Pathophysiological Aspects of Aging in Venous Thromboembolism: An Update
Abstract
1. Introduction
2. Definition of Aging and Old Age
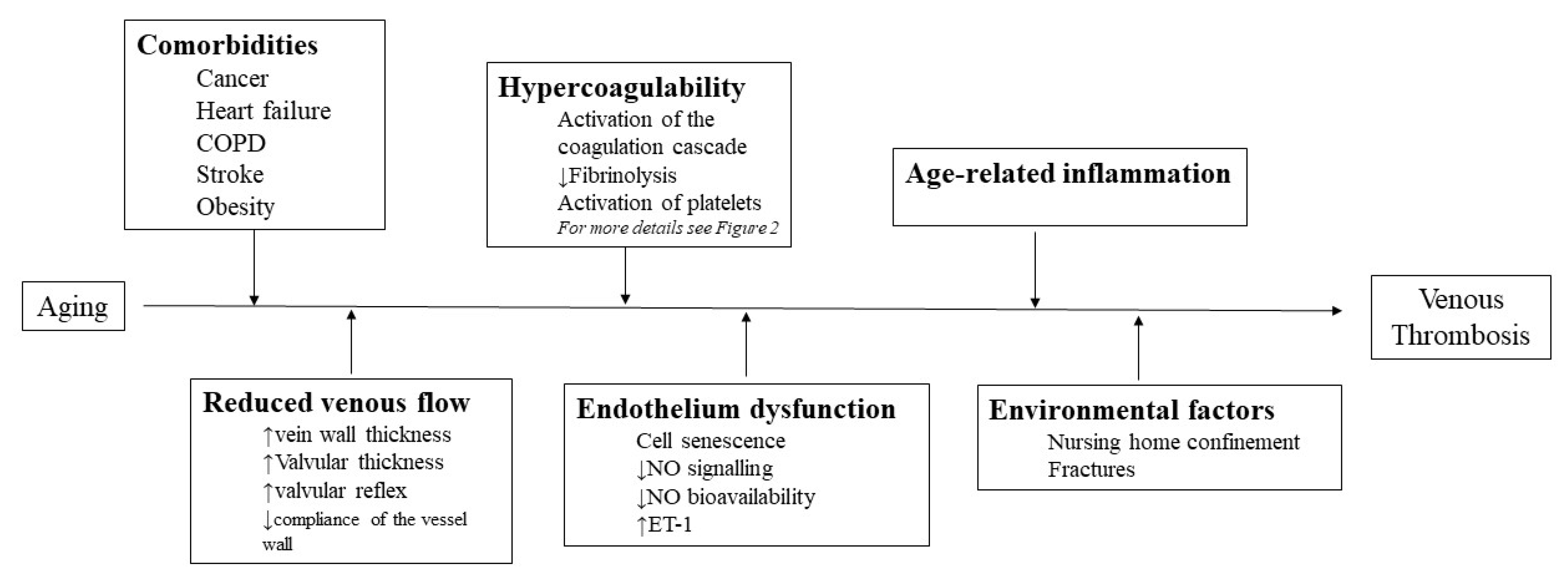
3. Pathophysiological Impact of Aging on VTE
3.1. Venous Stasis
3.2. Endothelium Dysfunction
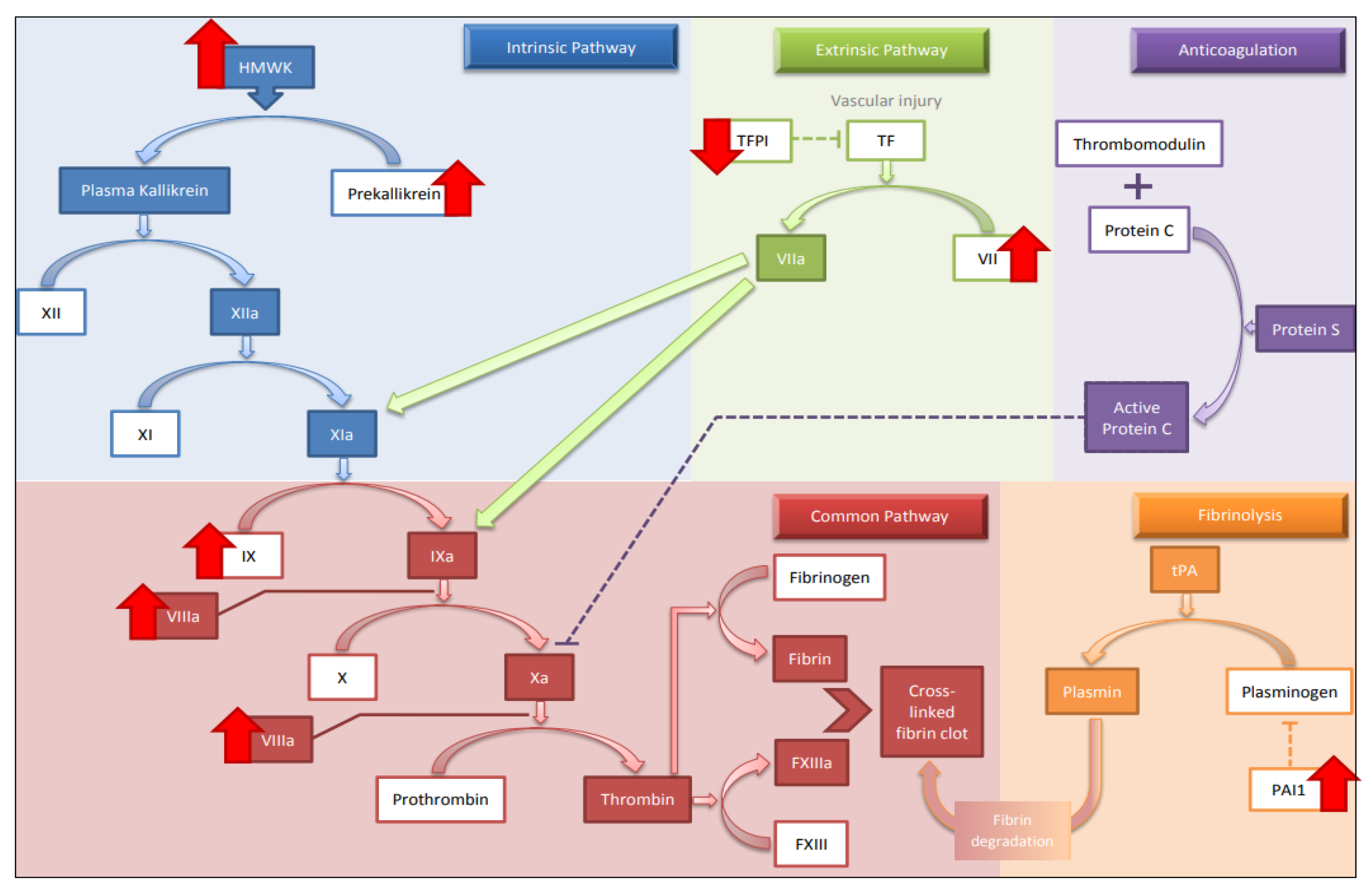
3.3. Hypercoagulability
3.3.1. Extrinsic, Intrinsic and Common Pathway
3.3.2. Fibrolytic Mechanism
3.3.3. vWF
3.3.4. Platelet Function
3.3.5. Coagulation Factors with Anticoagulant Properties
3.4. Impact of Age-Related Inflammation
4. Clinical Considerations in Elderly Patients Suffering from VTE
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gesammalte Abhandlungen zur Wissenschaftlichen Medtzin; Medinger Sohn &, Company, Ed.; Medinger Sohn & Company: Frankfurt, Germany, 1856. [Google Scholar]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.; Huisman, M.V.; Humbert, M.; Kucher, N. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3080. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; O’Fallon, W.M.; Petterson, T.M.; Lohse, C.M.; Silverstein, M.D.; Mohr, D.N.; Melton, L.J., III. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: A population-based study. Arch. Intern. Med. 2002, 162, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.A.; Spencer, F.A. Risk factors for venous thromboembolism. Circulation 2003, 107, I-9. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.Z. Pulmonary embolism in Black Americans. Am. J. Hematol. 2010, 85, 465–466. [Google Scholar] [CrossRef]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Trends in the Incidence of Deep Vein Thrombosis and Pulmonary Embolism: A 25-year population-based study. Arch. Intern. Med. 1998, 158, 585–593. [Google Scholar] [CrossRef]
- Næss, I.A.; Christiansen, S.C.; Romundstad, P.; Cannegieter, S.C.; Rosendaal, F.R.; Hammerstrøm, J. Incidence and mortality of venous thrombosis: A population-based study. J. Thromb. Haemost. 2007, 5, 692–699. [Google Scholar] [CrossRef]
- Tagalakis, V.; Patenaude, V.; Kahn, S.R.; Suissa, S. Incidence of and Mortality from Venous Thromboembolism in a Real-world Population: The Q-VTE Study Cohort. Am. J. Med. 2013, 126, 832.e13–832.e21. [Google Scholar] [CrossRef]
- Spencer, F.A.; Emery, C.; Joffe, S.W.; Pacifico, L.; Lessard, D.; Reed, G.; Gore, J.M.; Goldberg, R.J. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J. Thromb. Thrombolysis 2009, 28, 401–409. [Google Scholar] [CrossRef]
- Kniffin, W.D.; Baron, J.A.; Barrett, J.; Birkmeyer, J.D.; Anderson, F.A. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch. Intern. Med. 1994, 154, 861–866. [Google Scholar] [CrossRef]
- Hansson, P.O.; Welin, L.; Tibblin, G.; Eriksson, H. Deep vein thrombosis and pulmonary embolism in the general population. ’The Study of Men Born in 1913. Arch. Intern. Med. 1997, 157, 1665–1670. [Google Scholar] [CrossRef]
- Oger, E.; EPI-GETBO Study Group; Groupe d’Etude de la Thrombose de Bretagne Occidentale. Incidence of Venous Thromboembolism: A Community-based Study in Western France. Thromb. Haemost. 2000, 83, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Tsai, A.W.; White, R.H.; Heckbert, S.R.; Rosamond, W.D.; Enright, P.; Folsom, A.R. Deep vein thrombosis and pulmonary embolism in two cohorts: The longitudinal investigation of thromboembolism etiology. Am. J. Med. 2004, 117, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.A.; Wheeler, H.B.; Goldberg, R.J.; Hosmer, D.W.; Patwardhan, N.A.; Jovanovic, B.; Forcier, A.; Dalen, J.E. A Population-Based Perspective of the Hospital Incidence and Case-Fatality Rates of Deep Vein Thrombosis and Pulmonary Embolism: The Worcester DVT Study. Arch. Intern. Med. 1991, 151, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Cheng, C.-L.; Lin, L.-J.; Tsai, L.-M.; Yang, Y.-H.K. Epidemiology and Predictors of Short-Term Mortality in Symptomatic Venous Thromboembolism—A Nationwide Population-Based Study. Circ. J. 2011, 75, 1998–2004. [Google Scholar] [CrossRef]
