Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Markers for Advanced Non-Small-Cell Lung Cancer Treated with Immunotherapy: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design Study
2.2. Study Eligibility Criteria
2.3. Search Strategy
2.4. Study Selection
2.5. Data Extraction and Quality Assesment
2.6. Pooled Analysis
2.7. Subgroup Analysis
| No | Author; Year | Recruitment Period | Study Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Location | Study Design | Sample Size (Male/Female) | Age Median (Range)/Mean (SD) | Smoking | Types of NSCLC | Types of Immunotherapy | |||
| 1. | Katayama et al. (2017) [10] | April 2018-November 2019 | Japan | Cohort retrospective | 81 (44/37) | 71 (42–84) | Current/Former = 64 (79%) Never = 64 (79%) | Adenocarcinoma = 50; squamous cell carcinoma = 17; other = 14 | Atezolizumab |
| 2. | Suh et al. (2017) [21] | October 2013-April 2016 | Seoul | Cohort retrospective | 54 (42/12) | Median total = 68 Responder (n = 18) = 70 (55–78) Non-responder (n = 36) = 62 (43–80) | Current/former = 39 Non = 15 | Adenocarcinoma = 31; squamous cell carcinoma = 17; adenosquamous cell carcinoma = 2; NSCLC not specified = 2; pleomorphic carcinoma = 1; large cell carcinoma = 1 | Nivolumab (n = 31) or Pembrolizumab (n = 23) |
| 3. | Khunger et al. (2018) [22] | January 2013-October 2016 | United States of America | Cohort retrospective | 109 (56/53) | 67 (45–90) | Current = 14 (12.8%); Former = 78 (71.6%); Never = 17 (15.6%) | Adenocarcinoma = 71; squamous cell carcinoma = 26; other = 12 | Nivolumab |
| 4. | Nakaya et al. (2018) [23] | January 2015-December 2016 | Japan | Cohort retrospective | 101 (78/23) | 69 (45–84) | Current/Former = 84 Never = 16 | Squamous = 37; Nnon-squamous = 64 | Nivolumab |
