Effect of Blood Flow Restriction Technique on Delayed Onset Muscle Soreness: A Systematic Review
Abstract
1. Introduction
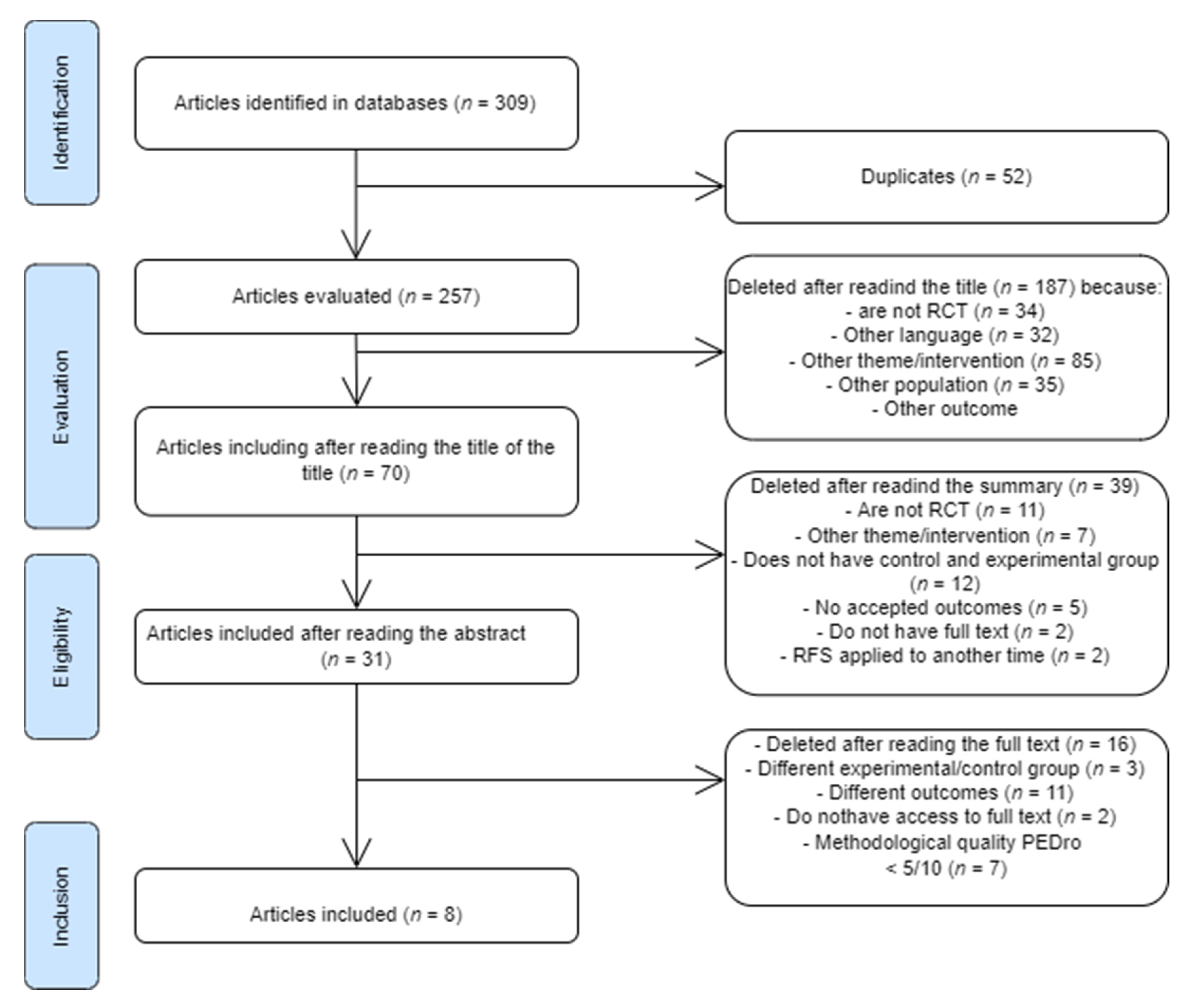
2. Materials and Methods
3. Results
Evaluation of Methodological Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1RM | 1 repetition maximum |
| AKE | Active Knee Extension |
| AOP | arterial occlusion pressure |
| ATP | adenosine triphosphate |
| BFR | blood flow restriction technique |
| BFR-C | blood flow restriction with low continuum pressure |
| BFR-I | blood flow restriction with high intermittent pressure |
| CG | Control group |
| CIR | Circumference |
| CK | creatine kinase |
| D | Dominant |
| DOMS | delayed onset muscular soreness |
| EG | Experimental group |
| G | Group |
| HL | High load |
| LDH | lactate dehydrogenase |
| LL | Light-load |
| M | Men |
| MVC | Maximum voluntary contraction |
| n | Sample size |
| ND | Non-dominant |
| NPS | Numeric pain scale |
| ns | Non-significant |
| PPT | Pressure pain threshold |
| RF | Rectus femoris |
| ROM | range of motion |
| RPE | ratings of perceived exertion |
| SBP | Systolic blood pressure |
| S | Significant |
| TF | tissue flossing |
| VAS | visual analog pain scale |
| VL | Vastus lateralis muscle |
| VM | Vastus medialis muscle |
| W | Women |
References
- Heiss, R.; Lutter, C.; Freiwald, J.; Hoppe, M.W.; Grim, C.; Poettgen, K.; Forst, R.; Bloch, W.; Hüttel, M.; Hotfiel, T. Advances in Delayed-Onset Muscle Soreness (DOMS)—Part II: Treatment and Prevention. Sportverletz Sportschaden 2019, 33, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Wohlfahrt, H.-W.; Haensel, L.; Mithoefer, K.; Ekstrand, J.; English, B.; McNally, S.; Orchard, J.; van Dijk, C.N.; Kerkhoffs, G.M.; Schamasch, P.; et al. Terminology and classification of muscle injuries in sport: The Munich consensus statement. Br. J. Sports Med. 2013, 47, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Hume, P.A.; Maxwell, L. Delayed Onset Muscle Soreness. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef]
- Cohen, J.; Cantecorp, K. Les DOMS: Compréhension d’un mécanisme en vue d’un traitement masso-kinésithérapique préventif: DOMS: Understanding the mechanism to enable preventive physiotherapy. Kinésithér. Rev. 2011, 11, 15–20. [Google Scholar] [CrossRef]
- Miles, M.P.; Clarkson, P.M. Exercise-induced muscle pain, soreness, and cramps. J. Sports Med. Phys. Fit. 1994, 34, 203–216. [Google Scholar]
- Abraham, W.M. Factors in delayed muscle soreness. Med. Sci. Sports 1977, 9, 11–20. [Google Scholar] [CrossRef]
- Coudreuse, J.M.; Dupont, P.; Nicol, C. Douleurs musculaires posteffort. Ann. Réadaptation Méd. Phys. 2004, 47, 290–298. [Google Scholar] [CrossRef]
- Armstrong, R.B.; Warren, G.L.; Warren, J.A. Mechanisms of Exercise-Induced Muscle Fibre Injury. Sports Med. 1991, 12, 184–207. [Google Scholar] [CrossRef]
- Hotfiel, T.; Freiwald, J.; Hoppe, M.W.; Lutter, C.; Forst, R.; Grim, C.; Bloch, W.; Hüttel, M.; Heiss, R. Advances in Delayed-Onset Muscle Soreness (DOMS): Part I: Pathogenesis and Diagnostics. Sportverletz. Sportschaden 2018, 32, 243–250. [Google Scholar] [CrossRef]