- Raptis, D.G.; Gourgoulianis, K.I.; Daniil, Z.; Malli, F. Time trends for pulmonary embolism incidence in Greece. Thromb. J. 2020, 18, 1. [Google Scholar] [CrossRef]
- White, R.H.; Zhou, H.; Romano, P. Incidence of Idiopathic Deep Venous Thrombosis and Secondary Thromboembolism among Ethnic Groups in California. Ann. Intern. Med. 1998, 128, 737–740. [Google Scholar] [CrossRef]
- Klatsky, A.L.; Armstrong, M.A.; Poggi, J. Risk of pulmonary embolism and/or deep venous thrombosis in Asian-Americans. Am. J. Cardiol. 2000, 85, 1334–1337. [Google Scholar] [CrossRef]
- Cheuk, B.L.Y.; Cheung, G.C.Y.; Cheng, S.W.K. Epidemiology of venous thromboembolism in a Chinese population. Br. J. Surg. 2004, 91, 424–428. [Google Scholar] [CrossRef]
- White, R.H.; Keenan, C.R. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb. Res. 2009, 123, S11–S17. [Google Scholar] [CrossRef]
- Zakai, N.A.; McClure, L.A.; Judd, S.E.; Safford, M.M.; Folsom, A.R.; Lutsey, P.L.; Cushman, M. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation 2014, 129, 1502–1509. [Google Scholar] [CrossRef]
- Gillum, R.F. Pulmonary embolism and thrombophlebitis in the United States, 1970–1985. Am. Hear. J. 1987, 114, 1262–1264. [Google Scholar] [CrossRef]
- Spencer, F.A.; Gurwitz, J.H.; Schulman, S.; Linkins, L.-A.; Crowther, M.A.; Ginsberg, J.S.; Lee, A.Y.; Saczynski, J.S.; Anand, S.; Lessard, D.; et al. Venous Thromboembolism in Older Adults: A Community-based Study. Am. J. Med. 2014, 127, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Posadas-Martínez, M.L.; Vázquez, F.J.; Grande-Ratti, M.F.; De Quirós, F.G.B.; Giunta, D.H. Inhospital mortality among clinical and surgical inpatients recently diagnosed with venous thromboembolic disease. J. Thromb. Thrombolysis 2015, 40, 225–230. [Google Scholar] [CrossRef]
- Verso, M.; Agnelli, G.; Ageno, W.; Imberti, D.; Moia, M.; Palareti, G.; Pistelli, R.; Cantone, V. Long-term death and recurrence in patients with acute venous thromboembolism: The MASTER registry. Thromb. Res. 2012, 130, 369–373. [Google Scholar] [CrossRef]
- Huang, W.; Goldberg, R.J.; Anderson, F.A.; Kiefe, C.I.; Spencer, F.A. Secular Trends in Occurrence of Acute Venous Thromboembolism: The Worcester VTE Study (1985–2009). Am. J. Med. 2014, 127, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.M.; Kim, J.S.; Han, S.W.; Hong, Y.S.; Kim, I.; Ha, J.; Kim, S.J.; Chung, J.W.; Park, J.H.; Lee, D.; et al. Clinical predictors of recurrent venous thromboembolism: A single institute experience in Korea. Thromb. Res. 2009, 123, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Mohr, D.N.; Silverstein, M.D.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Predictors of Recurrence After Deep Vein Thrombosis and Pulmonary Embolism. Arch. Intern. Med. 2000, 160, 761–768. [Google Scholar] [CrossRef]
- McRae, S.; Tran, H.; Schulman, S.; Ginsberg, J.; Kearon, C. Effect of patient’s sex on risk of recurrent venous thromboembolism: A meta-analysis. Lancet 2006, 368, 371–378. [Google Scholar] [CrossRef]
- Kyrle, P.A.; Minar, E.; Bialonczyk, C.; Hirschl, M.; Weltermann, A.; Eichinger, S. The Risk of Recurrent Venous Thromboembolism in Men and Women. N. Engl. J. Med. 2004, 350, 2558–2563. [Google Scholar] [CrossRef]
- Eichinger, S.; Heinze, G.; Jandeck, L.M.; Kyrle, P.A. Risk Assessment of Recurrence in Patients with Unprovoked Deep Vein Thrombosis or Pulmonary Embolism: The vienna prediction model. Circulation 2010, 121, 1630–1636. [Google Scholar] [CrossRef]
- Ageno, W.; Agnelli, G.; Imberti, D.; Moia, M.; Palareti, G.; Pistelli, R.; Rossi, R.; Verso, M. Risk factors for venous thromboembolism in the elderly: Results of the master registry. Blood Coagul. Fibrinolysis 2008, 19, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Bovill, E.G.; van der Vliet, A. Venous Valvular Stasis–Associated Hypoxia and Thrombosis: What Is the Link? Annu. Rev. Physiol. 2011, 73, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, R.T.; Raffetto, J.D. Chronic Venous Insufficiency. Circulation 2014, 130, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Engbers, M.J.; Blom, J.W.; Cushman, M.; Rosendaal, F.R.; Vlieg, A.V.H. The contribution of immobility risk factors to the incidence of venous thrombosis in an older population. J. Thromb. Haemost. 2013, 12, 290–296. [Google Scholar] [CrossRef]