| 5. | Russo et al. (2018) [24] | N/A | Italy | Cohort retrospective | 28 (25/3) | 69 (47–48) | Current/Former = 26 | Squamous cell carcinoma = 18; adenocarcinoma = 10 | Nivolumab (28) or Docetaxel (34) |
| 6. | Svaton et al. (2018) [25] | 2015–2016 | Czech Republic | Cohort retrospective | 120 (71/49) | ≤65 years (n = 53) >65 years (n = 67) | Current = 55 (45.8%) Former = 43 (35.8%) Non = 22 (18.3%) | Squamous cell carcinoma = 40; adenocarcinoma = 80 | Nivolumab |
| 7. | Takeda et al. (2018) [26] | January 2016-October 2017 | Japan | Cohort retrospective | 30 (19/11) | 71 (54–83) | Current = 9 (30%) Former = 17 (56.7%) Never = 4 (13.3%) | Adenocarcinoma = 21; squamous cell carcinoma = 9 | Nivolumab |
| 8. | Zer et al. (2018) [27] | May 2013-August 2016 | Canada | Cohort retrospective | 88 (43/45) | Median (range) = 63.9 (31.1–80.9) | Current = 11 Former = 56 Never = 21 | Adenocarcinoma = 66 squamous cell carcinoma = 15 large cell, other = 7 | PD-1 axis inhibitors |
| 9. | Pavan et al. (2019) [28] | August 2013-April 2018 | Italy | Cohort retrospective | 184 (125/59) | 67.3 (37.2–83.4) | Current = 160 (87%) | Adenocarcinoma = 108; squamous cell carcinoma = 59; NOS = 14; sarcomatioid = 3 | Nivolumab = 145; Pembrolizumab first line = 26, further lines = 6; Atezolizumab = 7 |
| 10. | Matsubara et al. (2020) [29] | January 2018-March 2019 | Japan | Cohort retrospective | 24 (17/7) | 64.5 ± 9.7 | Current/Former = 17 Never = 7 | Adenocarcinoma 18; squamous cell carcinoma = 4; other = 2 | Atezolizumab |
