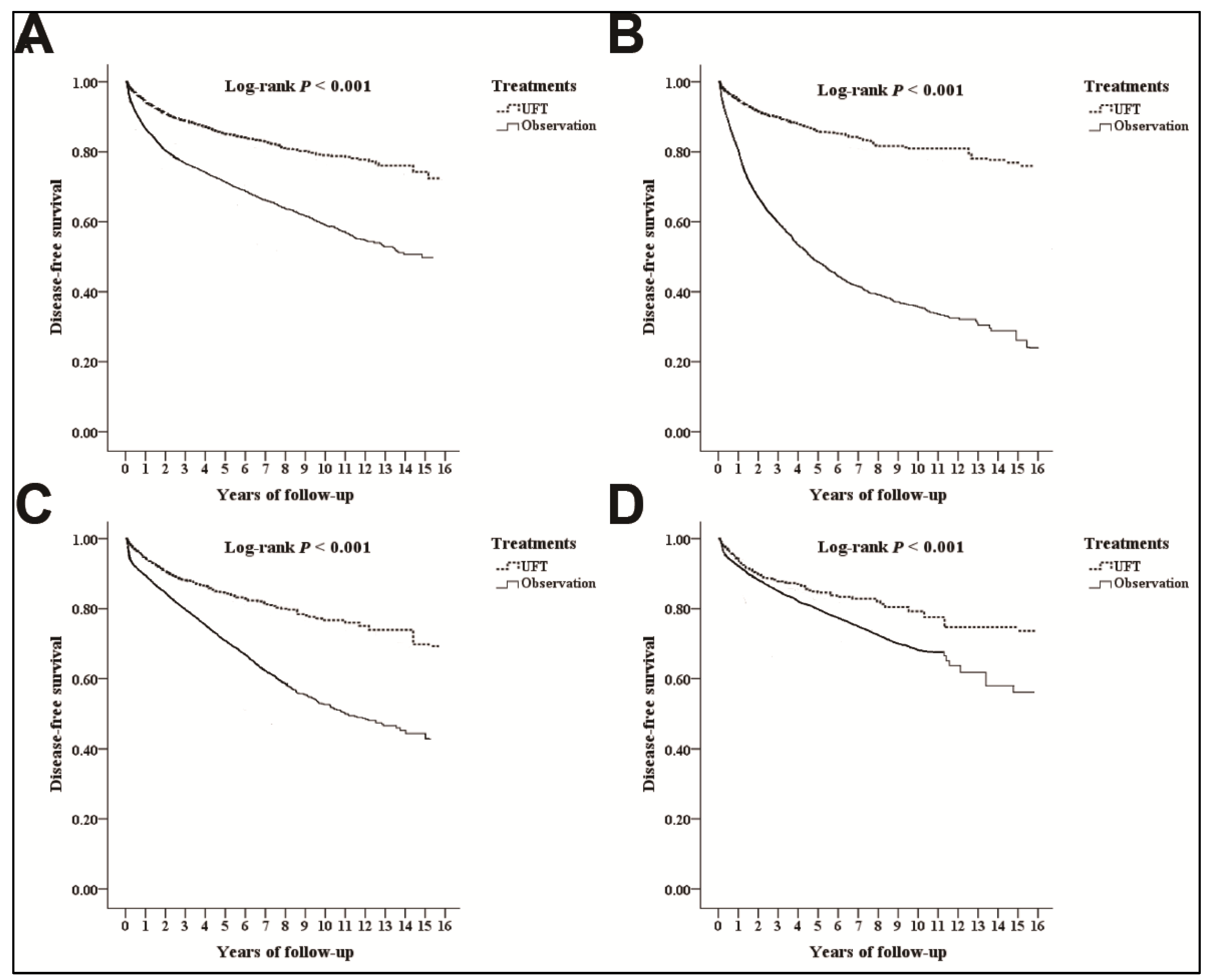
3.2. Disease-Free Survival
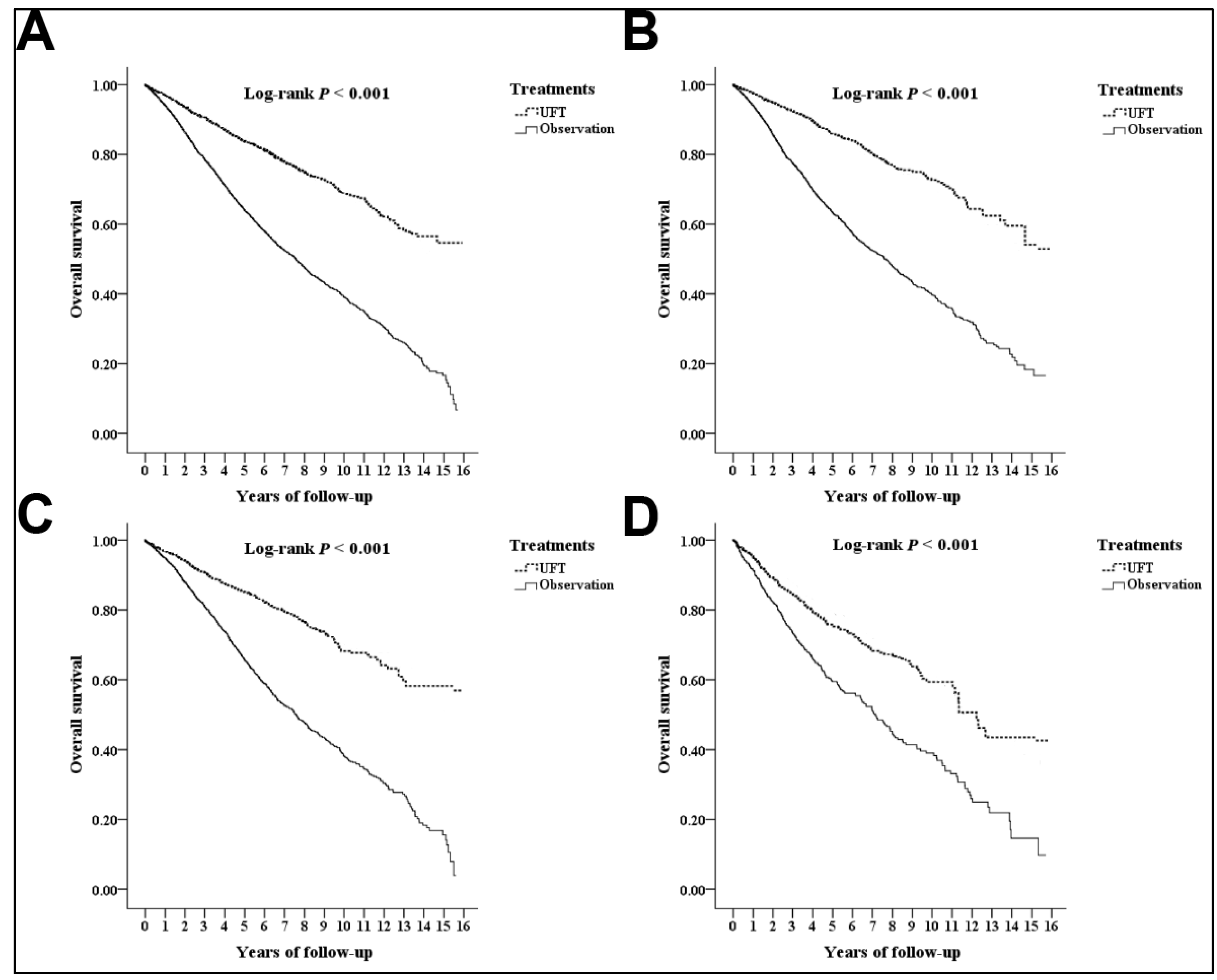
3.3. Overall Survival
3.4. Analyses for the Different Substages
4. Discussion
4.1. UFT Effectiveness
4.2. Survival Paradox in Patients with Localized Advanced Colon Cancer
4.3. Survival Risk Factors in Patients with Localized Advanced Colon Cancer
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Ethics approval
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Adjuvant Therapy for High-Risk Stage II or III Colon Adenocarcinoma: A Propensity Score-Matched, Nationwide, Population-Based Cohort Study. Cancers 2019, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Lonardi, S.; Sobrero, A.; Rosati, G.; Di Bartolomeo, M.; Ronzoni, M.; Aprile, G.; Scartozzi, M.; Banzi, M.; Zampino, M.G.; Pasini, F.; et al. Phase III trial comparing 3–6 months of adjuvant FOLFOX4/XELOX in stage II–III colon cancer: Safety and compliance in the TOSCA trial. Ann. Oncol. 2016, 27, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Wolmark, N.; Rockette, H.; Mamounas, E.; Jones, J.; Wieand, S.; Wickerham, D.L.; Bear, H.D.; Atkins, J.N.; Dimitrov, N.V.; Glass, A.G.; et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J. Clin. Oncol. 1999, 17, 3553–3559. [Google Scholar] [CrossRef]
- Lin, J.K.; Wang, W.S.; Hsieh, R.K.; Hsu, T.C.; Chiou, T.J.; Liu, J.H.; Fan, F.S.; Yen, C.C.; Lin, T.C.; Jiang, J.K.; et al. Phase II study of oral tegafur-uracil and folinic acid as first-line therapy for metastatic colorectal cancer: Taiwan experience. Jpn. J. Clin. Oncol. 2000, 30, 510–514. [Google Scholar] [CrossRef]
- Huang, W.Y.; Ho, C.L.; Lee, C.C.; Hsiao, C.W.; Wu, C.C.; Jao, S.W.; Yang, J.F.; Lo, C.H.; Chen, J.H. Oral tegafur-uracil as metronomic therapy following intravenous FOLFOX for stage III colon cancer. PLoS ONE 2017, 12, e0174280. [Google Scholar] [CrossRef]
- Chen, P.H.; Wu, Y.Y.; Lee, C.H.; Chung, C.H.; Chen, Y.G.; Huang, T.C.; Yeh, R.H.; Chang, P.Y.; Dai, M.S.; Lai, S.W.; et al. Uracil-tegafur vs fluorouracil as postoperative adjuvant chemotherapy in Stage II and III colon cancer: A nationwide cohort study and meta-analysis. Medicine 2021, 100, e25756. [Google Scholar] [CrossRef]
- Baxter, N.N.; Kennedy, E.B.; Bergsland, E.; Berlin, J.; George, T.J.; Gill, S.; Gold, P.J.; Hantel, A.; Jones, L.; Lieu, C.; et al. Adjuvant Therapy for Stage II Colon Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 892–910. [Google Scholar] [CrossRef]