- Engbers, M.J.; Karasu, A.; Blom, J.W.; Cushman, M.; Rosendaal, F.R.; Vlieg, A.V.H. Clinical features of venous insufficiency and the risk of venous thrombosis in older people. Br. J. Haematol. 2015, 171, 417–423. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Pfeifer, J.R.; Engle, J.S.; Schottenfeld, D. The Epidemiology of Chronic Venous Insufficiency and Varicose Veins. Ann. Epidemiol. 2005, 15, 175–184. [Google Scholar] [CrossRef]
- Ruckley, C.; Evans, C.J.; Allan, P.L.; Lee, A.J.; Fowkes, F.R. Chronic venous insufficiency: Clinical and duplex correlations. The Edinburgh Vein Study of venous disorders in the general population. J. Vasc. Surg. 2002, 36, 520–525. [Google Scholar] [CrossRef]
- Saphir, O.; Lev, M. The venous valve in the aged. Am. Hear. J. 1952, 44, 843–850. [Google Scholar] [CrossRef]
- Lev, M.; Saphir, O. Endophlebohypertrophy and phlebosclerosis: II. The external and common iliac veins. Am. J. Pathol. 1952, 28, 401. [Google Scholar]
- Fronek, A.; Criqui, M.H.; Denenberg, J.; Langer, R.D. Common femoral vein dimensions and hemodynamics including Valsalva response as a function of sex, age, and ethnicity in a population study. J. Vasc. Surg. 2001, 33, 1050–1056. [Google Scholar] [CrossRef]
- McLachlin, A.D.; McLachlin, J.A.; Jory, T.A.; Rawling, E.G. Venous Stasis in the Lower Extremities. Ann. Surg. 1960, 152, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Karasu, A.; Šrámek, A.; Rosendaal, F.R.; Van Der Geest, R.J.; Vlieg, A.V.H. Aging of the venous valves as a new risk factor for venous thrombosis in the elderly: The Batavia study. J. Thromb. Haemost. 2017, 16, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Schina, M.J.; Neumyer, M.M.; Healy, D.A.; Atnip, R.G.; Thiele, B.L. Influence of age on venous physiologic parameters. J. Vasc. Surg. 1993, 18, 749–752. [Google Scholar] [CrossRef][Green Version]
- Young, C.N.; Stillabower, M.E.; DiSabatino, A.; Farquhar, W.B. Venous smooth muscle tone and responsiveness in older adults. J. Appl. Physiol. 2006, 101, 1362–1367. [Google Scholar] [CrossRef]
- van Langevelde, K.; Šrámek, A.; Rosendaal, F.R. The Effect of Aging on Venous Valves. Arter. Thromb. Vasc. Biol. 2010, 30, 2075–2080. [Google Scholar] [CrossRef]
- Hamer, J.D.; Malone, P.C.; A Silver, I. The PO2 in venous valve pockets: Its possible bearing on thrombogenesis. Br. J. Surg. 1981, 68, 166–170. [Google Scholar] [CrossRef]
- Mackman, N. New insights into the mechanisms of venous thrombosis. J. Clin. Investig. 2012, 122, 2331–2336. [Google Scholar] [CrossRef]
- Poredos, P.; Jezovnik, M.K. Endothelial Dysfunction and Venous Thrombosis. Angiology 2017, 69, 564–567. [Google Scholar] [CrossRef]
- Bochenek, M.L.; Schütz, E.; Schäfer, K. Endothelial cell senescence and thrombosis: Ageing clots. Thromb. Res. 2016, 147, 36–45. [Google Scholar] [CrossRef]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Palomo, I.; Fuentes, E. Mechanisms of endothelial dysfunction during aging: Predisposition to thrombosis. Mech. Ageing Dev. 2017, 164, 91–99. [Google Scholar] [CrossRef]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell. Cardiol. 2015, 89, 122–135. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G.; Favaloro, E.J. Aging Hemostasis: Changes to Laboratory Markers of Hemostasis as We Age—A Narrative Review. Semin. Thromb. Hemost. 2014, 40, 621–633. [Google Scholar] [CrossRef]
- Toda, N. Age-related changes in endothelial function and blood flow regulation. Pharmacol. Ther. 2012, 133, 159–176. [Google Scholar] [CrossRef]
- Pantsulaia, I.; Ciszewski, W.M.; Niewiarowska, J. Senescent endothelial cells: Potential modulators of immunosenescence and ageing. Ageing Res. Rev. 2016, 29, 13–25. [Google Scholar] [CrossRef]