- Guo, J.; Li, L.; Gong, Y.; Zhu, R.; Xu, J.; Zou, J.; Chen, X. Massage Alleviates Delayed Onset Muscle Soreness after Strenuous Exercise: A Systematic Review and Meta-Analysis. Front. Physiol. 2017, 8, 747. [Google Scholar] [CrossRef]
- Hill, J.; Howatson, G.; Van Someren, K.; Leeder, J.; Pedlar, C. Compression garments and recovery from exercise-induced muscle damage: A meta-analysis. Br. J. Sports Med. 2014, 48, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Ziemann, E.; Banfi, G. Whole-Body Cryotherapy in Athletes: From Therapy to Stimulation. An Updated Review of the Literature. Front. Physiol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Hing, W.A.; White, S.G.; Bouaaphone, A.; Lee, P. Contrast therapy—A systematic review. Phys. Ther. Sport 2008, 9, 148–161. [Google Scholar] [CrossRef]
- Starrett, K.; Cordoza, G. Becoming a Supple Leopard: The Ultimate Guide to Resolving Pain, Preventing Injury, and Optimizing Athletic Performance, 2nd ed.; Victory Belt Publishing: Las Vegas, NV, USA, 2013; 400p. [Google Scholar]
- Driller, M.W.; Overmayer, R.G. The effects of tissue flossing on ankle range of motion and jump performance. Phys. Ther. Sport 2017, 25, 20–24. [Google Scholar] [CrossRef]
- Centner, C.; Lauber, B. A Systematic Review and Meta-Analysis on Neural Adaptations Following Blood Flow Restriction Training: What We Know and What We Don’t Know. Front. Physiol. 2020, 11, 887. [Google Scholar] [CrossRef]
- Pope, Z.K.; Willardson, J.M.; Schoenfeld, B.J. Exercise and blood flow restriction. J. Strength Cond. Res. 2013, 27, 2914–2926. [Google Scholar] [CrossRef]
- Charles, D.; White, R.; Reyes, C.; Palmer, D. A systematic review of the effects of blood flow restriction training on quadriceps muscle atrophy and circumference post ACL reconstruction. Int. J. Sports Phys. Ther. 2020, 15, 882. [Google Scholar] [CrossRef]
- Prill, R.; Schulz, R.; Michel, S. Tissue flossing: A new short-term compression therapy for reducing exercise-induced delayed-onset muscle soreness. A randomized, controlled and double-blind pilot crossover trial. J. Sports Med. Phys. Fit. 2018, 59, 861–867. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Blood flow restricted exercise and skeletal muscle health. Exerc. Sport Sci. Rev. 2009, 37, 78–85. [Google Scholar] [CrossRef]
- Bobes Álvarez, C.; Issa-Khozouz Santamaría, P.; Fernández-Matías, R.; Pecos-Martín, D.; Achalandabaso-Ochoa, A.; Fernández-Carnero, S.; Martínez-Amat, A.; Gallego-Izquierdo, T. Comparison of Blood Flow Restriction Training versus Non-Occlusive Training in Patients with Anterior Cruciate Ligament Reconstruction or Knee Osteoarthritis: A Systematic Review. J. Clin. Med. 2021, 10, 68. [Google Scholar] [CrossRef]
