The Successful Recovery of a Critically Ill COVID-19 Patient, Following the Combination of Therapeutic Plasma Exchange and Convalescent Plasma Transfusion: A Case Report
Abstract
:1. Introduction
2. Case Report
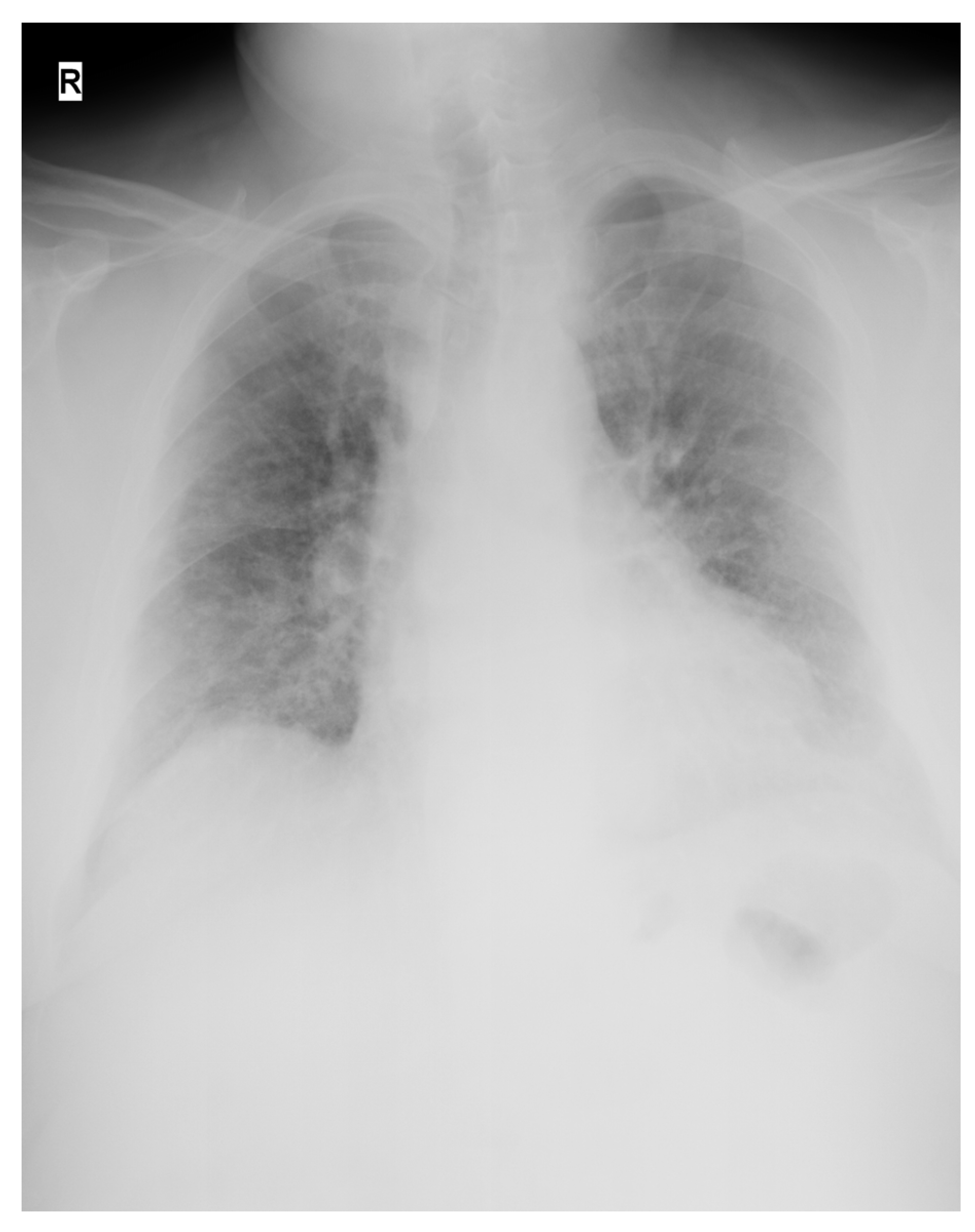
| Presentation | A 52-year-old man presented in the ER with malaise, fever, severe cough, tachypnea, tachycardia, and dyspnea, which started 2 days before the presentation. Upon rapid assessment, the patient had low oxygen saturation and showed signs of respiratory failure. The decision was made to transfer the patient to the ICU after preliminary radiological examination. |
| Initial treatment | Upon ICU admission, the patient was immediately started on high flow nasal oxygenation (60 L/min, FiO2 = 100%) combined with a non-rebreathing oxygen mask (oxygen flow rate 15 L/min). Antiviral therapy with remdesivir, high dose corticosteroid pulse therapy with methylprednisolone, and therapeutic anticoagulation with nadroparine were started, according to hospital and national guildelines. |
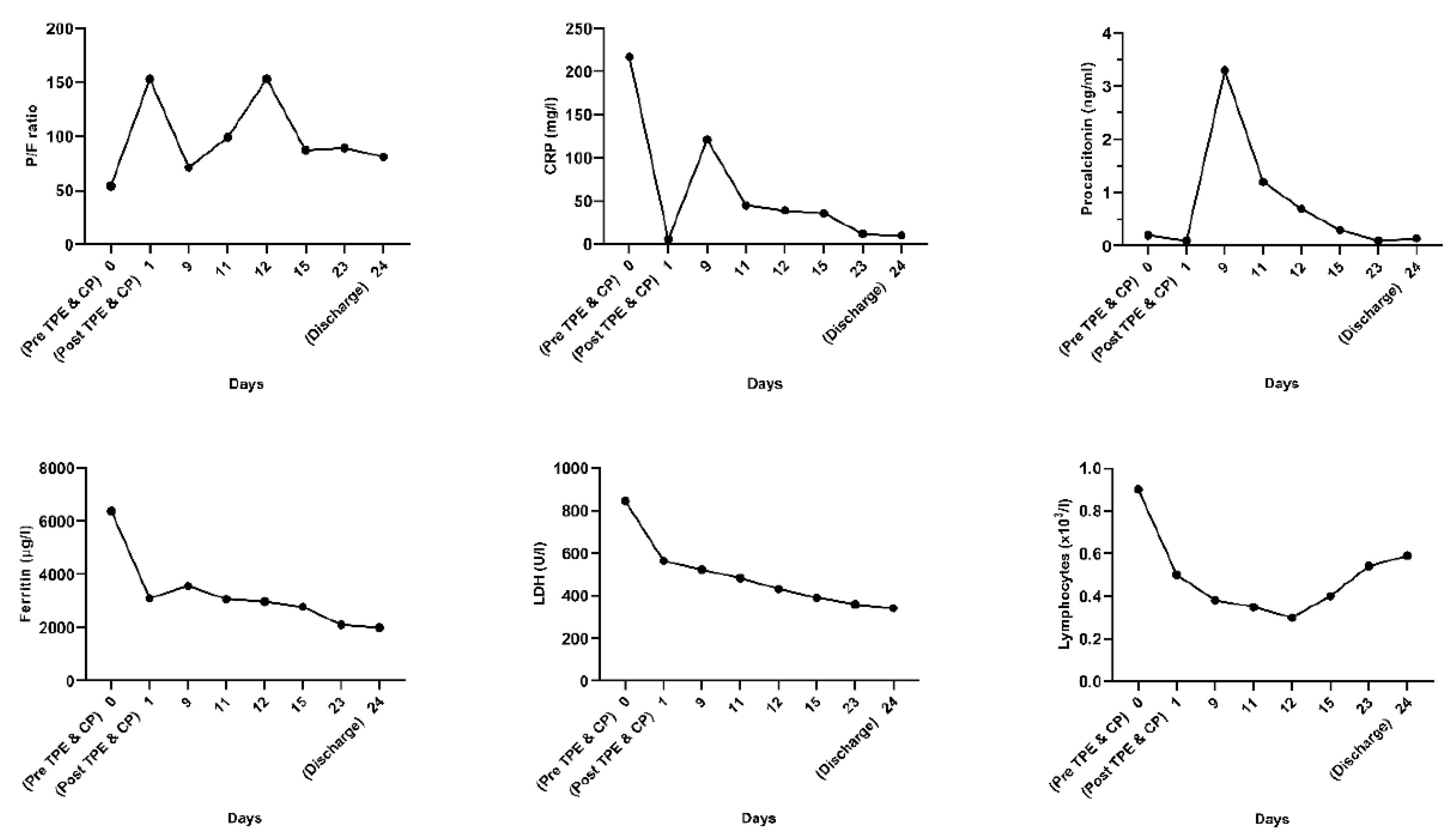
| Day 1 | Patient’s respiratory status worsened, requiring escalation to non-invasive ventilation with CPAP facemask and, 3 h later, after rapid assessment, intubation and mechanical ventilation were considered vital. A dual lumen 14 French dialysis catheter