Impacts of Outdoor Particulate Matter Exposure on the Incidence of Lung Cancer and Mortality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Published Study Search and Selection Criteria
2.2. Data Extraction
2.3. Statistical Analyses
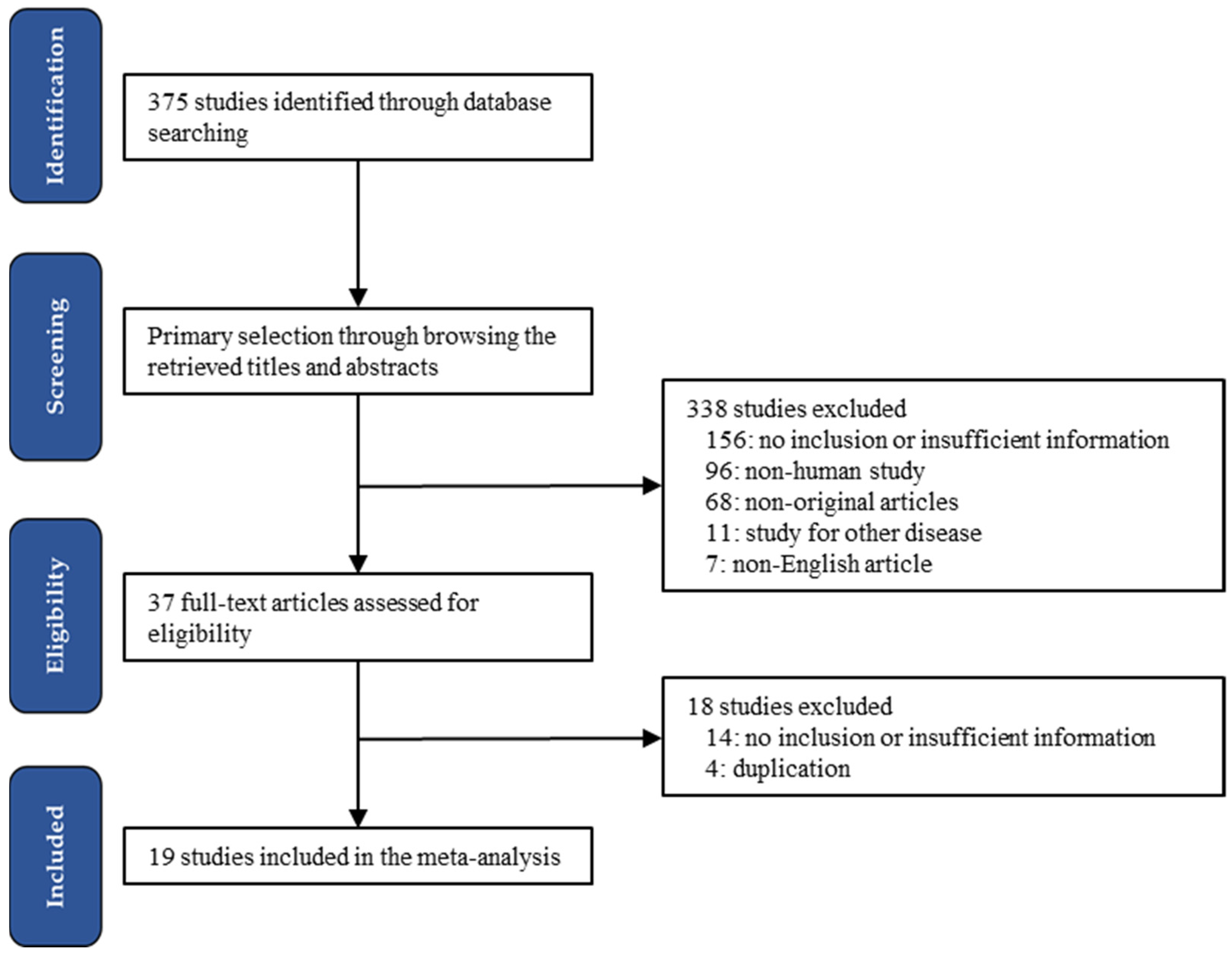
3. Results
3.1. Selection and Characteristics of Studies
3.2. The Incidence of Lung Cancers by PM Exposure
3.3. The Mortality by PM Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, K.J.; Ho, S.C.; Tsai, C.Y.; Chen, J.K.; Lee, C.N.; Lee, K.Y.; Chang, C.C.; Chen, T.T.; Feng, P.H.; Chen, K.Y.; et al. Exposure to PM(2.5) is associated with malignant pleural effusion in lung cancer patients. Ecotoxicol. Environ. Saf. 2021, 208, 111618. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.H.; Kwon, S.O.; Kim, S.Y.; Kim, W.J. Air Pollution and Incidence of Lung Cancer by Histological Type in Korean Adults: A Korean National Health Insurance Service Health Examinee Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 915. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Choi, K.S.; Inoue, S.; Shinozaki, R. Air pollution, temperature, and regional differences in lung cancer mortality in Japan. Arch. Environ. Health 1997, 52, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xue, T.; Wang, N.; Yuan, Y.; Liu, S.; Li, H.; Zhang, X.; Ren, A.; Ji, J. Burden of lung cancer attributable to ambient fine particles and potential benefits from air quality improvements in Beijing, China: A population-based study. Sci. Total Environ. 2020, 738, 140313. [Google Scholar] [CrossRef]
- Xing, D.F.; Xu, C.D.; Liao, X.Y.; Xing, T.Y.; Cheng, S.P.; Hu, M.G.; Wang, J.X. Spatial association between outdoor air pollution and lung cancer incidence in China. BMC Public Health 2019, 19, 1377. [Google Scholar] [CrossRef]
- Cao, M.; Chen, W. Epidemiology of lung cancer in China. Thorac. Cancer 2019, 10, 3–7. [Google Scholar] [CrossRef]
- Abbey, D.E.; Nishino, N.; McDonnell, W.F.; Burchette, R.J.; Knutsen, S.F.; Lawrence Beeson, W.; Yang, J.X. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am. J. Respir. Crit. Care Med. 1999, 159, 373–382. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A., 3rd; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Severi, G.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Boutron-Ruault, M.C.; Brandt, J.; Brunekreef, B.; Cesaroni, G.; et al. Long-term low-level ambient air pollution exposure and risk of lung cancer—A pooled analysis of 7 European cohorts. Environ. Int. 2021, 146, 106249. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.O. Deposition and clearance of inhaled particles. Environ. Health Perspect. 1976, 16, 41–53. [Google Scholar] [CrossRef]