- Procter, N.E.K.; Chong, C.-R.; Sverdlov, A.L.; Chan, W.P.A.; Chirkov, Y.Y.; Horowitz, J.D. Aging of Platelet Nitric Oxide Signaling: Pathogenesis, Clinical Implications, and Therapeutics. Semin. Thromb. Hemost. 2014, 40, 660–668. [Google Scholar] [CrossRef]
- Battistini, B. Modulation and roles of the endothelins in the pathophysiology of pulmonary embolism. Can. J. Physiol. Pharmacol. 2003, 81, 555–569. [Google Scholar] [CrossRef]
- Chou, T.-C.; Yen, M.-H.; Li, C.-Y.; Ding, Y.-A. Alterations of Nitric Oxide Synthase Expression with Aging and Hypertension in Rats. Hypertension 1998, 31, 643–648. [Google Scholar] [CrossRef]
- Loscalzo, J. Nitric Oxide Insufficiency, Platelet Activation, and Arterial Thrombosis. Circ. Res. 2001, 88, 756–762. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Palomo, I.; Fuentes, E. Primary and secondary haemostasis changes related to aging. Mech. Ageing Dev. 2015, 150, 46–54. [Google Scholar] [CrossRef]
- Mari, D.; Coppola, R.; Provenzano, R. Hemostasis factors and aging. Exp. Gerontol. 2008, 43, 66–73. [Google Scholar] [CrossRef]
- Franchini, M. Hemostasis and aging. Crit. Rev. Oncol./Hematol. 2006, 60, 144–151. [Google Scholar] [CrossRef]
- Wilkerson, W.R.; Sane, D.C. Aging and Thrombosis. Semin. Thromb. Hemost. 2002, 28, 555–568. [Google Scholar] [CrossRef]
- Balleisen, L.; Bailey, J.; Epping, P.-H.; Schulte, H.; van de Loo, J. Epidemiological Study on Factor VII, Factor VIII and Fibrinogen in an Industrial Population: I. Baseline Data on the Relation to Age, Gender, Body-Weight, Smoking, Alcohol, Pill-Using, and Menopause. Thromb. Haemost. 1985, 54, 475–479. [Google Scholar] [CrossRef]
- Tracy, R.P.; Bovill, E.G.; Fried, L.P.; Heiss, G.; Lee, M.H.; Polak, J.F.; Psaty, B.M.; Savage, P.J. The Distribution of coagulation factors VII and VIII and fibrinogen in adults over 65 years results from the cardiovascular health study. Ann. Epidemiol. 1992, 2, 509–519. [Google Scholar] [CrossRef]
- Lowe, G.D.O.; Rumley, A.; Woodward, M.; Morrison, C.E.; Philippou, H.; Lane, D.A.; Tunstall-Pedoe, H. Epidemiology of coagulation factors, inhibitors and activation markers: The third glasgow monica survey i. Illustrative reference ranges by age, sex and hormone use. Br. J. Haematol. 1997, 97, 775–784. [Google Scholar] [CrossRef]
- Tofler, G.H.; Massaro, J.; Levy, D.; Mittleman, M.; Sutherland, P.; Lipinska, I.; Muller, J.E.; D’Agostino, R.B. Relation of the Prothrombotic State to Increasing Age (from the Framingham Offspring Study). Am. J. Cardiol. 2005, 96, 1280–1283. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Soltani, S.; McDonald, J.; Grezchnik, E.; Easton, L. Cross-laboratory audit of normal reference ranges and assessment of ABO blood group, gender and age on detected levels of plasma coagulation factors. Blood Coagul. Fibrinolysis 2005, 16, 597–605. [Google Scholar] [CrossRef]
- Amin, H.; Mohsin, S.; Aslam, M.; Hussain, S.; Saeed, T.; Ullah, M.I.; Sami, W. Coagulation factors and antithrombin levels in young and elderly subjects in Pakistani population. Blood Coagul. Fibrinolysis 2012, 23, 745–750. [Google Scholar] [CrossRef]
- Hager, K.; Setzer, J.; Vogl, T.; Voit, J.; Platt, D. Blood coagulation factors in the elderly. Arch. Gerontol. Geriatr. 1989, 9, 277–282. [Google Scholar] [CrossRef]
- Koster, T.; Rosendaal, F.R.; Reitsma, P.H.; Van Der Velden, P.A.; Briët, E.; Vandenbroucke, J.P. Factor VII and fibrinogen levels as risk factors for venous thrombosis. A case-control study of plasma levels and DNA polymorphisms--the Leiden Thrombophilia Study (LETS). Thromb. Haemost. 1994, 71, 719–722. [Google Scholar]
- Koster, T.; Vandenbroucke, J.; Rosendaal, F.; Briët, E.; Blann, A. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995, 345, 152–155. [Google Scholar] [CrossRef]
- Aleman, M.M.; Walton, B.L.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and red blood cells in venous thrombosis. Thromb. Res. 2014, 133, S38–S40. [Google Scholar] [CrossRef]
