Choroidal Changes in Blood Flow in Patients with Intermediate AMD after Oral Dietary Supplement Based on Astaxanthin, Bromelain, Vitamin D3, Folic Acid, Lutein, and Antioxidants
Abstract
:1. Background
2. Methods
Retinal Imaging Procedures
3. Statistical Analysis
4. Results
4.1. Demographic Data
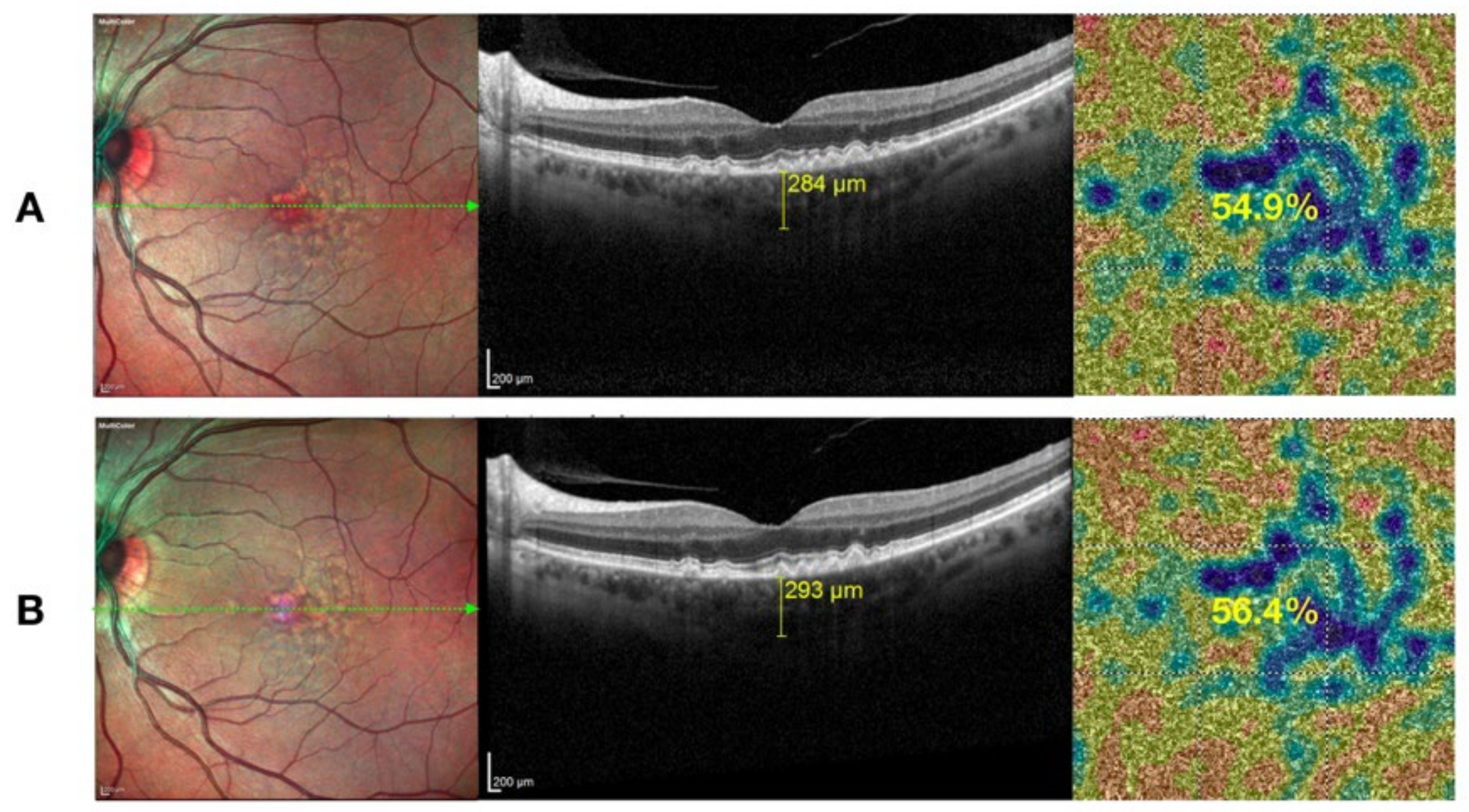
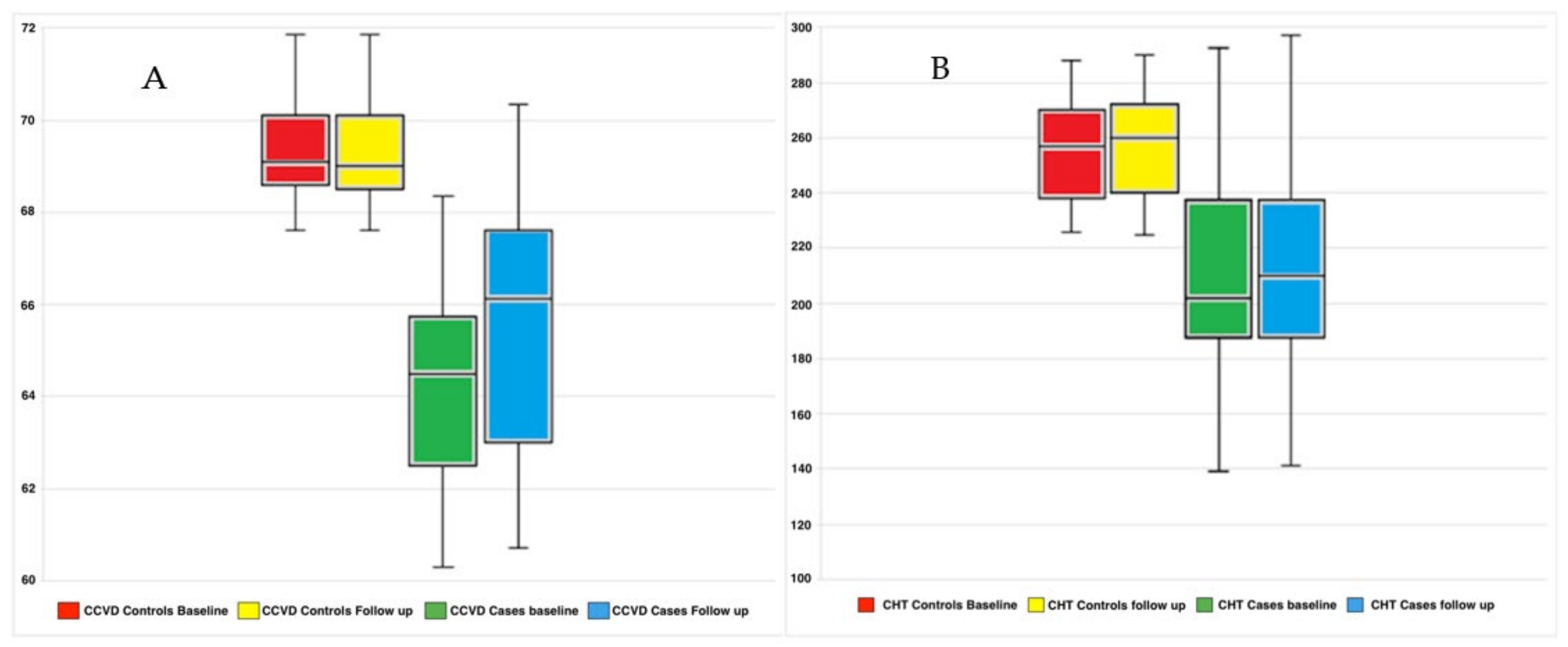
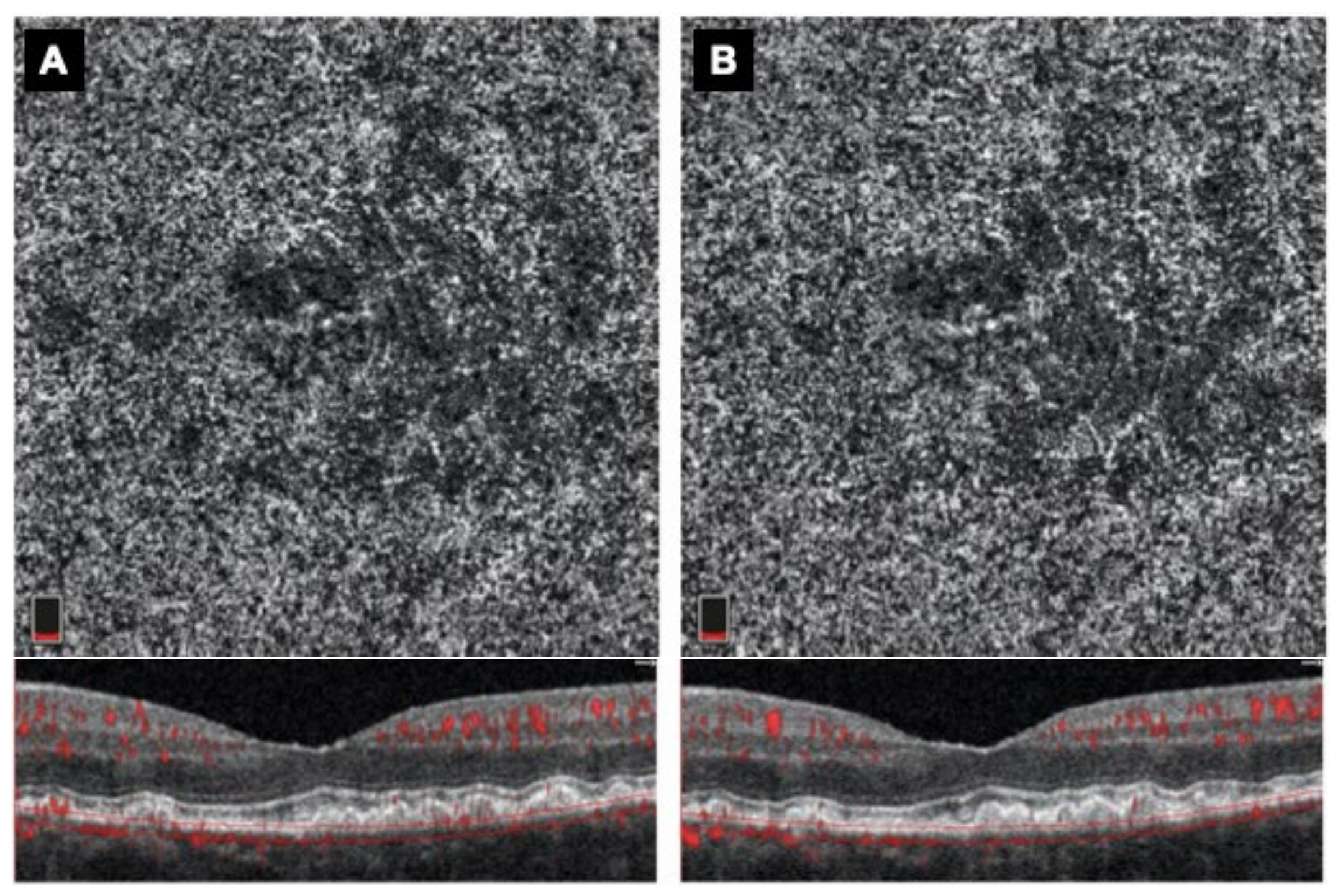
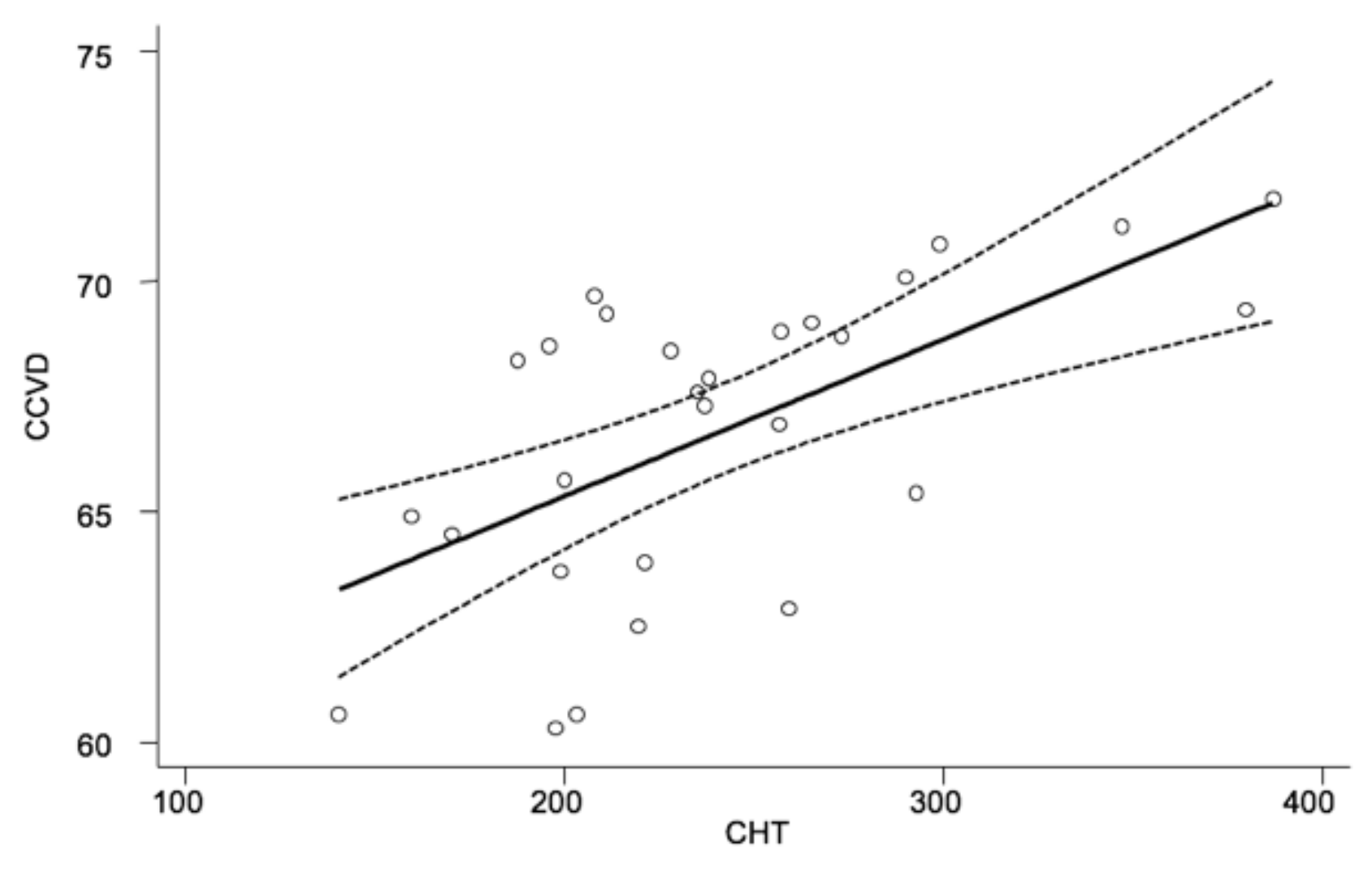
4.2. Functional and Morphological Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Astaxanthin | AXT |
| Age-related macular degeneration | AMD |
| Spectral domain optical coherence tomography | SD-OCT |
| Optical coherence tomography angiography | OCTA |
| Retinal pigment epithelium | RPE |
| Bruch’s membrane | BM |
| Geographic atrophy | GA |
| Best-corrected visual acuity | BCVA |
| Choroidal thickness | CHT |
| Choriocapillary vessel density | CCVD |
| Standard deviation | SD |
References
- Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.R.; Chan, C.C.; Ferris, F.L.; Chew, E.Y. Age-related macular degeneration. Lancet 2008, 372, 1835–1845. [Google Scholar] [CrossRef]
- Velez-Montoya, R.; Oliver, S.C.; Olson, J.L.; Fine, S.L.; Quiroz-Mercado, H.; Mandava, N. Current knowledge and trends in age-related macular degeneration: Genetics, epidemiology, and prevention. Retina 2014, 34, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A.; Rosenfeld, P.J. Pathway-based therapies for age-related macular degeneration: An integrated survey of emerging treatment alternatives. Retina 2010, 30, 1350–1367. [Google Scholar] [CrossRef]