- Kao, C.W.; Chiang, C.J.; Lin, L.J.; Huang, C.W.; Lee, W.C.; Lee, M.Y. Accuracy of long-form data in the Taiwan cancer registry. J. Formos. Med. Assoc. 2021, 120, 2037–2041. [Google Scholar] [CrossRef]
- Chiang, C.J.; You, S.L.; Chen, C.J.; Yang, Y.W.; Lo, W.C.; Lai, M.S. Quality assessment and improvement of nationwide cancer registration system in Taiwan: A review. Jpn. J. Clin. Oncol. 2015, 45, 291–296. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.-F.; Hsieh, C.-Y.; Hu, Y.-H. Two Decades of Research Using Taiwan’s National Health Insurance Claims Data: Bibliometric and Text Mining Analysis on PubMed. J. Med. Internet. Res. 2020, 22, e18457. [Google Scholar] [CrossRef]
- Simard, M.; Sirois, C.; Candas, B. Validation of the Combined Comorbidity Index of Charlson and Elixhauser to Predict 30-Day Mortality Across ICD-9 and ICD-10. Med. Care 2018, 56, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.C.; Kung, S.F.; Hu, S.C. The Relationship between Urbanization, the Built Environment, and Physical Activity among Older Adults in Taiwan. Int. J. Environ. Res. Public Health 2018, 15, 836. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.T.; Whang-Peng, J. Current status of clinical studies for colorectal cancer in Taiwan. Clin. Color. Cancer 2004, 4, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Jeng, Y.M.; Liang, J.T. Metronomic chemotherapy with tegafur-uracil following radical resection in stage II colorectal cancer. J. Formos. Med. Assoc. 2021, 120, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Shirao, K.; Moriya, Y.; Yoshida, S.; Kodaira, S.; Ohashi, Y. Final results of randomized trials by the National Surgical Adjuvant Study of Colorectal Cancer (NSAS-CC). Cancer Chemother. Pharmacol. 2011, 67, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Lembersky, B.C.; Wieand, H.S.; Petrelli, N.J.; O’Connell, M.J.; Colangelo, L.H.; Smith, R.E.; Seay, T.E.; Giguere, J.K.; Marshall, M.E.; Jacobs, A.D.; et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J. Clin. Oncol. 2006, 24, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.D.; Zhou, M.; Medeiros, K.L.; Peddi, P.; Kavanaugh, M.; Wu, X.C. Poor survival in stage IIB/C (T4N0) compared to stage IIIA (T1-2 N1, T1N2a) colon cancer persists even after adjusting for adequate lymph nodes retrieved and receipt of adjuvant chemotherapy. BMC Cancer 2016, 16, 460. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jeong, S.Y.; Choi, S.J.; Ryoo, S.B.; Park, J.W.; Park, K.J.; Oh, J.H.; Kang, S.B.; Park, H.C.; Heo, S.C.; et al. Survival paradox between stage IIB/C (T4N0) and stage IIIA (T1-2N1) colon cancer. Ann. Surg. Oncol. 2015, 22, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.D.; Zhou, M.; Medeiros, K.; Peddi, P. Positive surgical margins contribute to the survival paradox between patients with stage IIB/C (T4N0) and stage IIIA (T1-2N1, T1N2a) colon cancer. Surgery 2016, 160, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, K.M.; Lee, S.B.; Kim, G.R.; Han, Y.D.; Cho, M.S.; Hur, H.; Lee, K.Y.; Kim, N.K.; Min, B.S. Clinicopathological and biomolecular characteristics of stage IIB/IIC and stage IIIA colon cancer: Insight into the survival paradox. J. Surg. Oncol. 2019, 120, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fu, G.; Wei, W.; Huang, Y.; Wang, Z.; Liang, T.; Tian, S.; Chen, H.; Zhang, W. Re-Evaluation of the Survival Paradox Between Stage IIB/IIC and Stage IIIA Colon Cancer. Front. Oncol. 2020, 10, 595107. [Google Scholar] [CrossRef]
- Mo, S.; Dai, W.; Xiang, W.; Huang, B.; Li, Y.; Feng, Y.; Li, Q.; Cai, G. Survival Contradiction Between Stage IIA and Stage IIIA Rectal Cancer: A Retrospective Study. J. Cancer 2018, 9, 1466–1475. [Google Scholar] [CrossRef]
- Hu, J.M.; Chou, Y.C.; Wu, C.C.; Hsiao, C.W.; Lee, C.C.; Chen, C.T.; Hu, S.I.; Liu, W.T.; Jao, S.W. Adjuvant chemotherapy with tegafur/uracil for more than 1 year improves disease-free survival for low-risk Stage II colon cancer. J. Chin. Med. Assoc. 2016, 79, 477–488. [Google Scholar] [CrossRef]
- Costas-Chavarri, A.; Nandakumar, G.; Temin, S.; Lopes, G.; Cervantes, A.; Cruz Correa, M.; Engineer, R.; Hamashima, C.; Ho, G.F.; Huitzil, F.D.; et al. Treatment of Patients With Early-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Kotake, K.; Honjo, S.; Sugihara, K.; Hashiguchi, Y.; Kato, T.; Kodaira, S.; Muto, T.; Koyama, Y. Number of Lymph Nodes Retrieved is an Important Determinant of Survival of Patients with Stage II and Stage III Colorectal Cancer. Jpn. J. Clin. Oncol. 2012, 42, 29–35. [Google Scholar] [CrossRef]