- Klovaite, J.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Benn, M. Elevated Fibrinogen Levels Are Associated with Risk of Pulmonary Embolism, but Not with Deep Venous Thrombosis. Am. J. Respir. Crit. Care Med. 2013, 187, 286–293. [Google Scholar] [CrossRef]
- Gligorijević, N.; Križáková, M.Z.; Penezić, A.; Katrlík, J.; Nedić, O. Structural and functional changes of fibrinogen due to aging. Int. J. Biol. Macromol. 2018, 108, 1028–1034. [Google Scholar] [CrossRef]
- Key, N.S. Epidemiologic and clinical data linking factors XI and XII to thrombosis. Hematology 2014, 2014, 66–70. [Google Scholar] [CrossRef]
- Gallimore, M.J.; Harris, S.L.; Jones, D.W.; Winter, M. Plasma levels of factor XII, prekallikrein and high molecular weight kininogen in normal blood donors and patients having suffered venous thrombosis. Thromb. Res. 2004, 114, 91–96. [Google Scholar] [CrossRef]
- Ofosu, F.; Craven, S.; Dewar, L.; Anvari, N.; Andrew, M.; Blajchman, M.A. Age-related changes in factor VII proteolysis in vivo. Br. J. Haematol. 1996, 94, 407–412. [Google Scholar] [CrossRef]
- Maroney, S.A.; Mast, A.E. New insights into the biology of tissue factor pathway inhibitor. J. Thromb. Haemost. 2015, 13, S200–S207. [Google Scholar] [CrossRef]
- Camire, R.M. Rethinking events in the haemostatic process: Role of factor V and TFPI. Haemophilia 2016, 22, 3–8. [Google Scholar] [CrossRef]
- Fei, X.; Wang, H.; Yuan, W.; Wo, M.; Jiang, L. Tissue Factor Pathway Inhibitor-1 Is a Valuable Marker for the Prediction of Deep Venous Thrombosis and Tumor Metastasis in Patients with Lung Cancer. BioMed Res. Int. 2017, 2017, 8983763. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T.; De Groot, P.G.; Meijers, J.C.M.; Rosendaal, F.R. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood 2005, 105, 1102–1105. [Google Scholar] [CrossRef]
- Meltzer, M.E.; Lisman, T.; de Groot, P.G.; Meijers, J.C.M.; le Cessie, S.; Doggen, C.J.M.; Rosendaal, F.R. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood 2010, 116, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sprengers, E.; Kluft, C. Plasminogen activator inhibitors. Blood 1987, 69, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Suzuki, Y.; Iwaki, T.; Sano, H.; Honkura, N.; Castellino, F.J. Recognition of Plasminogen Activator Inhibitor Type 1 as the Primary Regulator of Fibrinolysis. Curr. Drug Targets 2019, 20, 1695–1701. [Google Scholar] [CrossRef]
- Comi, P.; Chiaramonte, R.; Maier, J.A. Senescence-Dependent Regulation of Type 1 Plasminogen Activator Inhibitor in Human Vascular Endothelial Cells. Exp. Cell Res. 1995, 219, 304–308. [Google Scholar] [CrossRef]
- Eren, M.; Boe, A.E.; Klyachko, E.A.; Vaughan, D.E. Role of Plasminogen Activator Inhibitor-1 in Senescence and Aging. Semin. Thromb. Hemost. 2014, 40, 645–651. [Google Scholar] [CrossRef]
- Takeshita, K.; Saito, H.; Yamamoto, K. Plasminogen Activator Inhibitor-1 in Aging. Semin. Thromb. Hemost. 2014, 40, 652–659. [Google Scholar] [CrossRef]
- Gleerup, G.; Winther, K. The Effect of Ageing on Platelet Function and Fibrinolytic Activity. Angiology 1995, 46, 715–718. [Google Scholar] [CrossRef]
- Nilsson, I.M.; Krook, H.; Sternby, N.-H.; Söderberg, E.; Söderström, N. Severe Thrombotic Disease in a Young Man with Bone Marrow and Skeletal Changes and with a High Content of an Inhibitor in the Fibrinolytic System. Acta Med. Scand. 2009, 169, 323–337. [Google Scholar] [CrossRef]
- Nilsson, I.M.; Ljungner, H.; Tengborn, L. Two different mechanisms in patients with venous thrombosis and defective fibrinolysis: Low concentration of plasminogen activator or increased concentration of plasminogen activator inhibitor. BMJ 1985, 290, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S.; Schönauer, V.; Weltermann, A.; Minar, E.; Bialonczyk, C.; Hirschl, M.; Schneider, B.; Quehenberger, P.; Kyrle, P.A. Thrombin-activatable fibrinolysis inhibitor and the risk for recurrent venous thromboembolism. Blood 2004, 103, 3773–3776. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.J.; Dawson, A.A.; Ogston, D.; Douglas, A.S. The effect of age on the fibrinolytic enzyme system. J. Clin. Pathol. 1974, 27, 326–329. [Google Scholar] [CrossRef]