- Buschini, A.; Cassoni, F.; Anceschi, E.; Pasini, L.; Poli, P.; Rossi, C. Urban airborne particulate: Genotoxicity evaluation of different size fractions by mutagenesis tests on microorganisms and comet assay. Chemosphere 2001, 44, 1723–1736. [Google Scholar] [CrossRef]
- Ghazipura, M.; Garshick, E.; Cromar, K.; Ma, Z. Ambient PM2.5 exposure and risk of lung cancer incidence in North America and Europe. Environ. Res. Commun. 2019, 1, 015004. [Google Scholar] [CrossRef]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Pitts, J.N., Jr.; Grosjean, D.; Mischke, T.M.; Simmon, V.F.; Poole, D. Mutagenic activity of airborne particulate organic pollutants. Toxicol. Lett. 1997, 1, 65–70. [Google Scholar] [CrossRef]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health 2008, 26, 339–362. [Google Scholar] [CrossRef]
- Carey, I.M.; Atkinson, R.W.; Kent, A.J.; van Staa, T.; Cook, D.G.; Anderson, H.R. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am. J. Respir. Crit. Care Med. 2013, 187, 1226–1233. [Google Scholar] [CrossRef]
- Cesaroni, G.; Badaloni, C.; Gariazzo, C.; Stafoggia, M.; Sozzi, R.; Davoli, M.; Forastiere, F. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ. Health Perspect. 2013, 121, 324–331. [Google Scholar] [CrossRef]
- Eckel, S.P.; Cockburn, M.; Shu, Y.H.; Deng, H.; Lurmann, F.W.; Liu, L.; Gilliland, F.D. Air pollution affects lung cancer survival. Thorax 2016, 71, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Gharibvand, L.; Lawrence Beeson, W.; Shavlik, D.; Knutsen, R.; Ghamsary, M.; Soret, S.; Knutsen, S.F. The association between ambient fine particulate matter and incident adenocarcinoma subtype of lung cancer. Environ. Health 2017, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.N.; DeRoos, A.J.; Hunt, R.P.; Gassett, A.J.; Mirabelli, M.C.; Bird, C.E.; Margolis, H.G.; Lane, D.; Bonner, M.R.; Anderson, G.; et al. Ambient air pollution and lung cancer risk among never-smokers in the Women’s Health Initiative. Environ. Epidemiol. 2019, 3, e076. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.E.; Garshick, E.; Dockery, D.W.; Smith, T.J.; Ryan, L.; Laden, F. Long-term ambient multipollutant exposures and mortality. Am. J. Respir. Crit. Care Med. 2011, 183, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.; Thiering, E.; Rzehak, P.; Krämer, U.; Hochadel, M.; Rauchfuss, K.M.; Gehring, U.; Wichmann, H.E. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup. Environ. Med. 2013, 70, 179–186. [Google Scholar] [CrossRef]
- Hystad, P.; Demers, P.A.; Johnson, K.C.; Carpiano, R.M.; Brauer, M. Long-term residential exposure to air pollution and lung cancer risk. Epidemiology 2013, 24, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Jerrett, M.; Burnett, R.T.; Beckerman, B.S.; Turner, M.C.; Krewski, D.; Thurston, G.; Martin, R.V.; van Donkelaar, A.; Hughes, E.; Shi, Y.; et al. Spatial analysis of air pollution and mortality in California. Am. J. Respir. Crit. Care Med. 2013, 188, 593–599. [Google Scholar] [CrossRef]