- Wang, J.J.; Mitchell, P.; Rochtchina, E.; Tan, A.G.; Wong, T.Y.; Klein, R. Retinal vessel wall signs and the 5 year incidence of age related maculopathy: The Blue Mountains Eye Study. Br. J. Ophthalmol. 2004, 88, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Remsch, H.; Spraul, C.W.; Lang, G.K.; Lang, G.E. Changes of retinal capillary blood flow in age-related maculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Toto, L.; Borrelli, E.; Di Antonio, L.; Carpineto, P.; Mastropasqua, R. Retinal vascular plexuses’ changes in dry age-related macular degeneration, evaluated by means of optical coherence tomography angiography. Retina 2016, 36, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Boltz, A.; Luksch, A.; Wimpissinger, B.; Maar, N.; Weigert, G.; Frantal, S.; Brannath, W.; Garhöfer, G.; Ergun, E.; Stur, M.; et al. Choroidal blood flow and progression of age-related macular degeneration in the fellow eye in patients with unilateral choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4220–4225. [Google Scholar] [CrossRef] [PubMed]
- Feigl, B. Age-related maculopathy—linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog. Retin. Eye Res. 2009, 28, 63–86. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yoshida, K.; Saito, W.; Fujiya, A.; Ohgami, K.; Kitaichi, N.; Tsukahara, H.; Ishida, S.; Ohno, S. Astaxanthin increases choroidal blood flow velocity. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, H.; Takahashi, J.; Tsukahara, H.; Takehara, I. Effects of astaxanthin on human blood rheology. J. Clin. Biochem. Nutr. 2008, 43, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, R.; Di Antonio, L.; Di Staso, S.; Agnifili, L.; Di Gregorio, A.; Ciancaglini, M.; Mastropasqua, L. Optical coherence tomography angiography in retinal vascular diseases and choroidal neovascularization. J. Ophthalmol. 