| 11. | Prelaj et al. (2020) [30] | August 2015-August 2018 | Italy | Cohort retrospective | 154 (126/28) | 67 (31–86) | Current/former = 128 | Squamous = 20; Non-squamous = 6 | Nivolumab and Pembrolizumab |
| 12. | Russo et al. (2020) [31] | April 2015-May 2018 | Italy | Cohort retrospective | 187 (137/50) | 67 (34–83) | Current/former = 163 | Squamous = 86; non-squamous = 101 | Nivolumab |
| 13. | Takada et al. (2020) [32] | January 2016-August 2018 | Japan | Cohort retrospective | 226 (184/42) | Median (range) = 66 (31–88) | Non = 37 Former = 95 Current = 94 | Adenocarcinoma = 146 squamous cell carcinoma = 62 others/unknown = 18 | Nivolumab (n = 131) or Pembrolizumab (n = 95) |
| 14. | Yang et al. (2020) [33] | January 2013-December 2017 | China | Cohort retrospective | 113 (52/61) | Median age: 50 years | Smoker = 33 Never = 80 | Adenocarcinoma = 107 others = 6 | Crizotinib |
| 15. | Ksienski et al. (2021) [34] | August 2017-June 2019 | Canada | Cohort retrospective | 220 (99/121) | 70 (62.8–76) | Current = 79 (35.9%) Former = 122 (55.5%) Never = 19 (8.6%) | Squamous = 45; non-squamous = 175 | Pembrolizumab |
| No | Author; Year | NLR Cut-Off Value | PLR Cut-Off Value | PFS | HR PFS | ||
|---|---|---|---|---|---|---|---|
| NLR | PLR | NLR | PLR | ||||
| 1. | Katayama et al. (2017) [10] | H-NLR > 5; L-NLR ≤ 5 | H-PLR > 262; L-NLR ≤ 262 | H-NLR = 42 days vs. L-NLR = 86 days [95% CI, p < 0.001] | H-PLR = 48.5 days vs. L-PLR = 90 days [95% CI, p = 0.033] | HR H-NLR vs. L-NLR = 2.47 [95%, 1.50–4.06, p < 0.001] | HR H-PLR vs. L-PLR = 1.67 [95% CI, 1.04–2.68, p = 0.035] |
| 2. | Suh et al. (2017) [21] | H-NLR ≥ 5; L-NLR <5 | H-PLR ≥ 169; L-PLR < 169 | L-NLR = 6.1 months vs. H-NLR = 1.3 months [p < 0.001] | N/A | HR H-NLR vs. L-NLR = 23.75/1 [95%, 7.56–74.66, p < 0.001] | HR H-PLR vs. L-PLR = 1.80/1 [95%, 0.92–3.52, p = 0.085] |