- Katanoda, K.; Sobue, T.; Satoh, H.; Tajima, K.; Suzuki, T.; Nakatsuka, H.; Takezaki, T.; Nakayama, T.; Nitta, H.; Tanabe, K.; et al. An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J. Epidemiol. 2011, 21, 132–143. [Google Scholar] [CrossRef]
- Lamichhane, D.K.; Kim, H.C.; Choi, C.M.; Shin, M.H.; Shim, Y.M.; Leem, J.H.; Ryu, J.S.; Nam, H.S.; Park, S.M. Lung Cancer Risk and Residential Exposure to Air Pollution: A Korean Population-Based Case-Control Study. Yonsei Med. J. 2017, 58, 1111–1118. [Google Scholar] [CrossRef]
- Lepeule, J.; Laden, F.; Dockery, D.; Schwartz, J. Chronic exposure to fine particles and mortality: An extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ. Health Perspect. 2012, 120, 965–970. [Google Scholar] [CrossRef]
- Lipsett, M.J.; Ostro, B.D.; Reynolds, P.; Goldberg, D.; Hertz, A.; Jerrett, M.; Smith, D.F.; Garcia, C.; Chang, E.T.; Bernstein, L. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am. J. Respir. Crit. Care Med. 2011, 184, 828–835. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, W.F.; Nishino-Ishikawa, N.; Petersen, F.F.; Chen, L.H.; Abbey, D.E. Relationships of mortality with the fine and coarse fractions of long-term ambient PM10 concentrations in nonsmokers. J. Expo. Anal. Environ. Epidemiol. 2000, 10, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Puett, R.C.; Hart, J.E.; Yanosky, J.D.; Spiegelman, D.; Wang, M.; Fisher, J.A.; Hong, B.; Laden, F. Particulate matter air pollution exposure, distance to road, and incident lung cancer in the nurses’ health study cohort. Environ. Health Perspect. 2014, 122, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, A.; Miller, A.B.; Weichenthal, S.A.; To, T.; Wall, C.; van Donkelaar, A.; Martin, R.V.; Crouse, D.L.; Villeneuve, P.J. Long-term exposure to fine particulate matter air pollution and the risk of lung cancer among participants of the Canadian National Breast Screening Study. Int. J. Cancer 2016, 139, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Nafstad, P.; Håheim, L.L.; Oftedal, B.; Gram, F.; Holme, I.; Hjermann, I.; Leren, P. Lung cancer and air pollution: A 27 year follow up of 16 209 Norwegian men. Thorax 2003, 58, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Avila-Tang, E.; Boffetta, P.; Hannan, L.M.; Olivo-Marston, S.; Thun, M.J.; Rudin, C.M. Lung cancer in never smokers: Clinical epidemiology and environmental risk factors. Clin. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef] [Green Version]
| Location | Period | Number of Patients | Subgroup | Outcome of Investigation: Concentration of PM (μg/m3) | ||
|---|---|---|---|---|---|---|
| PM2.5 | PM10 | |||||
| Carey 2013 [18] | UK | 2002 | 830,842 | 12.9 ± 1.4 | 19.7 ± 2.3 | |
| Cesaroni 2013 [19] | Italy | 1996–2010 | 1,265,058 | 23.0 ± 4.4 | NA | |