2015, 2015, 343515. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, R.; D’Aloisio, R.; De Nicola, C.; Ferro, G.; Senatore, A.; Libertini, D.; Di Marzio, G.; Di Nicola, M.; Di Martino, G.; Di Antonio, L.; et al. Widefield Swept Source OCTA in Retinitis Pigmentosa. Diagnostics 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, R.; D’Aloisio, R.; Di Antonio, L.; Erroi, E.; Borrelli, E.; Evangelista, F.; D’Onofrio, G.; Di Nicola, M.; Di Martino, G.; Toto, L. Widefield optical coherence tomography angiography in diabetic retinopathy. Acta Diabetol. 2019, 56, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Waheed, N.K.; Moult, E.M.; Fujimoto, J.G.; Rosenfeld, P.J. Optical Coherence Tomography Angiography of Dry Age-Related Macular Degeneration. Dev. Ophthalmol. 2016, 56, 91–100. [Google Scholar] [PubMed]
- Tan, C.S.; Fleckstein, M.; Schmitz-Valckenberg, S.; Holz, F.G. Clinical application of multicolor imaging technology. Ophthalmologica 2016, 236, 8–18. [Google Scholar] [CrossRef]
- Samara, W.A.; Shahlaee, A.; Sridhar, J.; Khan, M.A.; Ho, A.C.; Hsu, J. Quantitative Optical Coherence Tomography Angiography Features and Visual Function in Eyes with Branch Retinal Vein Occlusion. Am. J. Ophthalmol. 2016, 166, 76–83. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef]
- Borrelli, E.; Uji, A.; Sarraf, D.; Sadda, S.R. Alterations in the choriocapillaris in intermediate age-related macular degeneration. Invest. Opthalmol. Vis Sci. 2017, 58, 4792–4798. [Google Scholar] [CrossRef]