- Peyvandi, F.; Garagiola, I.; Baronciani, L. Role of von Willebrand factor in the haemostasis. Blood Transfus. 2011, 9, s3–s8. [Google Scholar] [CrossRef] [PubMed]
- Conlan, M.G.; Folsom, A.R.; Finch, A.; Davis, C.E.; Sorlie, P.; Marcucci, G.; Wu, K.K. For the ARIC Study Investigators Associations of Factor VIII and von Willebrand Factor with Age, Race, Sex, and Risk Factors for Atherosclerosis. Thromb. Haemost. 1993, 70, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.A.; Hathaway, L.S.; Collins, P.W.; Bowen, D.J. Von Willebrand factor: Demographics of plasma protein level in a large blood donor cohort from South Wales in the United Kingdom. Haemophilia 2012, 18, e79–e81. [Google Scholar] [CrossRef]
- Tsai, A.W.; Cushman, M.; Rosamond, W.D.; Heckbert, S.R.; Tracy, R.P.; Aleksic, N.; Folsom, A.R. Coagulation factors, inflammation markers, and venous thromboembolism: The longitudinal investigation of thromboembolism etiology (LITE). Am. J. Med. 2002, 113, 636–642. [Google Scholar] [CrossRef]
- Ocak, G.; Vossen, C.Y.; Verduijn, M.; Dekker, F.W.; Rosendaal, F.R.; Cannegieter, S.C.; Lijfering, W.M. Risk of venous thrombosis in patients with major illnesses: Results from the MEGA study. J. Thromb. Haemost. 2012, 11, 116–123. [Google Scholar] [CrossRef]
- McGill, S.N.; Ahmed, N.A.; Christou, N.V. Increased plasma von Willebrand factor in the systemic inflammatory response syndrome is derived from generalized endothelial cell activation. Crit. Care Med. 1998, 26, 296–300. [Google Scholar] [CrossRef]
- Gremmel, T.; Frelinger III, A.L.; Michelson, A.D. Platelet Physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [CrossRef]
- Johnson, M.; Ramey, E.; Ramwell, P.W. Sex and age differences in human platelet aggregation. Nature 1975, 253, 355–357. [Google Scholar] [CrossRef]
- Zahavi, J.; Jones, N.; Leyton, J.; Dubiel, M.; Kakkar, V. Enhanced in vivo platelet “release reaction” in old healthy individuals. Thromb. Res. 1980, 17, 329–336. [Google Scholar] [CrossRef]
- Sie, P.; Montagut, J.; Blanc, M.; Boneu, B.; Caranobe, C.; Cazard, J.C.; Biermé, R. Evaluation of Some Platelet Parameters in a Group of Elderly People. Thromb. Haemost. 1981, 45, 197–199. [Google Scholar] [CrossRef]
- Reilly, I.; FitzGerald, G. Eicosenoid biosynthesis and platelet function with advancing age. Thromb. Res. 1986, 41, 545–554. [Google Scholar] [CrossRef]
- Modesti, P.A.; Fortini, A.; Abbate, R.; Gensini, G.F. Age related changes of platelet prostacyclin receptors in humans. Eur. J. Clin. Investig. 1985, 15, 204–208. [Google Scholar] [CrossRef]
- Supiano, M.A.; Linares, O.A.; Halter, J.B.; Reno, K.M.; Rosen, S.G. Functional Uncoupling of the Plateletα2-Adrenergic Receptor-Adenylate Cyclase Complex in the Elderly. J. Clin. Endocrinol. Metab. 1987, 64, 1160–1164. [Google Scholar] [CrossRef]
- Supiano, M.A.; Hogikyan, R.V. High Affinity Platelet 2-Adrenergic Receptor Density Is Decreased in Older Humans. J. Gerontol. 1993, 48, B173–B179. [Google Scholar] [CrossRef]
- Zarbock, A.; Singbartl, K.; Ley, K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Investig. 2006, 116, 3211–3219. [Google Scholar] [CrossRef]
- Looney, M.R.; Nguyen, J.X.; Hu, Y.; Van Ziffle, J.A.; Lowell, C.A.; Matthay, M.A. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J. Clin. Investig. 2009, 119, 3450–3461. [Google Scholar] [CrossRef]
- Saeed, K.; Gould, I.; Esposito, S.; Ahmad-Saeed, N.; Ahmed, S.S.; Alp, E.; Bal, A.M.; Bassetti, M.; Bonnet, E.; Chan, M.; et al. Panton–Valentine leukocidin-positive Staphylococcus aureus: A position statement from the International Society of Chemotherapy. Int. J. Antimicrob. Agents 2018, 51, 16–25. [Google Scholar] [CrossRef]
- Schneider, D.J.; Taatjes, D.J.; Howard, D.B.; Sobel, B.E. Increased reactivity of platelets induced by fibrinogen independent of its binding to the IIb-IIIa surface glycoprotein: A potential contributor to cardiovascular risk. J. Am. Coll. Cardiol. 1999, 33, 261–266. [Google Scholar] [CrossRef]