was placed in the right femoral vein under echographic guidance and a single TPE session was performed using 40 mL/kg FFP as substitute. Upon completion of the TPE session, the patient was transfused with 500 mL ABO compatible CP under careful monitorization. (The procedures were performed on day 3 after the onset of symptoms). |
| Day 9 | Sedation and neuromuscular blockade were ceased and respiratory weaning from mechanical ventilation was started by switching the ventilation mode to spontaneous (CPAP). |
| Day 11 | Extubation was performed. |
| Day 12 | Uncontrollable hypercapnia leading to neurologic status aggravation determined intubation and the start of mechanical ventilation and continuous sedation. |
| Day 15 | Patient was weaned off mechanical ventilation and extubated. RT-PCR test resulted negative and the patient was transferred to the non-COVID-19 ICU. |
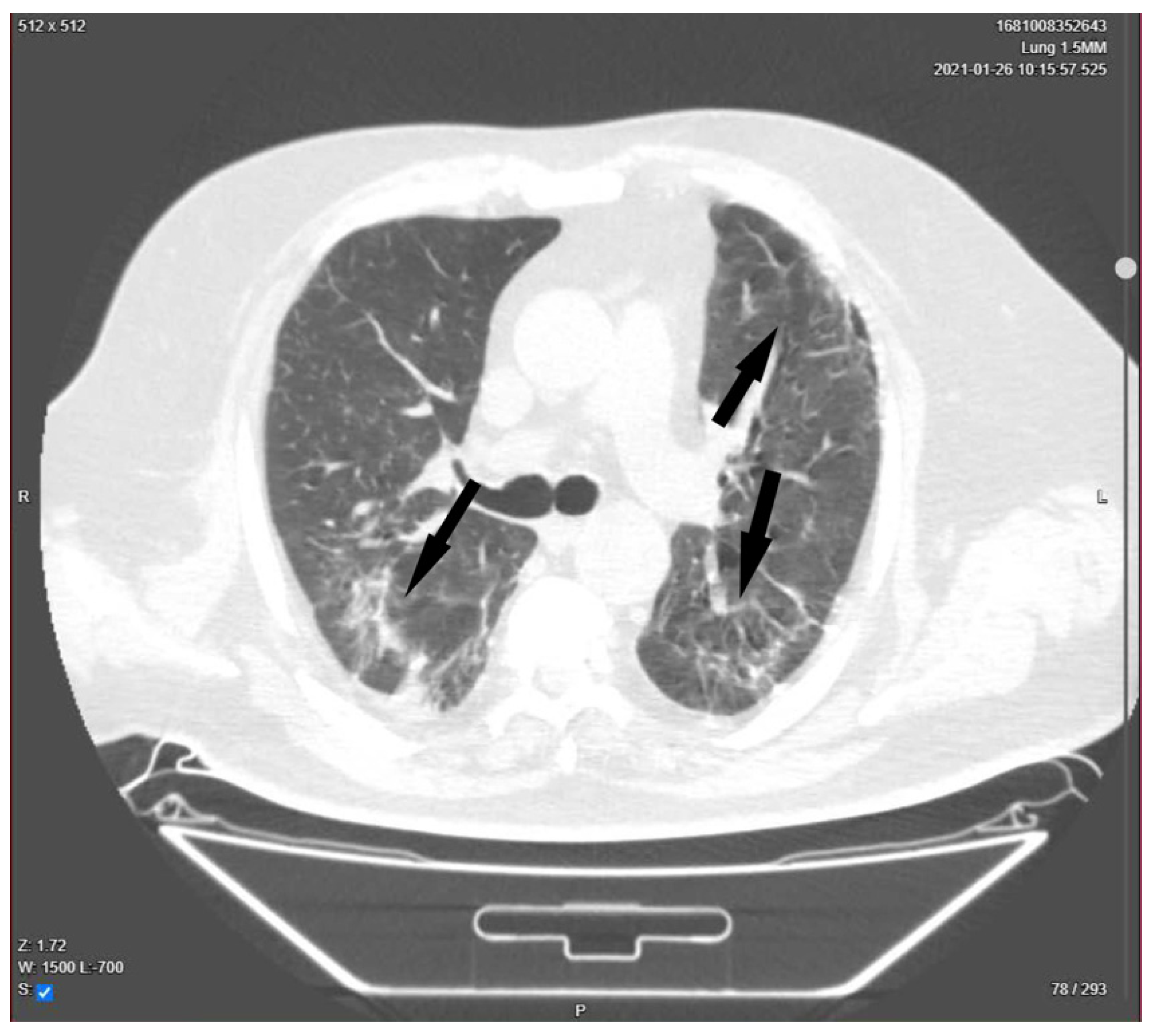
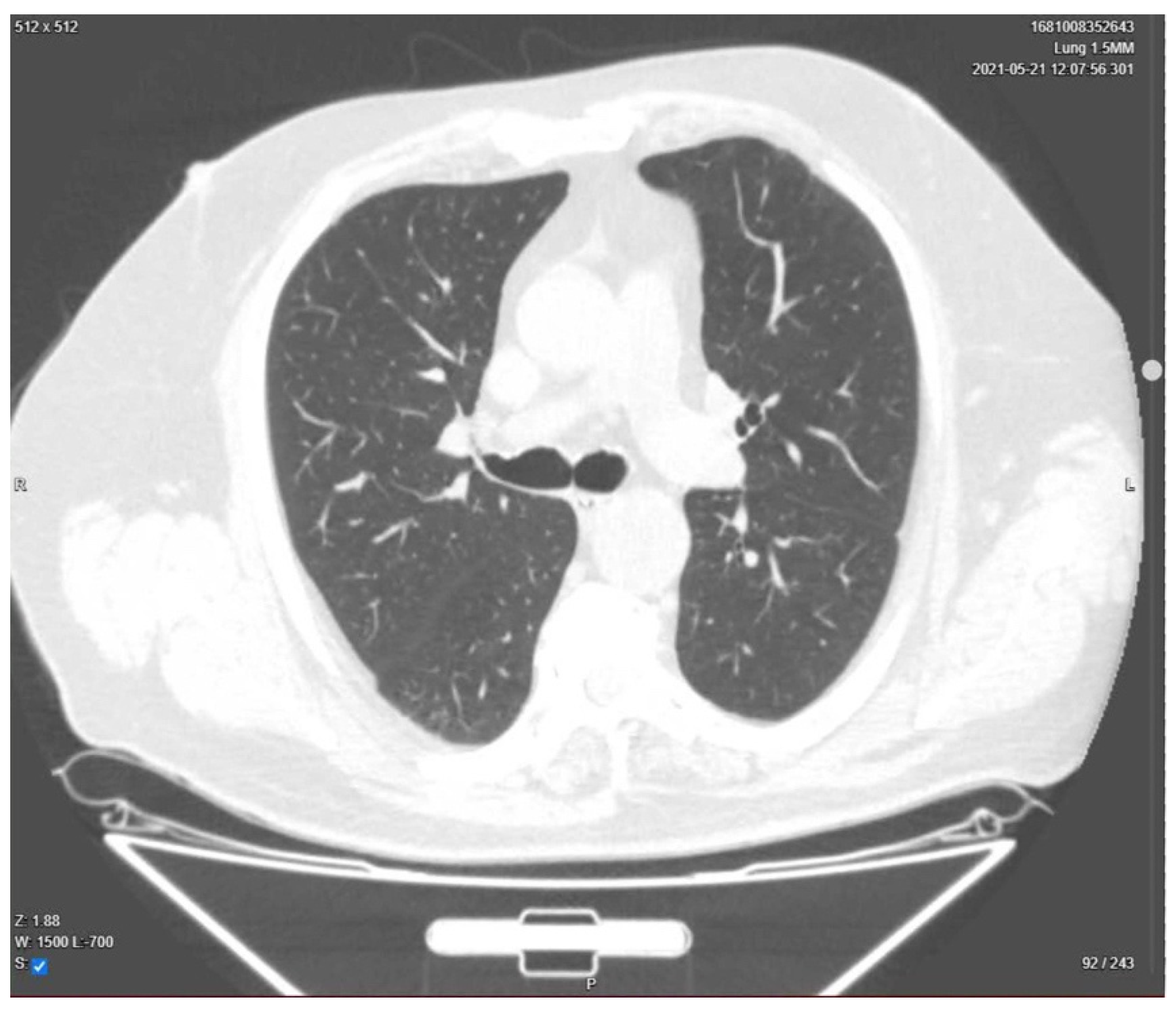
| Day 23 | Lung CT was performed for pulmonary re-evaluation. |
| Day 24 | The patient was discharged home. |
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): A review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Liumbruno, G.M. Convalescent plasma for the treatment of severe COVID-19. Biol. Targets Ther. 2021, 15, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, W.; Hu, Y.; Tong, X.; Zheng, S.; Yang, J.; Kong, Y.; Ren, L.; Wei, Q.; Mei, H.; et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA 2020, 324, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.T.H.; Lin, H.M.; Baine, I.; Wajnberg, A.; Gumprecht, J.P.; Rahman, F.; Rodriguez, D.; Tandon, P.; Bassily-Marcus, A.; Bander, J.; et al. Convalescent plasma treatment of severe COVID-19: A matched control study. Nat. Med. 2020, 26, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, K.; Wu, L.; Wang, Z.; Zhu, M.; Huang, B.; Li, J.; Wang, Z.; Wu, W.; Wu, M.; et al. Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood 2020, 136, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Keith, P.; Day, M.; Perkins, L.; Moyer, L.; Hewitt, K.; Wells, A. A novel treatment approach to the novel coronavirus: An argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit. Care 2020, 24, 128. [Google Scholar] [CrossRef] [PubMed]
- Khamis, F.; Al-Zakwani, I.; Al Hashmi, S.; Al Dowaiki, S.; Al Bahrani, M.; Pandak, N.; Al Khalili, H.; Memish, Z. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int. J. Infect. Dis. 2020, 99, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Gucyetmez, B.; Atalan, H.K.; Sertdemir, I.; Cakir, U.; Telci, L.; COVID-19 Study Group. Therapeutic plasma exchange in patients with COVID-19 pneumonia in intensive care unit: A retrospective study. Crit. Care 2020, 24, 492. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, A.; Singh-Makkar, S.; Sarfraz, Z.; Hathaway, D., III; Paul, T.; Sana, M.K.; Talalaev, M.; Perez-Fernandez, J.; Yatzkan, G. Therapeutic plasma exchange and COVID-19: A rapid review. Clin. Immunol. Immunother. 2020, 6, 041. [Google Scholar] [CrossRef]
- Balagholi, S.; Dabbaghi, R.; Eshghi, P.; Mousavi, S.A.; Heshmati, F.; Mohammadi, S. Potential of therapeutic plasmapheresis in treatment of COVID-19 patients: Immunopathogenesis and coagulopathy. Transfus. Apher. Sci. 2020, 59, 102993. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.M.; Mirza, Z.E.; Naseem, A.; Liaqat, J.; Fazal, I.; Alamgir, W.; Saeed, F.; Saleem, S.; Nisar, S.; Yousaf, M.A.; et al. Therapeutic plasma exchange for coronavirus disease-2019 triggered cytokine release syndrome; a retrospective propensity matched control study. PLoS ONE 2021, 16, e0244853. [Google Scholar] [CrossRef]
- Jaiswal, V.; Nasa, P.; Raouf, M.; Gupta, M.; Dewedar, H.; Mohammad, H.; Al Rais, Z.; Baqer, M.A.; Alsabbah, A.; Ibrahim, Y.; et al. Therapeutic plasma exchange followed by convalescent plasma transfusion in critical COVID-19—An exploratory study. Int. J. Infect. Dis. 2021, 102, 332–334. [Google Scholar] [CrossRef] [PubMed]
