Malassezia Folliculitis following Triple Therapy for Cystic Fibrosis
Abstract
:1. Introduction
2. Case Reports
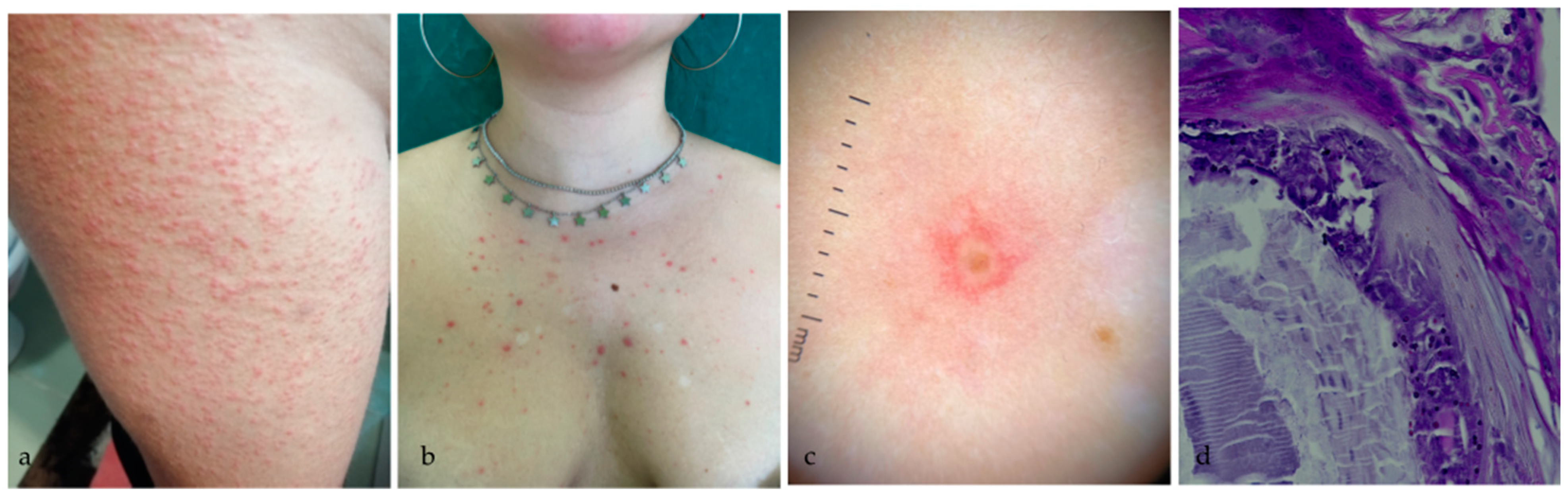
2.1. Case 1
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elborn, J.S. Cystic Fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic Fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef]
- Dickinson, K.M.; Collaco, J.M. Cystic Fibrosis. Pediatr. Rev. 2021, 42, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Scotet, V.; L’Hostis, C.; Férec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and Safety of the Elexacaftor plus Tezacaftor plus Ivacaftor Combination Regimen in People with Cystic Fibrosis Homozygous for the F508del Mutation: A Double-Blind, Randomised, Phase 3 Trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Barry, P.J.; Mall, M.A.; Álvarez, A.; Colombo, C.; de Winter-de Groot, K.M.; Fajac, I.; McBennett, K.A.; McKone, E.F.; Ramsey, B.W.; Sutharsan, S.; et al. Triple Therapy for Cystic Fibrosis Phe508del-Gating and -Residual Function Genotypes. N. Engl. J. Med. 2021, 385, 815–825. [Google Scholar] [CrossRef]
- Zemanick, E.T.; Taylor-Cousar, J.L.; Davies, J.; Gibson, R.L.; Mall, M.A.; McKone, E.F.; McNally, P.; Ramsey, B.W.; Rayment, J.H.; Rowe, S.M.; et al. A Phase 3 Open-Label Study of Elexacaftor/Tezacaftor/Ivacaftor in Children 6 through 11 Years of Age with Cystic Fibrosis and at Least One F508del Allele. Am. J. Respir. Crit. Care Med. 2021, 203, 1522–1532. [Google Scholar] [CrossRef]
- Leonhardt, K.; Autry, E.B.; Kuhn, R.J.; Wurth, M.A. CFTR Modulator Drug Desensitization: Preserving the Hope of Long Term Improvement. Pediatr. Pulmonol. 2021, 56, 2546–2552. [Google Scholar] [CrossRef]
- Hu, M.K.; Wood, G.; Dempsey, O. ‘Triple Therapy’ (Elexacaftor, Tezacaftor, Ivacaftor) Skin Rash in Patients with Cystic Fibrosis. Postgrad. Med. J. 2020, 98, 86. [Google Scholar] [CrossRef]
- Sosinski, L.M.; H, C.M.; Neugebauer, K.A.; Ghuneim, L.-A.J.; Guzior, D.V.; Castillo-Bahena, A.; Mielke, J.; Thomas, R.; McClelland, M.; Conrad, D.; et al. A Restructuring of Microbiome Niche Space Is Associated with Elexacaftor-Tezacaftor-Ivacaftor Therapy in the Cystic Fibrosis Lung. J. Cyst. Fibros. 2021. [Google Scholar] [CrossRef]
- Goldberg, R.H.; Matthews, N.H.; Hristov, A.C.; Wang, F. Urticaria Multiforme-like Eruption Due to a Novel Agent Elexacaftor/Tezacaftor/Ivacaftor in a Pediatric Patient with Cystic Fibrosis. JAAD Case Rep. 2021, 18, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Baker, O.; Hill, U. Elexacaftor, Tezacaftor and Ivacaftor: A Case of Severe Rash and Approach to Desensitisation. BMJ Case Rep. 2022, 15, e247042. [Google Scholar] [CrossRef] [PubMed]
- Stashower, J.; Carr, P.; Miller, V.; Zlotoff, B. Novel Reaction to New Cystic Fibrosis Medication Trikafta. Clin. Case Rep. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Balijepally, R.; Kwong, D.; Zhu, L.; Camacho, J.V.; Liu, A. Elexacaftor/Tezacaftor/Ivacaftor Outpatient Desensitization. Ann. Allergy Asthma Immunol. 2022, 128, 104–105. [Google Scholar] [CrossRef]
- Loyd, I.; Papac, N.; Hirshburg, J.; Levin, J.; Dannelley, J.; Dorris, J.; Stratton, J.; Mehdi, N. If At First You Don’t Succeed, Trikafta Again. J. Pediatr. Pharmacol. Ther. 2022, 27, 467–469. [Google Scholar] [CrossRef]
- Breneman, A.; Soliman, Y.S.; Gallitano, S.M. An Acneiform Eruption Associated with Elexacaftor/Tezacaftor/Ivacaftor Treatment. Dermatol. Online J. 2022, 27, 12. [Google Scholar] [CrossRef]