| 3. | Khunger et al. (2018) [22] | H-NLR ≥ 5; L-NLR < 5 | N/A | N/A | N/A | N/A | N/A |
| 4. | Nakaya et al. (2018) [23] | H-NLR ≥ 3; L-NLR < 3 | N/A | H-NLR = 2.1 months vs. L-NLR = 5.3 months [95% CI, p = 0.00528] (2 weeks) H-NLR = 2 months vs. L-NLR = 5.3 months [95% CI, p = 0.00515] (4 weeks) | N/A | N/A | N/A |
| 5. | Russo et al. (2018) [24] | H-NLR ≥ 3; L-NLR ≤ 3 | H-PLR ≥ 160; L-PLR ≤ 160 | L-NLR = 4.0 months vs. H-NLR = 1.0 months [95% CI, p = 0.204] | N/A | HR H-NLR vs. L-NLR = 1.05 [95% CI, p = 0.0924] | N/A |
| 6. | Svaton et al. (2018) [25] | H-NLR > 3.8; L-NLR ≤ 3.8 | H-PLR > 169.1; L-PLR ≤ 169.1 | L-NLR = 6.1 months vs. H-NLR = 4.1 months [95% CI, p = 0.321] | L-PLR = 6.6 months vs. H-PLR = 3.9 months [95% CI, p = 0.108] | 1.033 (0.985–1.083) [95% CI, p = 0.185] | 1.000 (0.999–1.001) [95% CI, p = 0.663] |
| 7. | Takeda et al. (2018) [26] | H-NLR ≥ 5; L-NLR < 5 | H-PLR > 300; M-PLR = 150–300; L-PLR < 150 | L-NLR = 67 days [95% CI, 27–111 days] vs. H-NLR = 109 days [95% CI, 4-NA days] (2 weeks) L-NLR = 95 days [95% CI, 50-NA days] vs. H-NLR = 10 days [95% CI, 6-NA days] (4 weeks) | L-PLR = 66 days [95% CI, 24–111 days] vs. M-PLR = 39 days [95% CI, 24-NA days] vs. H-PLR = 110 days [95% CI, 4-NA days] (2 weeks) L-PLR = 65 days [95% CI, 24-NA days] vs. M-PLR = 12 days [95% CI, 6–136 days] vs. H-PLR = 100.5 days [95% CI, 10-NA days] (4 weeks) | HR L-NLR vs. H-NLR = 4.02 [95% CI, 1.345–12.02] (4 weeks) | N/A |
| 8. | Zer et al. (2018) [27] | H-NLR > 4; L-NLR ≤ 4 | H-PLR > 200; L-PLR ≤ 200 | N/A | N/A | N/A | N/A |