- Cerqueira, M.S.; Nascimento, J.D.S.D.; Maciel, D.G.; Barboza, J.A.M.; Vieira, W.H.D.B. Effects of blood flow restriction without additional exercise on strength reductions and muscular atrophy following immobilization: A systematic review. J. Sport Health Sci. 2020, 9, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Formiga, M.F.; Fay, R.; Hutchinson, S.; Locandro, N.; Ceballos, A.; Lesh, A.; Buscheck, J.; Meanor, J.; Owens, J.G.; Cahalin, L.P. Effect of Aerobic Exercise Training with and Without Blood Flow Restriction on Aerobic Capacity in Healthy Young Adults: A Systematic Review with Meta-Analysis. Int. J. Sports Phys. Ther. 2020, 15, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.; Stannard, M.S.; Duren, D.L.; Cook, J.L.; Stannard, J.P. Does Blood Flow Restriction Therapy in Patients Older Than Age 50 Result in Muscle Hypertrophy, Increased Strength, or Greater Physical Function? A Systematic Review. Clin. Orthop. Relat. Res. 2020, 478, 593–606. [Google Scholar] [CrossRef] [PubMed]
- de Queiros, V.S.; dos Santos, Í.K.; Almeida-Neto, P.F.; Dantas, M.; de França, I.M.; Vieira, W.H.D.B.; Neto, G.R.; Dantas, P.M.S.; Cabral, B.G.D.A.T. Effect of resistance training with blood flow restriction on muscle damage markers in adults: A systematic review. PLoS ONE 2021, 16, e0253521. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Y.; Yang, J.; Li, X.; Zeng, N.; Martin, R.L. The effectiveness of low intensity exercise and blood flow restriction without exercise on exercise induced muscle damage: A systematic review. Phys. Ther. Sport 2020, 46, 77–88. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Brandner, C.R.; Warmington, S.A. Delayed Onset Muscle Soreness and Perceived Exertion After Blood Flow Restriction Exercise. J. Strength Cond. Res. 2017, 31, 3101–3108. [Google Scholar] [CrossRef]
- Curty, V.M.; Melo, A.B.; Caldas, L.; Guimarães-Ferreira, L.; De Sousa, N.F.; Vassallo, P.F.; Vasquez, E.C.; Barauna, V.G. Blood flow restriction attenuates eccentric exercise-induced muscle damage without perceptual and cardiovascular overload. Clin. Physiol. Funct. Imaging 2017, 38, 468–476. [Google Scholar] [CrossRef]
- Freitas, E.D.S.; Bemben, M.G.; Silva, A.S.; Aniceto, R.R.; Ferreira-Junior, J.B.; Cirilo-Sousa, M.S. Resistance Exercise Performed at Different Degrees of Arterial Occlusion Pressure does not Induce Prolonged Oxidative Stress or Muscle Damage. Int. J. Sports Exerc. Med. 2017, 3, 1–9. [Google Scholar] [CrossRef][Green Version]
- Page, W.; Swan, R.; Patterson, S.D. The effect of intermittent lower limb occlusion on recovery following exercise-induced muscle damage: A randomized controlled trial. J. Sci. Med. Sport 2017, 20, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Penailillo, L.; Santander, M.; Zbinden-Foncea, H.; Jannas-Vela, S. Metabolic Demand and Indirect Markers of Muscle Damage After Eccentric Cycling with Blood Flow Restriction. Res. Q. Exerc. Sport 2020, 91, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, R.S.; Loenneke, J.; Fahs, C.; Kim, D.; Ye, X.; Abe, T.; Nosaka, K.; Bemben, M. Muscle damage after low-intensity eccentric contractions with blood flow restriction. Acta Physiol. Hung. 2014, 101, 150–157. [Google Scholar] [CrossRef]
- Wernbom, M.; Järrebring, R.; Andreasson, M.A.; Augustsson, J. Acute Effects of Blood Flow Restriction on Muscle Activity and Endurance During Fatiguing Dynamic Knee Extensions at Low Load. J. Strength Cond. Res. 2009, 23, 2389–2395. [Google Scholar] [CrossRef]