- Diseroad, E.R.; Mogayzel, P.J.; Pan, A. Rechallenge of Elexacaftor/Tezacaftor/Ivacaftor After Skin Rash in Two Pediatric Patients. J. Pediatr. Pharmacol. Ther. 2022, 27, 463–466. [Google Scholar] [CrossRef]
- Rubenstein, R.M.; Malerich, S.A. Malassezia (Pityrosporum) Folliculitis. J. Clin. Aesthet. Dermatol. 2014, 7, 37–41. [Google Scholar]
- Prohic, A.; Jovovic Sadikovic, T.; Krupalija-Fazlic, M.; Kuskunovic-Vlahovljak, S. Malassezia Species in Healthy Skin and in Dermatological Conditions. Int. J. Dermatol. 2016, 55, 494–504. [Google Scholar] [CrossRef]
- Vlachos, C.; Henning, M.A.S.; Gaitanis, G.; Faergemann, J.; Saunte, D.M. Critical Synthesis of Available Data in Malassezia Folliculitis and a Systematic Review of Treatments. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Kim, S.K.; Kim, Y.C. Comparison between Malassezia Folliculitis and Non- Malassezia Folliculitis. Ann. Dermatol. 2014, 26, 598. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.L.; Gaitanis, G.; Hay, R.J. Malassezia-Associated Skin Diseases, the Use of Diagnostics and Treatment. Front. Cell. Infect. Microbiol. 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]

| First Author, Reference, Year | Sex | Age | Rash-Type | Onset of the Rash (Days) | Re-Administration | New Rash | Therapeutic Decision |
|---|---|---|---|---|---|---|---|
| Leonhardt K [9], 2021 | F F | 20 47 | Morbilliform Urticarial-like | 7 9 | Yes Yes | Yes, after 11 days No | Desensitization Interruption |
| Goldberg RH [12], 2021 | M | 12 | Urticarial-like | 4 | No | Interruption | |
| Cheng A [13], 2022 | M | ? | Urticarial-like | 9 | Yes | Yes, after 24 h | Desensitization |
| Stashower J [14], 2021 | F | 24 | Urticarial-like | 7 | No | Discontinuation | |
| Hu MK [10], 2020 | F | 24 | Urticarial-like | 8 | Yes | No | Continuation |
| Balijepally R [15], 2022 | F F | 48 37 | Urticarial-like Urticarial-like | 7 6 | Yes Yes | Yes, only on full-dose Yes, after 8 h | Desensitization Desensitization |
| Loyd I [16], 2022 | M | 7 | Urticarial-like | 7 | Yes | Yes, the same day | Desensitization |
| Breneman A [17], 2021 | M | 29 | Acneiform | 120 | Yes | Yes, after 9 days | Continuation |
| Diseroad E [18], 2022 | F M | 12 14 | Maculo-papular Maculo-papular | 1 8 | Yes Yes | No No | Desensitization Desensitization |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li Pomi, F.; Di Bartolomeo, L.; Vaccaro, M.; Lentini, M.; Cristadoro, S.; Lucanto, M.C.; Lombardo, M.; Costa, S.; Borgia, F. Malassezia Folliculitis following Triple Therapy for Cystic Fibrosis. Medicina 2022, 58, 1204. https://doi.org/10.3390/medicina58091204
Li Pomi F, Di Bartolomeo L, Vaccaro M, Lentini M, Cristadoro S, Lucanto MC, Lombardo M, Costa S, Borgia F. Malassezia Folliculitis following Triple Therapy for Cystic Fibrosis. Medicina. 2022; 58(9):1204. https://doi.org/10.3390/medicina58091204
Chicago/Turabian StyleLi Pomi, Federica, Luca Di Bartolomeo, Mario Vaccaro, Maria Lentini, Simona Cristadoro, Maria Cristina Lucanto, Mariangela Lombardo, Stefano Costa, and Francesco Borgia. 2022. "Malassezia Folliculitis following Triple Therapy for Cystic Fibrosis" Medicina 58, no. 9: 1204. https://doi.org/10.3390/medicina58091204
APA StyleLi Pomi, F., Di Bartolomeo, L., Vaccaro, M., Lentini, M., Cristadoro, S., Lucanto, M. C., Lombardo, M., Costa, S., & Borgia, F. (2022). Malassezia Folliculitis following Triple Therapy for Cystic Fibrosis. Medicina, 58(9), 1204. https://doi.org/10.3390/medicina58091204