- Borrelli, E.; Mastropasqua, R.; Senatore, A.; Palmieri, M.; Toto, L.; Sadda, S.R.; Mastropasqua, L. Impact of Choriocapillaris Flow on Multifocal Electroretinography in Intermediate Age-Related Macular Degeneration Eye. Invest. Ophthalmol. Vis. Sci. 2018, 59, AMD25–AMD30. [Google Scholar] [CrossRef] [PubMed]
- Moult, E.M.; Waheed, N.K.; Novais, E.A.; Choi, W.; Lee, B.; Ploner, S.B.; Cole, E.D.; Louzada, R.N.; Lu, C.D.; Rosenfeld, P.J.; et al. Swept-source optical coherence tomography angiography reveals choriocapillaris alterations in eyes with nascent geographic atrophy and drusen-associated geographic atrophy. Retina 2016, 36, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Jurgens, G.; Quehenberger, O.; Koller, E. Autoxidation of human low density lipoprotein: Loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J. Lipid Res. 1987, 28, 495–509. [Google Scholar] [CrossRef]
- Li, W.; Hellsten, A.; Jacobsson, L.S.; Blomqvist, H.M.; Olsson, A.G.; Yuan, X.M. Alpha-tocopherol and astaxanthin decrease macrophage infiltration, apoptosis and vulnerability in atheroma of hyperlipidaemic rabbits. J. Mol. Cell. Cardiol. 2004, 37, 969–978. [Google Scholar] [CrossRef]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of astaxanthin on lipopolysaccharideinduced inflammation in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Isobe, A.; Otaka, Y.; Abematsu, Y.; Nakata, D.; Honma, C.; Sakurai, S.; Shimada, Y.; Horiguchi, M. Change in visual function from astaxanthin. Jpn. J. Clin. Ophthalmol. 2004, 58, 1051–1054. [Google Scholar]