| 9. | Pavan et al. (2019) [28] | H-NLR ≥ 3; L-NLR ≤ 3 | H-PLR ≥ 180; L-PLR ≤ 180 | L-NLR = 7.4 months [95% CI, 5.0–9,8 months] vs. H-NLR = 3.1 months [95% CI, 2.2–3.9 months) | L-PLR = 7.3 months [95% CI, 4.4–10.2 months] vs. H-PLR = 2.9 months [95% CI, 1.9–4.0 months] | HR L-NLR vs. H-NLR = 0.557 [95% CI, 0.378–0.820, p = 0.003] | HR H-PLR vs. L-PLR = 1.709 [95% CI, 1.178–2.478, p = 0.005] |
| 10. | Matsubara et al. (2020) [29] | H-NLR ≥ 5; L-NLR < 5 | H-PLR ≥ 150; L-PLR < 150 | N/A | N/A | N/A | N/A |
| 11. | Prelaj et al. (2020) [30] | H-NLR ≥ 4; L-NLR ≤ 4 | N/A | L-NLR = 4.7 months vs. H-NLR = 2.2 months | N/A | HR H-NLR vs. L-NLR = 2.52 [95% CI, 1.72–3.69, p < 0.001] | N/A |
| 12. | Russo et al. (2020) [31] | H-NLR ≥ 5; L-NLR ≤ 5 | H-PLR ≥ 200; L-PLR ≤ 200 | L-NLR = 7.0 months vs. H-NLR = 4.0 months | L-PLR = 7.0 months vs. H-PLR = 4.0 months | HR L-NLR vs. H-NLR = 0.64 [95% CI, p = 0.028] | HR L-PLR vs. H-PLR = 0.67 [95% CI, p = 0.05] |
| 13. | Takada et al. (2020) [32] | H-NLR ≥ 6.05; L-NLR <6.05 | H-PLR ≥ 245; L-PLR < 245 | N/A | N/A | H-NLR/L-NLR = 2.13 (1.55–2.92) p < 0.0001 | H-PLR/L-PLR = 1.32 (0.99–1.76) p = 0.0596 |
| 14. | Yang et al. (2020) [33] | H-NLR > 2.4; L-NLR ≤ 2.4 | H-PLR > 195; L-PLR ≤ 195 | L-NLR = 12.73 months [95% CI, 9.9–15.6 months] vs. H-NLR = 7.73 months [95% CI,6.7–8.7] (p = 0.018) | L-PLR = 12.57 months [95% CI, 9.9–15.3] vs. H-PLR = 7 months [95% CI,6.2–7.8] (p = 0.002) | HR L-NLR vs. H-NLR = 1.576 [95% CI (1.078–2.304), p =0.018] | HR L-PLR vs. H-PLR = 1.862 [95% CI (1.257–2.757), p = 0.002] |