- The ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Lin, L.; Li, T.S. Interpretation of “Guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the national health commission (Trial Version 5)”. Zhonghua Yi Xue Za Zhi 2020, 100, E001. [Google Scholar]
- Martínez Chamorro, E.; Díez Tascón, A.; Ibáñez Sanz, L.; Ossaba Vélez, S.; Borruel Nacenta, S. Radiologic diagnosis of patients with COVID-19. Radiología 2021, 63, 56–73. [Google Scholar] [CrossRef]
- Honore, P.M.; Barreto Gutierrez, L.; Kugener, L.; Redant, S.; Attou, R.; Gallerani, A.; de Bels, D. Plasma exchange in critically ill COVID-19 patients improved inflammation, microcirculatory clot formation, and hypotension, thereby improving clinical outcomes: Fact or fiction? Crit. Care 2020, 24, 551. [Google Scholar] [CrossRef] [PubMed]
- Novacescu, A.N.; Duma, G.; Buzzi, B.; Baditoiu, L.M.; Bedreag, O.; Papurica, M.; Sandesc, D.; Sorescu, T.; Vlad, D.; Licker, M. Therapeutic plasma exchange followed by convalescent plasma transfusion in severe and critically ill COVID-19 patients: A single centre non-randomized controlled trial. Exp. Ther. Med. 2022, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Ventilator-associated pneumonia (VAP) caused by carbapenem-resistant Acinetobacter baumannii in patients with COVID-19: Two problems, one solution? Med. Hypotheses 2020, 144, 110139. [Google