- Nitta, T.; Ohgami, K.; Shiratori, K.; Shinmei, Y.; Chin, S.; Yoshida, K. Effects of astaxanthin on accommodation and asthenopia—Dose finding study in healthy volunteers. J. Clin. Ther. Med. 2005, 21, 543–556. [Google Scholar]
- Chitchumroonchokchai, C.; Bomser, J.A.; Glamm, J.E.; Failla, M.L. Xanthophylls and alpha-tocopherol decrease UVB-induced lipid peroxidation and stress signaling in human lens epithelial cells. J. Nutr. 2004, 134, 3225–3232. [Google Scholar] [CrossRef]
- Ang, M.; Wong, C.W.; Hoang, Q.V.; Cheung, G.C.M.; Lee, S.Y.; Chia, A.; Saw, S.M.; Ohno-Matsui, K.; Schmetterer, L. Imaging in myopia: Potential biomarkers, current challenges and future developments. Br. J. Ophthalmol. 2019, 103, 855–862. [Google Scholar] [CrossRef]
- Ambati, J.; Ambati, B.K.; Yoo, S.H.; Ianchulev, S.; Adamis, A.P. Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Surv. Ophthalmol. 2003, 48, 257–293. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef] [PubMed]

| Baseline | Follow Up | ||||
|---|---|---|---|---|---|
| Controls | Cases | p-Value 1 | Cases | p-Value 2 | |
| Numbers | 13 | 15 | 15 | ||
| Age (yr), mean ± SD | 68.92 ± 8.62 | 70.20 ± 8.13 | n.s. | 70.20 ± 8.13 | n.s. |
| Gender, n (%) | n.s. | ||||
| Male | 6 (46) | 7 (33) | 7 (33) | n.s. | |
| Female | 7 (54) | 8 (66) | 8 (66) | n.s. | |
| Eye, n (%) | n.s. | ||||
| R | 8 (62) | 10 (67) | 10 (67) | n.s. | |
| L | 5 (38) | 5 (33) | 5 (33) | n.s. | |
| BCVA (LogMAR) | 0.0 ± 0.0 | 0.1 ± 0.1 | n.s. | 0.1 ± 0.1 | n.s. |
| IOP (mmHg) | 17.42 ± 3.21 | 16.77 ± 3.85 | n.s. | 15.65 ± 3.72 | n.s. |