| 15. | Ksienski et al. (2021) [34] | H-NLR ≥ 6.4; L-NLR < 6.4 | H-PLR ≥ 441.8; L-PLR < 441.8 | H-NLR = 3.5 months vs. L-NLR = 10 months [95% CI, p < 0.001] | H-PLR = 2.9 months vs. L-PLR = 6.7 months [95% CI, p < 0.001] | N/A | N/A |
| No | Author; Year | NLR Cut-Off Value | PLR Cut-Off Value | OS | HR OS | ||
|---|---|---|---|---|---|---|---|
| NLR | PLR | NLR | PLR | ||||
| 1. | Katayama et al. (2017) [10] | H-NLR > 5; L-NLR ≤ 5 | H-PLR > 262; L-NLR ≤ 262 | H-NLR = 98 days vs. L-NLR = NA [95% CI, p < 0.001] | H-NLR = 106 days vs. L-PLR = NA [95% CI, p < 0.001] | H-NLR = 98 days vs. L-NLR = NA [95% CI, p < 0.001] | H-NLR = 106 days vs. L-PLR = NA [95% CI, p < 0.001] |
| 2. | Suh et al. (2017) [21] | H-NLR ≥ 5; L-NLR <5 | H-PLR ≥ 169; L-PLR < 169 | L-NLR = 14.0 months vs. H-NLR = 2.1 months [p < 0.001] | N/A | L-NLR = 14.0 months vs. H-NLR = 2.1 months [p < 0.001] | N/A |
| 3. | Khunger et al. (2018) [22] | H-NLR ≥ 5; L-NLR < 5 | N/A | H-NLR = 24.2 months [95% CI, 16.1–36.2 months] vs. L-NLR = 29.1 months [95% CI, 16.2–40.9 months], p < 0.001 | N/A | H-NLR = 24.2 months [95% CI, 16.1–36.2 months] vs. L-NLR = 29.1 months [95% CI, 16.2–40.9 months], p < 0.001 | N/A |
| 4. | Nakaya et al. (2018) [23] | H-NLR ≥ 3; L-NLR < 3 | N/A | N/A | N/A | N/A | N/A |
| 5. | Russo et al. (2018) [24] | H-NLR ≥ 3; L-NLR ≤ 3 | H-PLR ≥ 160; L-PLR ≤ 160 | L-NLR = 6.0 months vs. H-NLR = 2.0 months [p = 0.789] | L-PLR = 10.0 months vs. H-PLR = 6.0 months [p = 0.756] | L-NLR = 6.0 months vs. H-NLR = 2.0 months [p = 0.789] | L-PLR = 10.0 months vs. H-PLR = 6.0 months [p = 0.756] |
| 6. | Svaton et al. (2018) [25] | H-NLR > 3.8; L-NLR ≤ 3.8 | H-PLR > 169.1; L-PLR ≤ 169.1 | L-NLR = 14.2 months vs. H-NLR = 9.2 months [95% CI, p = 0.020] | L-PLR = 14.2 months vs. H-PLR = 9.2 months [95% CI, p = 0.014] | L-NLR = 14.2 months vs. H-NLR = 9.2 months [95% CI, p = 0.020] | L-PLR = 14.2 months vs. H-PLR = 9.2 months [95% CI, p = 0.014] |