- Chang, G.J.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Moyer, V.A. Lymph node evaluation and survival after curative resection of colon cancer: Systematic review. J. Natl. Cancer Inst. 2007, 99, 433–441. [Google Scholar] [CrossRef]
- Compton, C.; Fenoglio-Preiser, C.M.; Pettigrew, N.; Fielding, L.P. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer 2000, 88, 1739–1757. [Google Scholar] [CrossRef]
- Compton, C.C.; Greene, F.L. The staging of colorectal cancer: 2004 and beyond. CA A Cancer J. Clin. 2004, 54, 295–308. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Bentrem, D.J.; Stewart, A.K.; Talamonti, M.S.; Winchester, D.P.; Russell, T.R.; Ko, C.Y. Lymph node evaluation as a colon cancer quality measure: A national hospital report card. J. Natl. Cancer Inst. 2008, 100, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Babcock, B.D.; Aljehani, M.A.; Jabo, B.; Choi, A.H.; Morgan, J.W.; Selleck, M.J.; Luca, F.; Raskin, E.; Reeves, M.E.; Garberoglio, C.A.; et al. High-Risk Stage II Colon Cancer: Not All Risks Are Created Equal. Ann. Surg. Oncol. 2018, 25, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Loprinzi, C.L.; Sargent, D.J.; Thomé, S.D.; Alberts, S.R.; Haller, D.G.; Benedetti, J.; Francini, G.; Shepherd, L.E.; Francois Seitz, J.; et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J. Clin. Oncol. 2004, 22, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 359–369. [Google Scholar] [CrossRef]
- Lin, L.Y.; Warren-Gash, C.; Smeeth, L.; Chen, P.C. Data resource profile: The National Health Insurance Research Database (NHIRD). Epidemiol. Health 2018, 40, e2018062. [Google Scholar] [CrossRef]
- Tsai, F.-J.; Junod, V. Medical research using governments’ health claims databases: With or without patients’ consent? J. Public Health 2018, 40, 871–877. [Google Scholar] [CrossRef]
- Hsing, A.W.; Ioannidis, J.P. Nationwide Population Science: Lessons from the Taiwan National Health Insurance Research Database. JAMA Intern. Med. 2015, 175, 1527–1529. [Google Scholar] [CrossRef]

| Treatment Variables | UFT | Observation | p-Value | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Total | 1137 | 29.03 | 2779 | 70.97 | ||
| Gender | 0.582 | |||||
| Male | 681 | 59.89 | 1638 | 58.94 | ||
| Female | 456 | 40.11 | 1141 | 41.06 | ||
| Age (years ± SD) | 63.40 ± 10.25 | 65.12 ± 11.12 | <0.001 | |||