- Meade, T.W.; North, W.R.S.; Chakrabarti, R.; Haines, A.; Stirlig, Y. Population-Based Distributions of Haemostatic Variables. Br. Med. Bull. 1977, 33, 283–288. [Google Scholar] [CrossRef]
- Dolan, G.; Neal, K.; Cooper, P.; Brown, P.; Preston, F.E. Protein C, antithrombin III and plasminogen: Effect of age, sex and blood group. Br. J. Haematol. 1994, 86, 798–803. [Google Scholar] [CrossRef]
- Bauer, K.A.; Weiss, L.M.; Sparrow, D.; Vokonas, P.S.; Rosenberg, R.D. Aging-associated changes in indices of thrombin generation and protein C activation in humans. Normative Aging Study. J. Clin. Investig. 1987, 80, 1527–1534. [Google Scholar] [CrossRef]
- Conlan, M.G.; Folsom, A.R.; Finch, A.; Davis, C.E.; Marcucci, G.; Sorlie, P.; Wu, K.K. Antithrombin III: Associations with age, race, sex and cardiovascular disease risk factors. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Thromb. Haemost. 1994, 72, 551–556. [Google Scholar]
- Tait, R.; Walker, I.D.; Islam, S.I.A.M.; McCall, F.; Conkie, J.A.; Mitchell, R.; Davidson, J.F. Influence of demographic factors on antithrombin III activity in a healthy population. Br. J. Haematol. 1993, 84, 476–480. [Google Scholar] [CrossRef]
- Tahara, C.; Kazama, M.; Miyajima, Y.; Matsumoto, K.; Abe, T. Reference values of hemostasis related factors of healthy Japanese adults II. protein C. Thromb. Res. 1991, 62, 345–351. [Google Scholar] [CrossRef]
- Tait, R.C.; Walker, I.D.; Islam, S.I.A.M.; McCall, F.; Conkie, J.A.; Wight, M.; Mitchell, R.; Davidson, J.F. Protein C Activity in Healthy Volunteers—Influence of Age, Sex, Smoking and Oral Contraceptives. Thromb. Haemost. 1993, 70, 281–285. [Google Scholar] [CrossRef]
- Pintao, M.C.; Ribeiro, D.D.; Bezemer, I.D.; Garcia, A.A.; de Visser, M.C.H.; Doggen, C.J.M.; Lijfering, W.M.; Reitsma, P.H.; Rosendaal, F.R. Protein S levels and the risk of venous thrombosis: Results from the MEGA case-control study. Blood 2013, 122, 3210–3219. [Google Scholar] [CrossRef]
- Mestries, J.C.; Kruithof, E.K.; Gascon, M.P.; Herodin, F.; Agay, D.; Ythier, A. In vivo modulation of coagulation and fibrinolysis by recombinant glycosylated human interleukin-6 in baboons. Eur. Cytokine Netw. 1994, 5, 275–281. [Google Scholar]
- Bartlett, D.B.; Firth, C.M.; Whittaker, A.; Moss, P.; Baylis, D.; Syddall, H.; Sayer, A.A.; Cooper, C.; Lord, J.M. The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging Cell 2012, 11, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Schlaudecker, J.; Becker, R. Inflammatory Response and Thrombosis in Older Individuals. Semin. Thromb. Hemost. 2014, 40, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Biwas, I.; Rezaie, A.R. Vascular inflammation in aging. Aging 2018, 10, 3634–3635. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Branchford, B.R.; Carpenter, S.L.; Branchford, B.R.; Carpenter, S.L. The Role of Inflammation in Venous Thromboembolism. Front. Pediatr. 2018, 6, 142. [Google Scholar] [CrossRef]
- Wakefield, T.W.; Myers, D.D.; Henke, P.K. Mechanisms of Venous Thrombosis and Resolution. Arter. Thromb. Vasc. Biol. 2008, 28, 387–391. [Google Scholar] [CrossRef]
- Fillenbaum, G.G.; Pieper, C.F.; Cohen, H.J.; Cornoni-Huntley, J.C.; Guralnik, J.M. Comorbidity of five chronic health conditions in elderly communityresidents: Determinants and impact on mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M84–M89. [Google Scholar] [CrossRef]
- Iverson, G. Interpretation of mini-mental state examination scores in community-dwelling elderly and geriatric neuropsychiatry patients. Int. J. Geriatr. Psychiatry 1998, 13, 661–666. [Google Scholar] [CrossRef]
- Mallappallil, M.; A Friedman, E.; Delano, B.G.; McFarlane, S.I.; Salifu, M.O. Chronic kidney disease in the elderly: Evaluation and management. Clin. Pract. 2014, 11, 525–535. [Google Scholar] [CrossRef]