- Close, G.L.; Ashton, T.; McArdle, A.; MacLaren, D.P. The emerging role of free radicals in delayed onset muscle soreness and contraction-induced muscle injury. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 142, 257–266. [Google Scholar] [CrossRef]
- Dankel, S.J.; Mattocks, K.T.; Jessee, M.B.; Buckner, S.L.; Mouser, J.G.; Loenneke, J.P. Do metabolites that are produced during resistance exercise enhance muscle hypertrophy? Eur. J. Appl. Physiol. 2017, 117, 2125–2135. [Google Scholar] [CrossRef]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Monica, J.M.S. Exercise-Induced Muscle Damage in Humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Thiebaud, R.S.; Abe, T. Does blood flow restriction result in skeletal muscle damage? A critical review of available evidence. Scand. J. Med. Sci. Sports 2014, 24, e415–422. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef] [PubMed]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S. Studies Comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for Assessment of Pain Intensity in Adults: A Systematic Literature Review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
| Author(s) | Present Criteria | PEDro Score |
|---|---|---|
| Brandner e Warmington [30] | 2, 4, 9, 10, 11 | 5/10 |
| Curty et al. [31] | 2, 4, 9, 10, 11 | 5/10 |
| Freitas et al. [32] | 2, 4, 8, 9, 10, 11 | 6/10 |
| Page, Swan e Patterson [33] | 2, 5, 7, 8, 9, 10, 11 | 7/10 |
| Penailillo et al. [34] | 2, 4, 8, 9, 10, 11 | 6/10 |
| Prill, Schulz and Michel [19] | 1, 2, 3, 5, 7, 8, 10, 11 | 7/10 |
| Thiebaud et al. [35] | 2, 4, 9, 10, 11 | 5/10 |
| Wernbom et al. [36] | 2, 4, 8, 9, 10, 11 | 6/10 |
| Author | Sample | Objective | Description of the Intervention | Outcomes | Results | Main Conclusion |
|---|---|---|---|---|---|---|
| Brandner e Warmington [30] | N = 17 M untrained healthy/23 ± 3 years. Each performs the 4 different protocols G1: HL (80% 1 RM) without BFR G2: LL (20% 1 RM) without BFR G3: BFR-C: LL with BFR (20% 1 RM) G4: BFR-I: LL with BFR (20% 1 RM) | Determine and compare the perception and DOMS responses to resistance training with HL and LL with and without BFR | Exercise protocol Biceps curl (2 s of concentric contraction and 2 s eccentric) G1: 4 × 6–8 repetitions, 2.5 min rest G2–4: 1 × 30 reps and 3 × 15 reps with 30 s rest. BFR protocol: applied to the most proximal part of the arm. Pressure cycle: 50 mmHg for 30 s and then released for 10 s adding 20 mmHg to each inflation until it reaches 80% of resting PSS (G3) and 130%/0% at rest time (G4). |
| Pain
| The BFR combined with exercise causes higher DOMS. BFR-I causes more DOMS with longer recovery time than BFR-C |
| Curty et al. [31] | N = 9 M healthy active (26 ± 1 years) CG (without BFR) on a member EG (with BFR) on the other | Evaluate the acute effect of eccentric exercise with BFR on DOMS markers | Exercise protocol: Unilateral elbow extension (eccentric phase only), 3 × 10 reps at 130% of 1RM, 1 min rest. 30 min between the two groups. BFR protocol: pressure of ≈80% to have complete BFR in resting condition. The pressure was about 121 ± 7 mmHg in the dominant arm and 122 ± 4 mmHg in the non-dominant arm. |
| CIR