| 7. | Takeda et al. (2018) [26] | H-NLR ≥ 5; L-NLR < 5 | H-PLR > 300; M-PLR = 150–300; L-PLR < 150 | N/A | N/A | N/A | N/A |
| 8. | Zer et al. (2018) [27] | H-NLR > 4; L-NLR ≤ 4 | H-PLR > 200; L-PLR ≤ 200 | L-NLR = 21.4 months vs. H-NLR = 6.8 months [95% CI, p = 0.019] | L-PLR = 14.2 months vs. H-PLR = 9.2 months [p = 0.019] | L-NLR = 21.4 months vs. H-NLR = 6.8 months [95% CI, p = 0.019] | L-PLR = 14.2 months vs. H-PLR = 9.2 months [p = 0.019] |
| 9. | Pavan et al. (2019) [28] | H-NLR ≥ 3; L-NLR ≤ 3 | H-PLR ≥ 180; L-PLR ≤ 180 | L-NLR = 49.3 months [95% CI, 7.4–91.3 months] vs. H-NLR = 17.3 months [95% CI, 12.1–22.5 months] | L-PLR = 36.4 months [95% CI, 16.4–56.4 months] vs. H-PLR = 14.7 months [95% CI, 9.6–19.7 months] | L-NLR = 49.3 months [95% CI, 7.4–91.3 months] vs. H-NLR = 17.3 months [95% CI, 12.1–22.5 months] | L-PLR = 36.4 months [95% CI, 16.4–56.4 months] vs. H-PLR = 14.7 months [95% CI, 9.6–19.7 months] |
| 10. | Matsubara et al. (2020) [29] | H-NLR ≥ 5; L-NLR < 5 | H-PLR ≥ 150; L-PLR < 150 | N/A | N/A | N/A | N/A |
| 11. | Prelaj et al. (2020) [30] | H-NLR ≥ 4; L-NLR ≤ 4 | N/A | L-NLR = 10.1 months vs. H-NLR = 2.6 months | N/A | L-NLR = 10.1 months vs. H-NLR = 2.6 months | N/A |
| 12. | Russo et al. (2020) [31] | H-NLR ≥ 5; L-NLR ≤ 5 | H-PLR ≥ 200; L-PLR ≤ 200 | L-NLR = 15.0 months vs. H-NLR = 6.0 months | L-PLR = 15.0 months vs. H-PLR = 11.0 months | L-NLR = 15.0 months vs. H-NLR = 6.0 months | L-PLR = 15.0 months vs. H-PLR = 11.0 months |