| HTN | With | 330 | 29.02 | 781 | 28.10 | 0.562 |
| Without | 807 | 70.98 | 1998 | 71.90 | ||
| DM | With | 201 | 17.68 | 455 | 16.37 | 0.321 |
| Without | 936 | 82.32 | 2324 | 83.63 | ||
| COPD | With | 55 | 4.84 | 108 | 3.89 | 0.186 |
| Without | 1082 | 95.16 | 2671 | 96.11 | ||
| CKD | With | 17 | 1.50 | 36 | 1.30 | 0.648 |
| Without | 1120 | 98.50 | 2743 | 98.70 | ||
| IHD | With | 60 | 5.28 | 145 | 5.22 | 0.937 |
| Without | 1077 | 94.72 | 2634 | 94.78 | ||
| CHD | With | 24 | 2.11 | 54 | 1.94 | 0.733 |
| Without | 1113 | 97.89 | 2725 | 98.06 | ||
| Stroke | With | 33 | 2.90 | 80 | 2.88 | 0.968 |
| Without | 1104 | 97.10 | 2699 | 97.12 | ||
| CCI_R | 1.03 ± 0.19 | 1.03 ± 0.15 | 0.998 | |||
| Prognosis | Disease-Free Survival (DFS) | Overall Survival (OS) | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Crude HR (95% CI) | p | Adjusted HR (95% CI) | p | Crude HR (95% CI) | p | Adjusted HR (95% CI) | p |
| Treatments | ||||||||
| UFT vs. observation | 0.613 (0.377–0.806) | <0.001 | 0.702 (0.489–1.024) | 0.074 | 0.785 (0.426–0.894) | <0.001 | 0.894 (0.542–1.186) | 0.477 |
| Gender | ||||||||
| Male vs. female | 1.365 (1.124–1.503) | <0.001 | 1.265 (1.106 –1.482) | <0.001 | 1.489 (1.303 –1.677) | <0.001 | 1.420 (1.298–1.583) | <0.001 |
| Age Groups (Years) | ||||||||
| <30 | Reference | |||||||
| 30–39 | 1.124 (0.822–1.825) | 0.182 | 1.024 (0.724–1.781) | 0.389 | 2.561 (1.786 –4.486) | <0.001 | 2.008 (1.025–3.349) | 0.035 |
| 40–49 | 1.304 (0.913–1.911) | 0.094 | 1.203 (0.902–1.924) | 0.172 | 3.789 (2.229–5.702) | <0.001 | 2.186 (1.097–3.570) | 0.001 |
| 50–59 | 1.386 (0.972–1.934) | 0.067 | 1.186 (0.851–1.876) | 0.234 | 5.978 (3.224–9.972) | <0.001 | 4.299 (2.004–8.301) | <0.001 |
| ≧60 | 1.402 (1.020–2.020) | 0.030 | 1.354 (0.989–1.986) | 0.069 | 7.124 (4.809–13.312) | <0.001 | 5.038 (2.897–9.896) | <0.001 |
| HTN | 1.678 (1.307–1.882) | <0.001 | 1.562 (1.265–1.782) | <0.001 | 1.863 (1.511–2.104) | <0.001 | 1.782 (1.428–2.006) | <0.001 |
| DM | 1.831 (1.367–2.010) | <0.001 | 1.762 (1.303–1.977) | <0.001 | 2.030 (1.724–2.308) | <0.001 | 1.975 (1.629–2.210) | <0.001 |
| COPD | 1.382 (0.986–1.769) | 0.072 | 1.283 (0.865–1.677) | 0.277 | 1.397 (1.002–1.784) | 0.049 | 1.270 (0.852–1.624) | 0.289 |
| CKD | 1.482 (1.153–1.780) | <0.001 | 1.293 (1.021–1.445) | 0.029 | 2.156 (1.503–2.970) | <0.001 | 2.011 (1.452–2.897) | <0.001 |