- Fedullo, P.F.; Tapson, V.F. The Evaluation of Suspected Pulmonary Embolism. N. Engl. J. Med. 2003, 349, 1247–1256. [Google Scholar] [CrossRef]
- Duffett, L.; Castellucci, L.A.; Forgie, M.A. Pulmonary embolism: Update on management and controversies. BMJ 2020, 370. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Righini, M.; Goehring, C.; Bounameaux, H.; Perrier, A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am. J. Med. 2000, 109, 357–361. [Google Scholar] [CrossRef]
- Righini, M.P.; Van Es, J.; Exter, P.L.D.; Roy, P.-M.; Verschuren, F.; Ghuysen, A.; Rutschmann, O.T.; Sanchez, O.; Jaffrelot, M.; Trinh-Duc, A.; et al. Age-Adjusted D-Dimer Cutoff Levels to Rule Out Pulmonary Embolism. JAMA 2014, 311, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Matta, F. Treatment of Unstable Pulmonary Embolism in the Elderly and Those with Comorbid Conditions. Am. J. Med. 2013, 126, 304–310. [Google Scholar] [CrossRef]
- Mikkola, K.M.; Patel, S.R.; Parker, J.; Grodstein, F.; Goldhaber, S.Z. Increasing age is a major risk factor for hemorrhagic complications after pulmonary embolism thrombolysis. Am. Heart J. 1997, 134, 69–72. [Google Scholar] [CrossRef]
- Damanti, S.; Braham, S.; Pasina, L. Anticoagulation in frail older people. J. Geriatr. Cardiol. 2019, 16, 844–846. [Google Scholar] [CrossRef]
- Mitchell, A.; Watson, M.C.; Welsh, T.; McGrogan, A. Effectiveness and Safety of Direct Oral Anticoagulants versus Vitamin K Antagonists for People Aged 75 Years and over with Atrial Fibrillation: A Systematic Review and Meta-Analyses of Observational Studies. J. Clin. Med. 2019, 8, 554. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 41, 407–477. [Google Scholar] [CrossRef]
- Hoffman, R.; Monreal, M.; Tzoran, I. Hemostasis and Thrombosis in the Oldest Old. Semin. Thromb. Hemost. 2018, 44, 624–631. [Google Scholar] [CrossRef]
| Study (Year) | Antithrombin III Levels | Antithrombin III Activity |
|---|---|---|
| Maede (1977) [62] | Decrease with age—♂ No change with age—♀ | - |
| Bauer (1987) [115] | No change with age (male subjects only) | Increase in FPA levels with age, thus decrease in ATIII activity |
| Hager (1989) [71] | Decrease with age | Significant increase in FPA levels with age |
| Tait (1993) [117] | - | Significant decrease with age—♂ Increase with age—♀ |
| Dolan (1994) [114] | ♂ > ♀ Increase with age—♀ | |
| Conlan (1994) [116] | Significant difference ♀ > ♂ | Decrease with age—♂ Increase with age—♀ |
| Amin (2012) [70] | - | Decrease with age |
| Study (Year) | Protein C Levels | Protein C Activity |
|---|---|---|
| Bauer (1987) [115] | No change with age (male subjects only) | Significant increase in PCP levels with age, thus increase in PC activity |
| Hager (1989) [71] | No change with age | - |
| Tahara (1991) [118] | No change with age—♂ Increase with age—♀ | No significant difference—♀ ♂ |
| Conlan (1993) [121] | ♀ > ♂ No significant change with age | - |
| Tait (1993) [117] | - | Significant increase |
| Dolan (1994) [114] | ♂ > ♀ Significant increase with age | - |
| Lowe (1997) [67] | Increase with age—♀ | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akrivou, D.; Perlepe, G.; Kirgou, P.; Gourgoulianis, K.I.; Malli, F. Pathophysiological Aspects of Aging in Venous Thromboembolism: An Update. Medicina 2022, 58, 1078. https://doi.org/10.3390/medicina58081078
Akrivou D, Perlepe G, Kirgou P, Gourgoulianis KI, Malli F. Pathophysiological Aspects of Aging in Venous Thromboembolism: An Update. Medicina. 2022; 58(8):1078. https://doi.org/10.3390/medicina58081078
Chicago/Turabian StyleAkrivou, Dimitra, Garifallia Perlepe, Paraskevi Kirgou, Konstantinos I. Gourgoulianis, and Foteini Malli. 2022. "Pathophysiological Aspects of Aging in Venous Thromboembolism: An Update" Medicina 58, no. 8: 1078. https://doi.org/10.3390/medicina58081078
APA StyleAkrivou, D., Perlepe, G., Kirgou, P., Gourgoulianis, K. I., & Malli, F. (2022). Pathophysiological Aspects of Aging in Venous Thromboembolism: An Update. Medicina, 58(8), 1078. https://doi.org/10.3390/medicina58081078