| Eckel 2016 [20] | USA | 1988–2009 | 352,053 | 13.7 ± 5.3 | 31.8 ± 12.1 | |
| Gharibvand 2017 [21] | USA | 2000–2001 | 80,044 | LC cases | 13.11 ± 3.98 | NA |
| Non-LC cases | 12.88 ± 3.7 | NA | ||||
| Gowda 2019 [22] | USA | 1993–1998 | 65,419 | LC cases | 13.1 ± 2.9 | NA |
| Non-LC cases | 13.3 ± 3.1 | NA | ||||
| Hart 2011 [23] | USA | 1985–2000 | 53,814 | 14.1 ± 4.0 | 26.8 ± 6.0 | |
| Heinrich 2013 [24] | Germany | 1985–1994 | 4752 | NA | (34.8–52.5) * | |
| Hystad 2013 [25] | Canada | 1975–1994 | 8897 | 11.9 ± 3.0 | NA | |
| Jerrett 2013 [26] | USA | 1998–2002 | 73,711 | 14.1 ± 12.4 | NA | |
| Katanoda 2011 [27] | Japan | 1974–1983 | 63,520 | (16.8–41.9) * | NA | |
| Lamichhane 2017 [28] | Korea | 1995–2014 | 1816 | Adenocarcinoma | NA | 55.3 ± 7.8 |
| Lepeule 2012 [29] | USA | 1979–2009 | 8096 | 15.9 | NA | |
| Lipsett 2011 [30] | USA | 1996–2005 | 133,479 | 15.6 ± 4.5 | 29.2 ± 9.7 | |
| McDonnell 2000 [31] | USA | 1973–1977 | 6338 | 31.9 ± 10.7 | 59.2 ± 16.8 | |
| Moon 2020 [2] | Korea | 2002–2007 | 6,567,909 | NA | 55.8 ± 6.3 | |
| Pope CA 3rd 2002 [32] | USA | 1979–1983 | 1,200,000 | 21.1 ± 4.6 | NA | |
| 1999–2000 | 14.0 ± 3.0 | NA | ||||
| 1982–1998 | NA | 28.8 ± 5.9 | ||||
| Puett 2014 [33] | USA | 1994–2010 | 1,510,027 | NA | NA | |
| Tomczak 2016 [34] | Canada | 1980–2005 | 89,835 | 9.1 † (1.3–17.6) * | NA | |
| Yang 2020 [5] | China | 2001–2016 | 12,150,000 | 77.3 ± 17.7 | NA | |
| Number of References | Heterogeneity Test (p-Value) | Random Effect (95% CI) | Egger’s Test (p-Value) | |
|---|---|---|---|---|
| PM2.5 | 6 | 0.002 | 1.081 (0.939, 1.245) | 0.848 |
| per 10 μg/m3 increment | 6 | 0.002 | 1.081 (0.939, 1.245) | 0.848 |
| Asia | 1 | 1.000 | 1.061 (1.044, 1.078) | NA |
| North America | 5 | 0.001 | 1.082 (0.853, 1.372) | 0.831 |
| Male | 1 | 1.000 | 1.590 (1.052, 2.404) | NA |
| Female | 2 | 0.423 | 0.973 (0.694, 1.362) | NA |
| Never smoker | 4 | 0.759 | 1.016 (0.769, 1.340) | 0.992 |
| Former smoker | 2 | 0.483 | 1.278 (1.032, 1.584) | NA |
| Current smoker | 3 | 0.002 | 1.147 (0.790, 1.665) | 0.985 |
| Adenocarcinoma | 5 | 0.017 | 1.210 (0.971, 1.508) | 0.331 |
| Squamous cell carcinoma | 2 | 0.500 | 1.151 (1.107, 1.198) | NA |
| Large cell carcinoma | 1 | 1.000 | 0.650 (0.406, 1.040) | NA |
| Small cell carcinoma | 1 | 1.000 | 1.650 (1.040, 2.619) | NA |
| PM10 | 6 | 0.308 | 0.972 (0.914, 1.034) | 0.489 |
| per 10 μg/m3 increment | 2 | 0.890 | 1.062 (0.932, 1.210) | NA |
| Asia | 6 | 0.308 | 0.972 (0.914, 1.034) | 0.489 |
| Male | 2 | 0.092 | 0.971 (0.831, 1.134) | NA |
| Female | 4 | 0.517 | 0.990 (0.925, 1.060) | 0.811 |
| Never smoker | 9 | 0.885 | 0.919 (0.871, 0.970) | 0.717 |
| Current smoker | 5 | 0.298 | 0.936 (0.771, 1.137) | 0.552 |
| Adenocarcinoma | 19 | 0.132 | 0.970 (0.920, 1.022) | 0.337 |
| Squamous cell carcinoma | 7 | 0.103 | 0.999 (0.927, 1.076) | 0.904 |