Scholar] [CrossRef]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novacescu, A.N.; Duma, G.; Sandesc, D.; Sorescu, T.; Licker, M. The Successful Recovery of a Critically Ill COVID-19 Patient, Following the Combination of Therapeutic Plasma Exchange and Convalescent Plasma Transfusion: A Case Report. Medicina 2022, 58, 1088. https://doi.org/10.3390/medicina58081088
Novacescu AN, Duma G, Sandesc D, Sorescu T, Licker M. The Successful Recovery of a Critically Ill COVID-19 Patient, Following the Combination of Therapeutic Plasma Exchange and Convalescent Plasma Transfusion: A Case Report. Medicina. 2022; 58(8):1088. https://doi.org/10.3390/medicina58081088
Chicago/Turabian StyleNovacescu, Alexandru Noris, Georgiana Duma, Dorel Sandesc, Teodora Sorescu, and Monica Licker. 2022. "The Successful Recovery of a Critically Ill COVID-19 Patient, Following the Combination of Therapeutic Plasma Exchange and Convalescent Plasma Transfusion: A Case Report" Medicina 58, no. 8: 1088. https://doi.org/10.3390/medicina58081088
APA StyleNovacescu, A. N., Duma, G., Sandesc, D., Sorescu, T., & Licker, M. (2022). The Successful Recovery of a Critically Ill COVID-19 Patient, Following the Combination of Therapeutic Plasma Exchange and Convalescent Plasma Transfusion: A Case Report. Medicina, 58(8), 1088. https://doi.org/10.3390/medicina58081088