| IHD | 1.686 (1.112–1.897) | <0.001 | 1.553 (1.086–1.795) | 0.002 | 1.918 (1.628–2.774) | <0.001 | 1.897 (1.583–2.610) | <0.001 |
| CHD | 1.735 (1.442–1.975) | <0.001 | 1.652 (1.352–1.896) | <0.001 | 1.993 (1.586–2.601) | <0.001 | 1.824 (1.550–2.533) | <0.001 |
| Stroke | 1.808 (1.553–2.030) | <0.001 | 1.771 (1.448–1.909) | <0.001 | 2.030 (1.724–2.789) | <0.001 | 1.902 (1.652–2.672) | <0.001 |
| CCI_R | 1.304 (1.205–1.488) | <0.001 | 1.246 (1.112–1.304) | <0.001 | 1.372 (1.289–1.483) | <0.001 | 1.297 (1.158–1.372) | <0.001 |
| UFT vs. Observation | Disease-Free Survival (DFS) | ||||
|---|---|---|---|---|---|
| Stage | Patients | Adjusted HR | 95% CI | 95% CI | p |
| Overall | 3916 | 0.702 | 0.489 | 1.024 | 0.074 |
| Stage IIA | 2326 | 0.652 | 0.352 | 0.951 | 0.001 |
| Stage IIB | 819 | 0.713 | 0.492 | 1.029 | 0.079 |
| Stage IIC | 871 | 0.804 | 0.575 | 1.125 | 0.184 |
| Overall Survival (OS) | |||||
| Stage | Patients | Adjusted HR | 95% CI | 95% CI | p |
| Overall | 3916 | 0.894 | 0.542 | 1.186 | 0.477 |
| Stage IIA | 2326 | 0.734 | 0.475 | 1.093 | 0.503 |
| Stage IIB | 819 | 0.877 | 0.531 | 1.153 | 0.483 |
| Stage IIC | 871 | 0.904 | 0.638 | 1.256 | 0.425 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-H.; Jhou, H.-J.; Chung, C.-H.; Wu, Y.-Y.; Huang, T.-C.; Lee, C.-H.; Chien, W.-C.; Chen, J.-H. Benefit of Uracil–Tegafur Used as a Postoperative Adjuvant Chemotherapy for Stage IIA Colon Cancer. Medicina 2023, 59, 10. https://doi.org/10.3390/medicina59010010
Chen P-H, Jhou H-J, Chung C-H, Wu Y-Y, Huang T-C, Lee C-H, Chien W-C, Chen J-H. Benefit of Uracil–Tegafur Used as a Postoperative Adjuvant Chemotherapy for Stage IIA Colon Cancer. Medicina. 2023; 59(1):10. https://doi.org/10.3390/medicina59010010
Chicago/Turabian StyleChen, Po-Huang, Hong-Jie Jhou, Chi-Hsiang Chung, Yi-Ying Wu, Tzu-Chuan Huang, Cho-Hao Lee, Wu-Chien Chien, and Jia-Hong Chen. 2023. "Benefit of Uracil–Tegafur Used as a Postoperative Adjuvant Chemotherapy for Stage IIA Colon Cancer" Medicina 59, no. 1: 10. https://doi.org/10.3390/medicina59010010
APA StyleChen, P.-H., Jhou, H.-J., Chung, C.-H., Wu, Y.-Y., Huang, T.-C., Lee, C.-H., Chien, W.-C., & Chen, J.-H. (2023). Benefit of Uracil–Tegafur Used as a Postoperative Adjuvant Chemotherapy for Stage IIA Colon Cancer. Medicina, 59(1), 10. https://doi.org/10.3390/medicina59010010