| CHT (micron) CCVD (%) | 277.15 ± 61.80 69.42 ± 1.26 | 210.29 ± 39.81 64.45 ± 7.79 | 0.002 <0.001 | 213.65 ± 40.50 65.55 ± 3.13 | <0.001 <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Aloisio, R.; Di Antonio, L.; Toto, L.; Rispoli, M.; Di Iorio, A.; Delvecchio, G.; Mastropasqua, R. Choroidal Changes in Blood Flow in Patients with Intermediate AMD after Oral Dietary Supplement Based on Astaxanthin, Bromelain, Vitamin D3, Folic Acid, Lutein, and Antioxidants. Medicina 2022, 58, 1092. https://doi.org/10.3390/medicina58081092
D’Aloisio R, Di Antonio L, Toto L, Rispoli M, Di Iorio A, Delvecchio G, Mastropasqua R. Choroidal Changes in Blood Flow in Patients with Intermediate AMD after Oral Dietary Supplement Based on Astaxanthin, Bromelain, Vitamin D3, Folic Acid, Lutein, and Antioxidants. Medicina. 2022; 58(8):1092. https://doi.org/10.3390/medicina58081092
Chicago/Turabian StyleD’Aloisio, Rossella, Luca Di Antonio, Lisa Toto, Marco Rispoli, Angelo Di Iorio, Giancarlo Delvecchio, and Rodolfo Mastropasqua. 2022. "Choroidal Changes in Blood Flow in Patients with Intermediate AMD after Oral Dietary Supplement Based on Astaxanthin, Bromelain, Vitamin D3, Folic Acid, Lutein, and Antioxidants" Medicina 58, no. 8: 1092. https://doi.org/10.3390/medicina58081092
APA StyleD’Aloisio, R., Di Antonio, L., Toto, L., Rispoli, M., Di Iorio, A., Delvecchio, G., & Mastropasqua, R. (2022). Choroidal Changes in Blood Flow in Patients with Intermediate AMD after Oral Dietary Supplement Based on Astaxanthin, Bromelain, Vitamin D3, Folic Acid, Lutein, and Antioxidants. Medicina, 58(8), 1092. https://doi.org/10.3390/medicina58081092