| Large cell carcinoma | 6 | 0.116 | 0.938 (0.843, 1.044) | 0.461 |
| Small cell carcinoma | 6 | 0.879 | 0.860 (0.683, 1.081) | 0.680 |
| Number of References | Heterogeneity Test (p-Value) | Random Effect (95% CI) | Egger’s Test (p-Value) | |
|---|---|---|---|---|
| PM2.5, all causes | 7 | <0.001 | 1.143 (1.011, 1.291) | 0.428 |
| per 5.3 μg/m3 increment | 2 | <0.001 | 1.194 (0.898, 1.587) | NA |
| per 10 μg/m3 increment | 5 | <0.001 | 1.101 (1.029, 1.178) | 0.021 |
| Asia | 1 | 1.000 | 1.240 (1.121, 1.371) | NA |
| Europe | 1 | 1.000 | 1.010 (1.000, 1.020) | NA |
| North America | 5 | <0.001 | 1.156 (0.989, 1.350) | 0.586 |
| PM2.5, lung cancer | 8 | <0.001 | 1.144 (1.002, 1.307) | 0.169 |
| per 4 μg/m3 increment | 1 | 1.000 | 1.021 (0.950, 1.097) | NA |
| per 5.3 μg/m3 increment | 2 | <0.001 | 1.221 (0.938, 1.590) | NA |
| per 10 μg/m3 increment | 7 | 0.055 | 1.166 (1.055, 1.288) | 0.182 |
| per 24.3 μg/m3 increment | 1 | 1.000 | 2.230 (0.558, 8.910) | NA |
| Europe | 8 | <0.001 | 1.144 (1.002, 1.307) | 0.169 |
| PM10, all causes | 4 | 0.066 | 1.091 (1.023, 1.162) | 0.204 |
| per 6 μg/m3 increment | 1 | 1.000 | 1.043 (1.011, 1.077) | NA |
| per 7 μg/m3 increment | 1 | 1.000 | 1.190 (1.080, 1.311) | NA |
| per 10 μg/m3 increment | 1 | 1.000 | 1.070 (0.988, 1.158) | NA |
| per 29.5 μg/m3 increment | 1 | 1.000 | 1.150 (0.939, 1.408) | NA |
| Europe | 2 | 0.095 | 1.124 (1.013, 1.247) | NA |
| North America | 2 | 0.351 | 1.045 (1.013, 1.079) | NA |
| PM10, lung cancer | 5 | <0.001 | 1.168 (0.962, 1.419) | 0.491 |
| per 6 μg/m3 increment | 1 | 1.000 | 0.999 (0.922, 1.083) | NA |
| per 10 μg/m3 increment | 2 | 0.001 | 1.307 (0.640, 2.668) | NA |
| per 12.1 μg/m3 increment | 1 | 1.000 | 1.270 (1.250, 1.290) | NA |
| per 29.5 μg/m3 increment | 1 | 1.000 | 1.840 (0.594, 5.704) | NA |
| Europe | 1 | 1.000 | 1.930 (1.294, 2.879) | NA |
| North America | 4 | <0.001 | 1.082 (0.881, 1.328) | 0.311 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyo, J.-S.; Kim, N.Y.; Kang, D.-W. Impacts of Outdoor Particulate Matter Exposure on the Incidence of Lung Cancer and Mortality. Medicina 2022, 58, 1159. https://doi.org/10.3390/medicina58091159
Pyo J-S, Kim NY, Kang D-W. Impacts of Outdoor Particulate Matter Exposure on the Incidence of Lung Cancer and Mortality. Medicina. 2022; 58(9):1159. https://doi.org/10.3390/medicina58091159
Chicago/Turabian StylePyo, Jung-Soo, Nae Yu Kim, and Dong-Wook Kang. 2022. "Impacts of Outdoor Particulate Matter Exposure on the Incidence of Lung Cancer and Mortality" Medicina 58, no. 9: 1159. https://doi.org/10.3390/medicina58091159
APA StylePyo, J.-S., Kim, N. Y., & Kang, D.-W. (2022). Impacts of Outdoor Particulate Matter Exposure on the Incidence of Lung Cancer and Mortality. Medicina, 58(9), 1159. https://doi.org/10.3390/medicina58091159