| There was no significant difference between the groups, however, it is noted that rom recovery occurs earlier in EG than in the CG. Thus, the BFR technique could be of benefit in the prevention of DOMS. |
| Freitas et al. [32] | N = 20 M healthy and trained/20.58 ± 2.39 years. Each performs the 4 protocols
| Investigate whether exercise combined with BFR with different pressures causes oxidative stress and muscle damage | Exercise Protocol (G1–4) Unilateral knee extension at 20% of 1 RM, 4 × 10 reps (1.5 s each concentric and eccentric phase) 30 s rest BFR Protocol (G2–4): cuff positioned in the inguinal part of the limb and inflated before the beginning of the first series until the end of the 4th series. The pressure according to % of the total AOP. |
| In all groups, there was an increase (p = 0.08) in the 24 h MVC after exercise compared to 1 h post-exercise, as well as a lower LDH level (p < 0.01) 24 h post-exercise than 48 h post-exercise. However, there is no significant difference between the groups at the level of pain, MVC and in CK and LDH levels at 1, 24 or 48 h post-exercise. | BFR combined with exercise has no effect on DOMS. |
| Page, Swan e Patterson [33] | N = 16 M healthy and physically active/22.6 ± 2.8 years EG with BFR after exercise (220 mmHg) N = 8 CG with BFR after exercise (20 mmHg) N = 8 | Evaluate the efficacy of BFR in recovery from exercise-induced muscle damage | Exercise protocol: 100 drop-jumps from a 0.6 m box 5 × 20 reps, 2 min rest BFR protocol: applied after exercise 3 × 5 min occlusion/5 min reperfusion. bilaterally in the proximal portion of the leg 220 mmHg (EG) 20 mmHg (CG) |
| The decrease in MVC is significantly higher in CF than EG at 24, 48 and 72 h after exercise (p < 0.05), CK levels are lower (p < 0.05) in EG at 24 and 48 h after exercise. For pain despite having a score of DOMS at 24 h post exercise for CG and EG (p < 0.05), pain is lower in EG at 24, 48 and 72 h after exercise (p < 0.05). There was no significant difference in CIR between the groups. | The BFR technique applied after exercise decreases DOMS. |
| Penailillo et al. [34] | N = 21 M healthy and active/24.0 ± 3.2 years CG without BFR N = 10 EG with BFR N = 10 | Compare the effects of an eccentric cycling session with and without BFR at the level of changes in cardiometabolic demand and indirect markers of muscle damage | Exercise protocol: Warm up (30–60 rpm to about 50 W) for 5 min on the eccentric ergometer followed by a 30 min workout always at 60 rpm (participants must resist movement to maintain % of Max Power Output. BFR Protocol: Application to the most proximal portion of each thigh with a pressure of ≈60% of arterial occlusion (estimated from the circumference of the thigh). The mean pressure used was 192 ± 24 mmHg. |
Measured before, soon after, and 24, 48, 72 and 96 h post-exercise |
| There was a reduction in MVC, PPT and ROM and an increase in CK and pain in both groups, however there is a greater increase in pain in the EG than in the CG and a longer ROM recovery time in the EG than the CG. Thus, the BFR technique combined with exercise induces greater DOMS. |
| Prill, Schulz and Michel [19] | Healthy, trained N = 15 (7 F and 8 M)/21.9 years (±2.3) 1st day Arm D/ND receives BFR, and another arm serves as CG. 2nd day arm that received BFR 7 days ago is CG, and the other receives the BFR | Assess whether the technical application of TF after exercise can reduce DOMS | Training protocol: Difficult exercises for the biceps 3 × 5–8 repetitions until failure, 1 min rest BFR Protocol: TF, 15 min after training around the arm (at 50 and 75% of maximum elongation) for 3 min combined with elbow (flexion/extension) and shoulder (RI with AB/RE pronation) movements. |
| 62% of the participants had lower DOMS with TF than without, at 24 h (p = 0.036) and 48h (p = 0.035) after exercise. | The TF technique plus exercise induces lower DOMS. |
| Thiebaud et al. [35] | N = 9 M active, but untrained/between 18–26 years. BFR group on one arm CG without BFR on the other arm | Evaluate the effects of BFR on indirect DOMS markers | Exercise protocol: Only eccentric contraction (2 s) of elbow flexors at 30% of 1RM, 4 × 30/15/15/15 reps, 30 s rest. 30 min of rest between the two groups. BFR Protocol: With initial pressure of 35 mmHg gradually increased to a final pressure of 120 mmHg |
|
| The BFR technique combined with exercise has no effect on DOMS. |
| Wernbom [36] | N = 11 (8 M and 3 F) trained/between 20–39 years. Each participant has one control leg: training without BFR (CG) and another experimental training with BFR (EG) | Investigate the differences in activity and muscle hardening in exercise with/without BFR. | Exercise protocol: Unilateral knee extension 30% of 1RM of 3× maximum reps (up to failure), 45 s rest (1.5 s for eccentric and concentric phase). BFR Protocol: applied at a pressure of 100 mmHg before exercise until the end of the |
|
| Pain is significantly lower in EG than in CG. The BFR combined with exercise relieves DOMS symptoms. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, S.; Forte, P.; Dewaele, E.; Branquinho, L.; Teixeira, J.E.; Ferraz, R.; Barbosa, T.M.; Monteiro, A.M. Effect of Blood Flow Restriction Technique on Delayed Onset Muscle Soreness: A Systematic Review. Medicina 2022, 58, 1154. https://doi.org/10.3390/medicina58091154
Rodrigues S, Forte P, Dewaele E, Branquinho L, Teixeira JE, Ferraz R, Barbosa TM, Monteiro AM. Effect of Blood Flow Restriction Technique on Delayed Onset Muscle Soreness: A Systematic Review. Medicina. 2022; 58(9):1154. https://doi.org/10.3390/medicina58091154
Chicago/Turabian StyleRodrigues, Sandra, Pedro Forte, Eva Dewaele, Luís Branquinho, José E. Teixeira, Ricardo Ferraz, Tiago M. Barbosa, and António M. Monteiro. 2022. "Effect of Blood Flow Restriction Technique on Delayed Onset Muscle Soreness: A Systematic Review" Medicina 58, no. 9: 1154. https://doi.org/10.3390/medicina58091154
APA StyleRodrigues, S., Forte, P., Dewaele, E., Branquinho, L., Teixeira, J. E., Ferraz, R., Barbosa, T. M., & Monteiro, A. M. (2022). Effect of Blood Flow Restriction Technique on Delayed Onset Muscle Soreness: A Systematic Review. Medicina, 58(9), 1154. https://doi.org/10.3390/medicina58091154