| 13. | Takada et al. (2020) [32] | H-NLR ≥ 6.05; L-NLR <6.05 | H-PLR ≥ 245; L-PLR < 245 | N/A | N/A | N/A | N/A |
| 14. | Yang et al. (2020) [33] | H-NLR > 2.4; L-NLR ≤ 2.4 | H-PLR > 195; L-PLR ≤ 195 | L-NLR = Not reached vs. H-NLR = 15.77 months [95% CI, 11.4–20.1] (p = 0.000) | L-PLR = Not reached vs. H-PLR = 12.00 months [95% CI, 9.0–15.1] (p = 0.000) | L-NLR = Not reached vs. H-NLR = 15.77 months [95% CI, 11.4–20.1] (p = 0.000) | L-PLR = Not reached vs. H-PLR = 12.00 months [95% CI, 9.0–15.1] (p = 0.000) |
| 15. | Ksienski et al. (2021) [34] | H-NLR ≥ 6.4; L-NLR < 6.4 | H-PLR ≥ 441.8; L-PLR < 441.8 | H-NLR = 5.4 months [95% CI, 3.6–8.4 months] vs. L-NLR = 18.9 months [95% CI, 14.2-NR months] | H-PLR = 4 months [95% CI, 3.1–6.2 months] vs. L-NLR = 15.4 months [95% CI, 11.4–20.4 months] | H-NLR = 5.4 months [95% CI, 3.6–8.4 months] vs. L-NLR = 18.9 months [95% CI, 14.2-NR months] | H-PLR = 4 months [95% CI, 3.1–6.2 months] vs. L-NLR = 15.4 months [95% CI, 11.4–20.4 months] |
3. Results
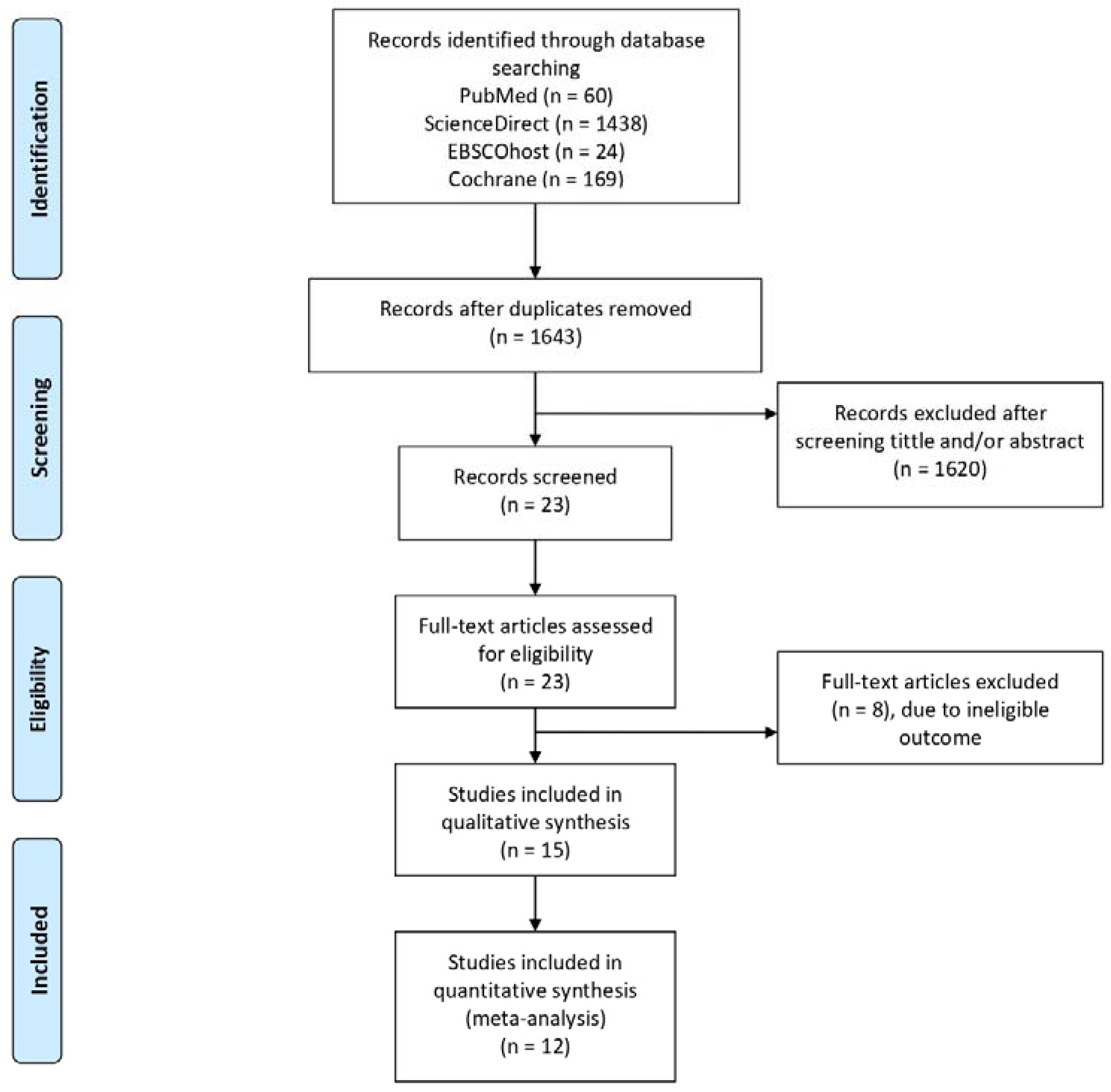
3.1. Search Results
3.2. Characteristics of Included Study and Critical Appraisal
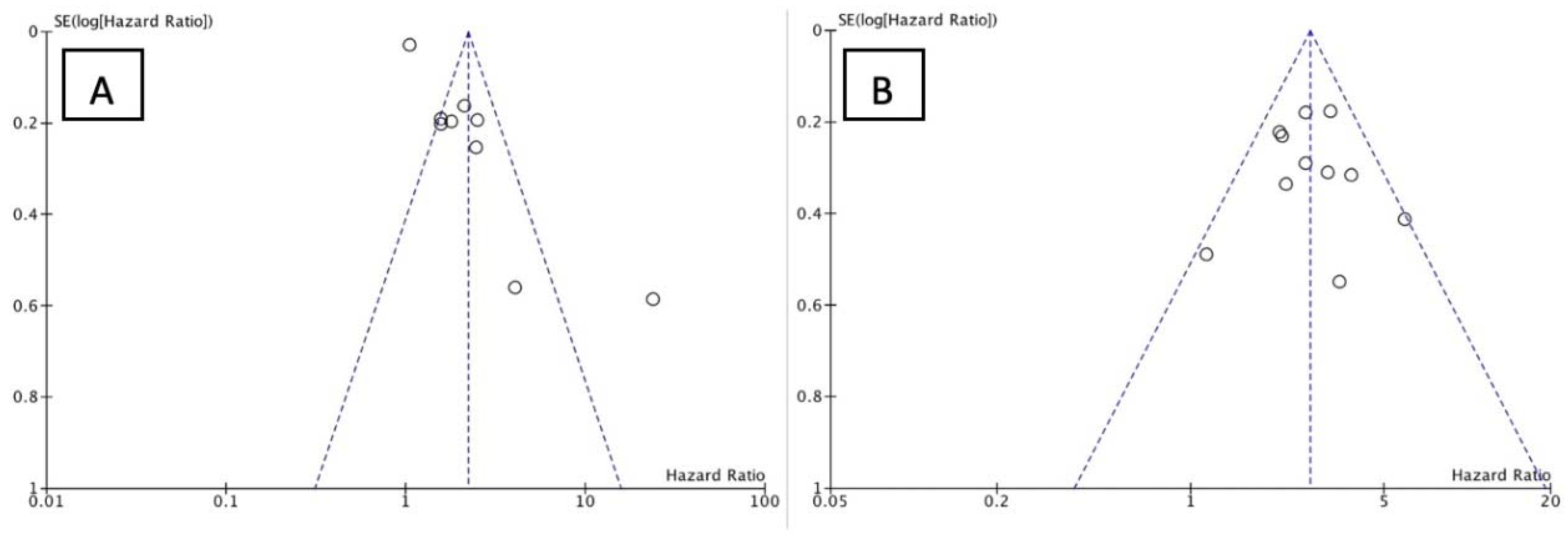
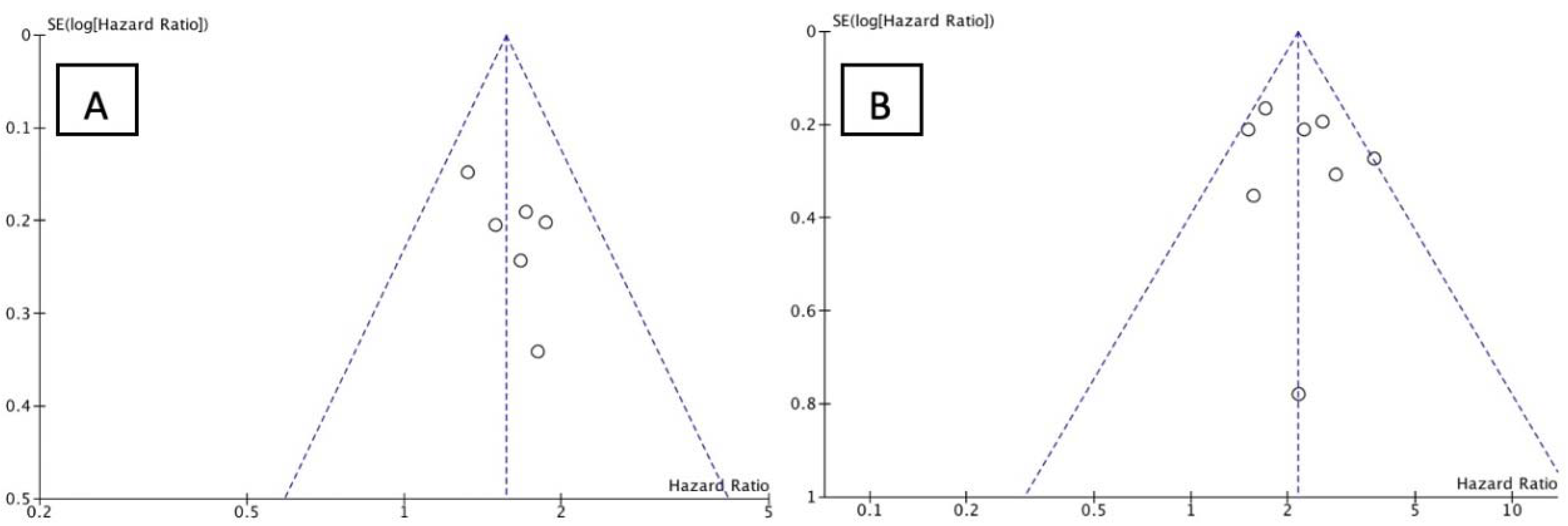
3.3. Study Outcome
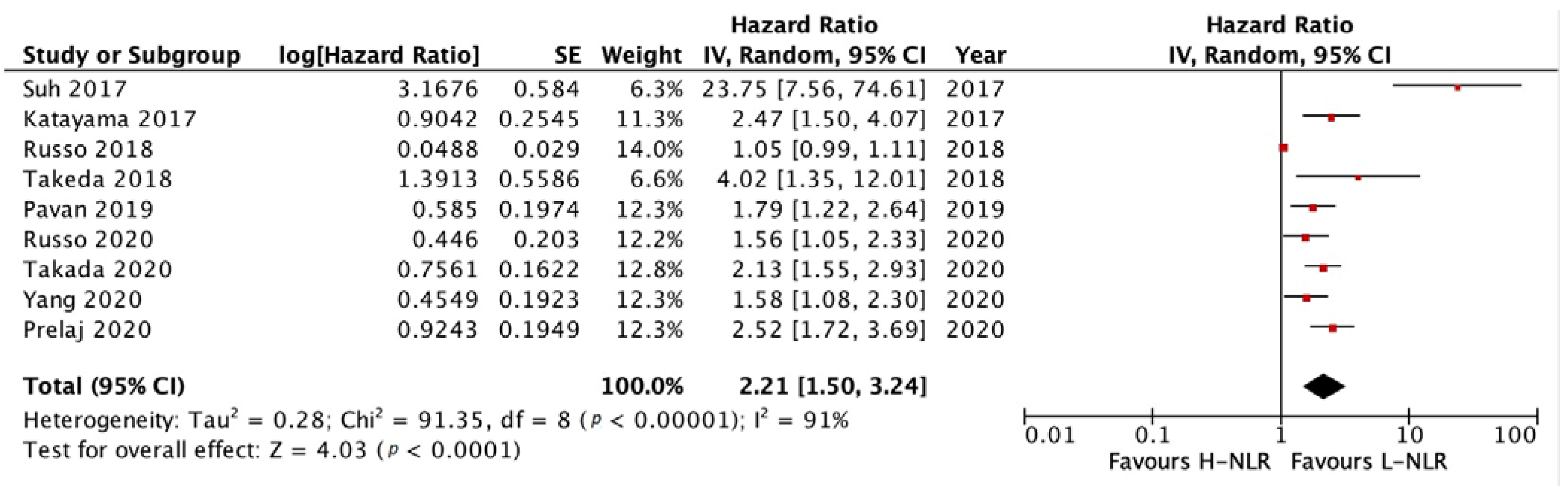
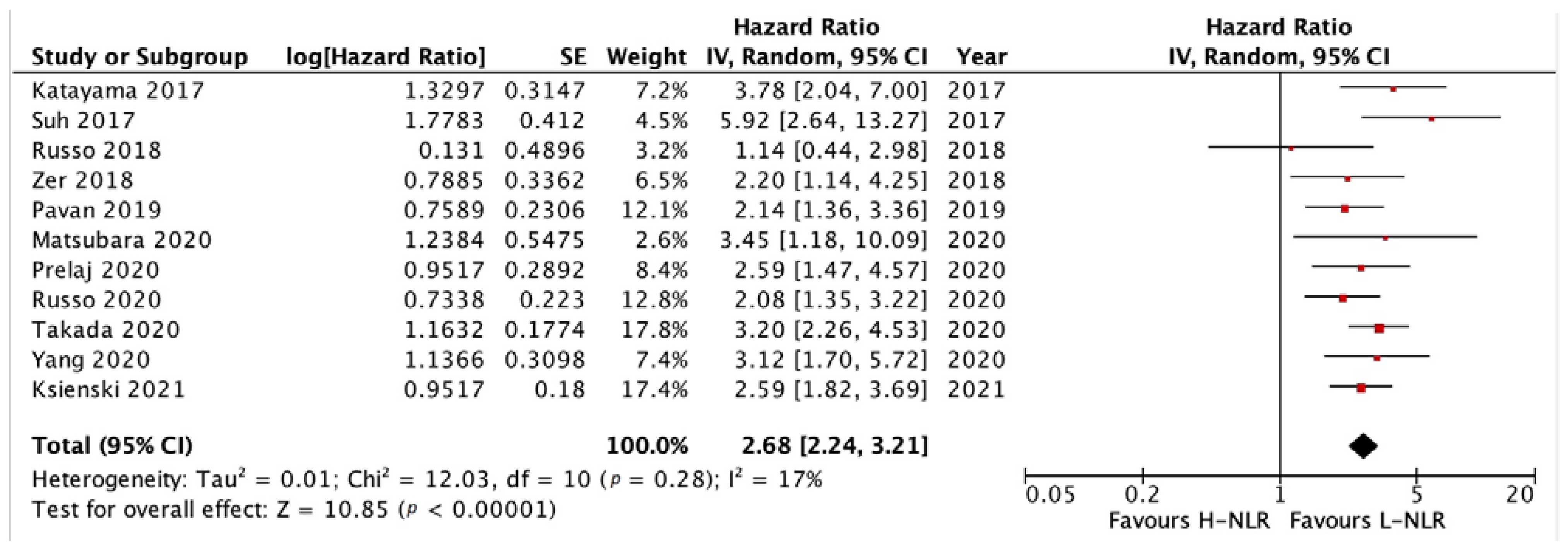
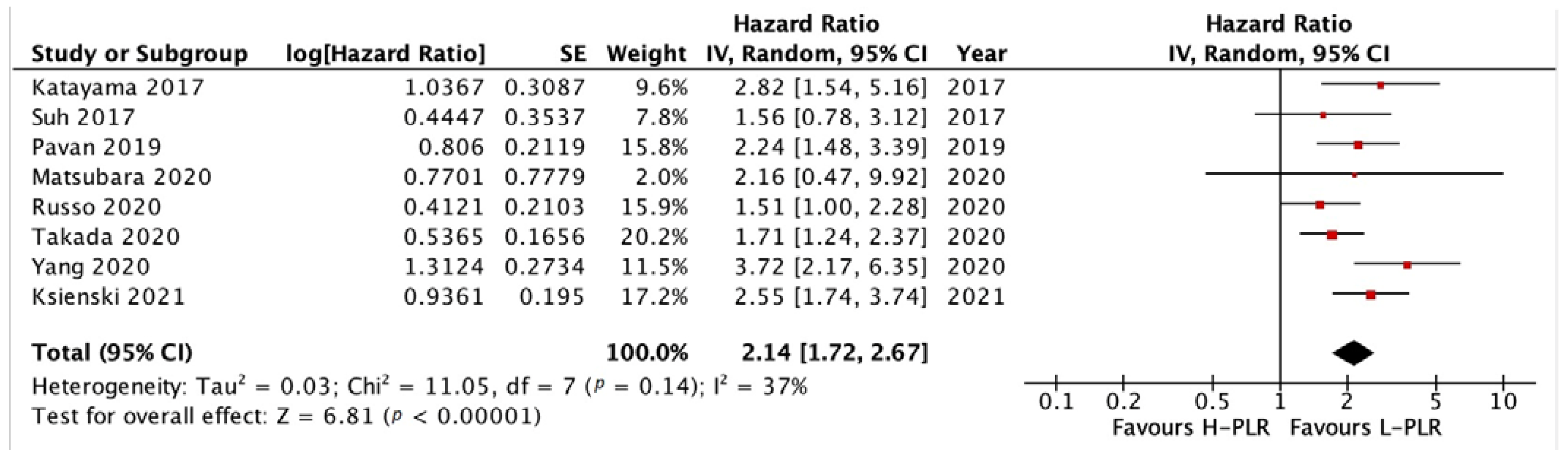
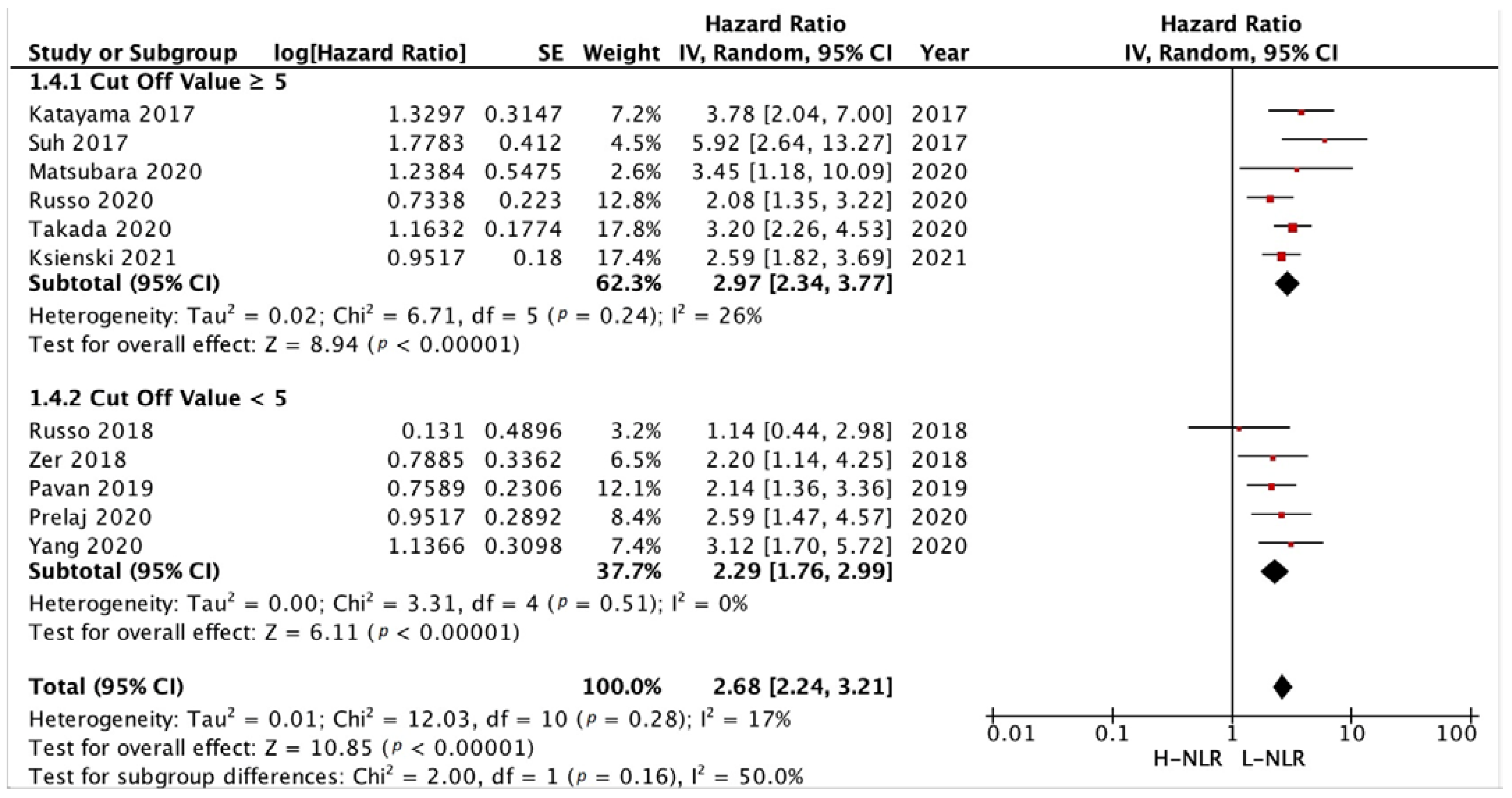
3.3.1. PFS and OS of NLR
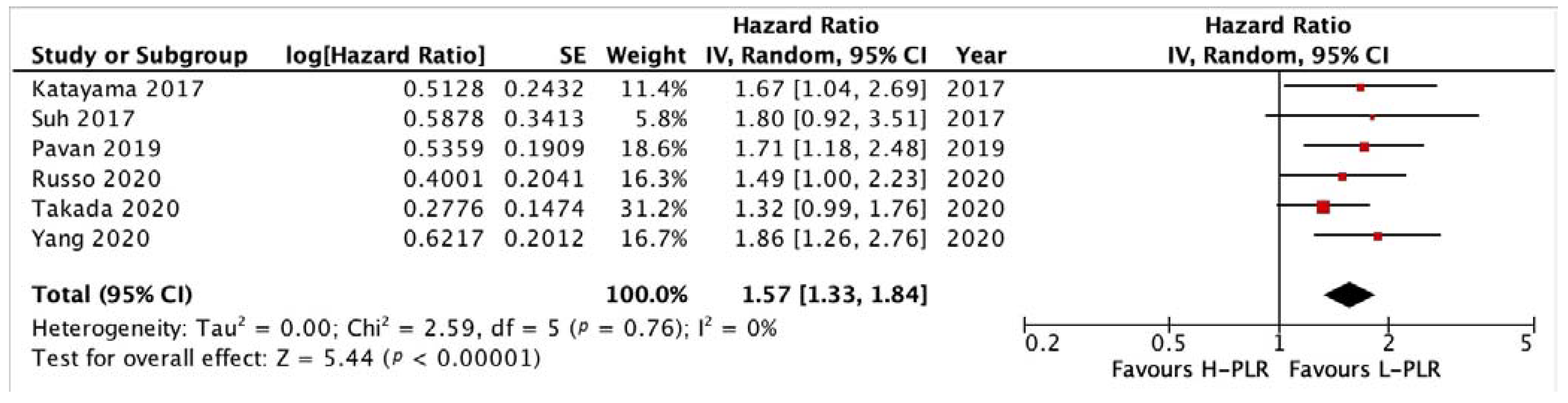
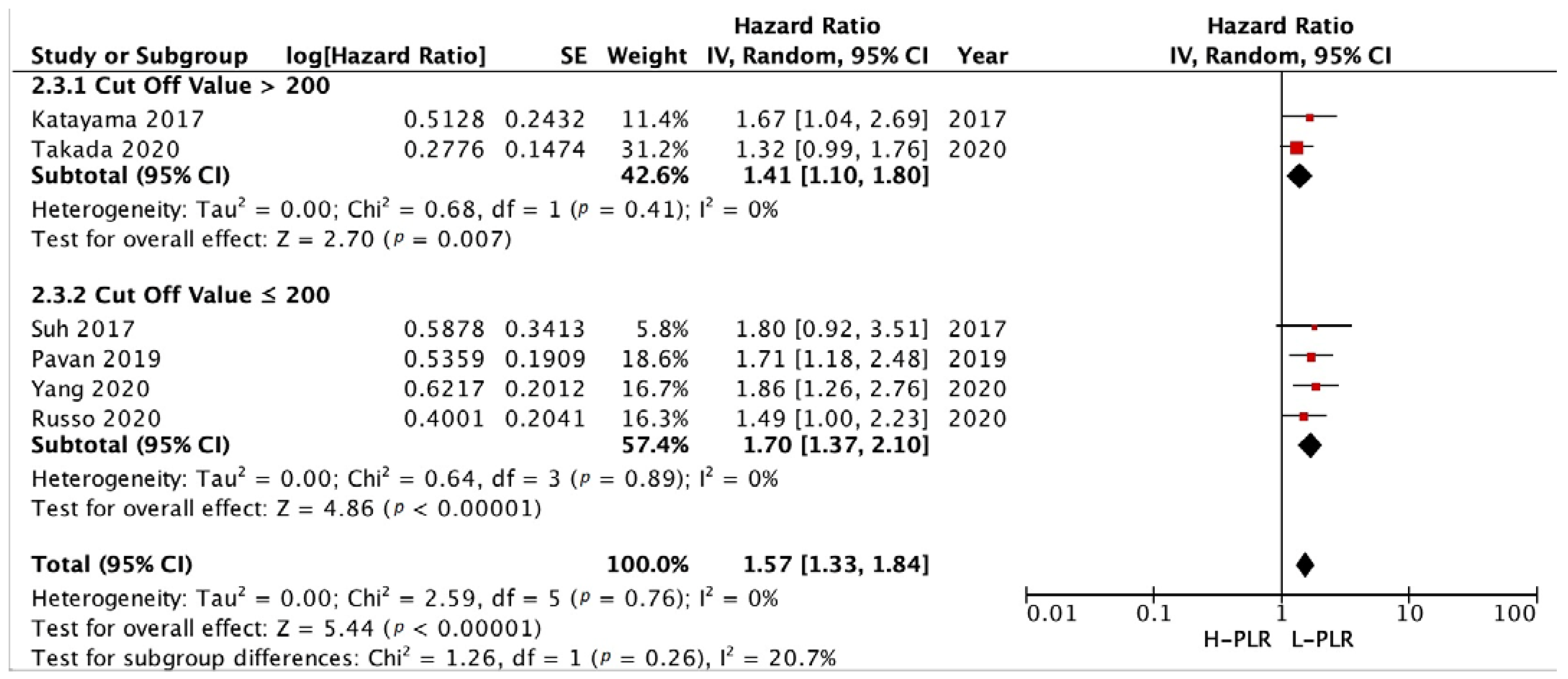
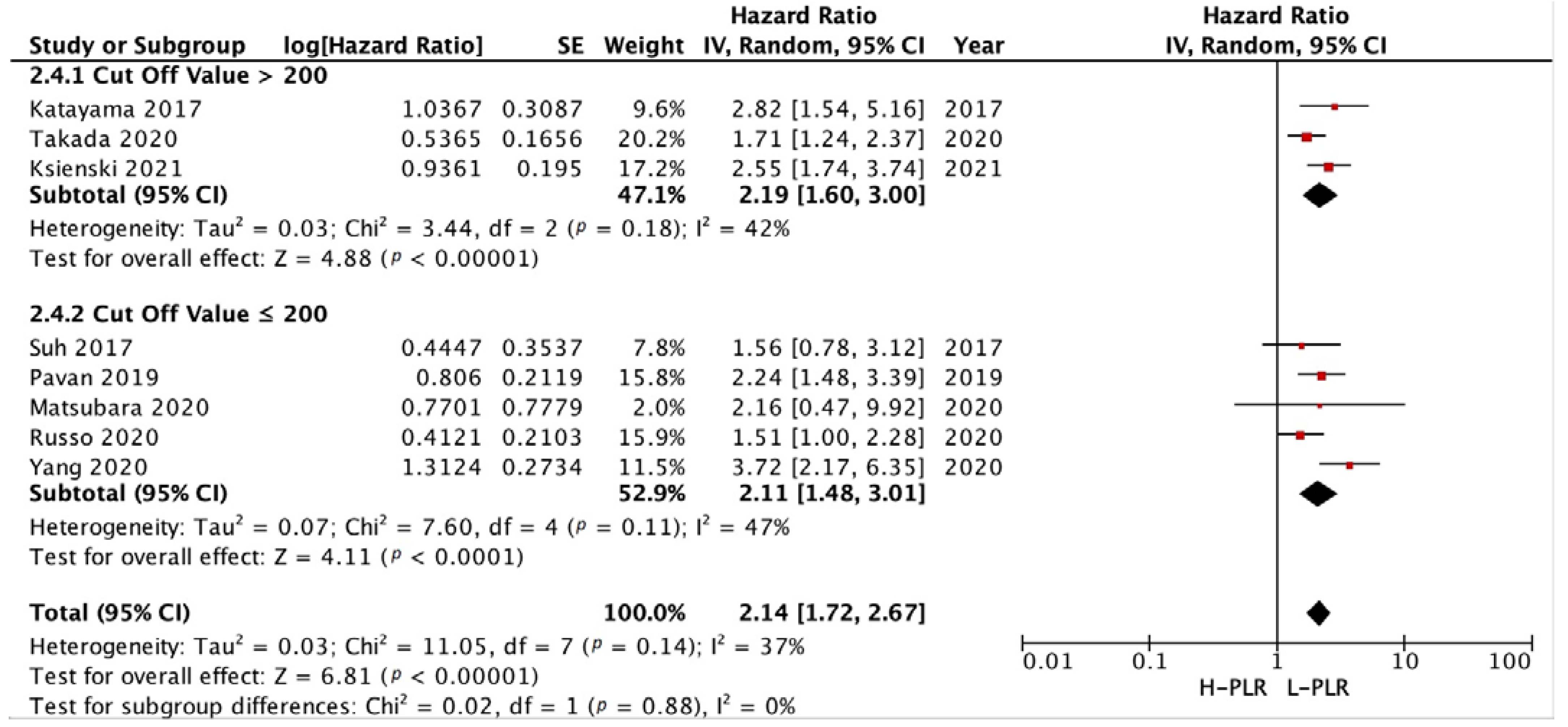
3.3.2. PFS and OS of PLR
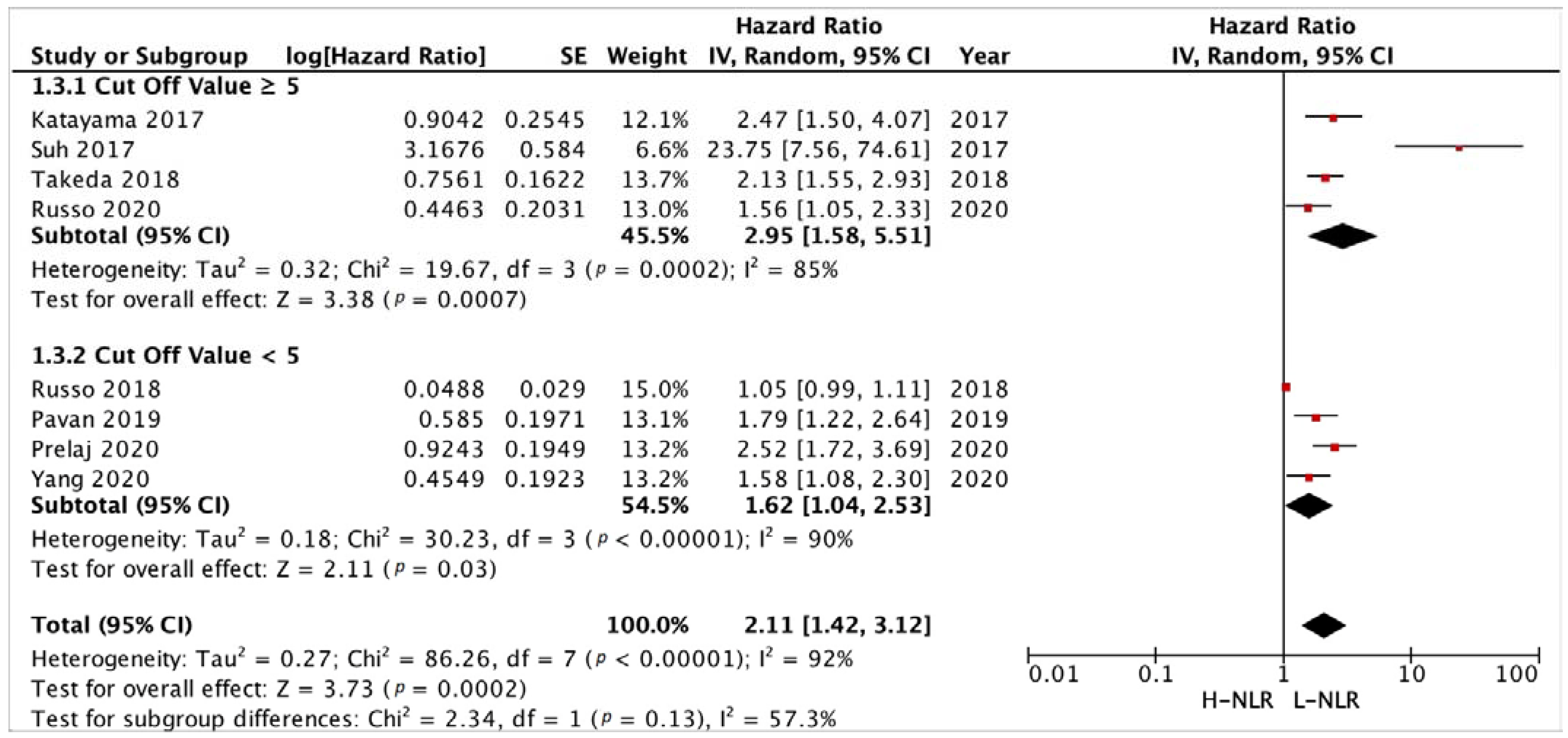
3.3.3. Subgroup Analysis in NLR and PLR Cut-Off
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune Checkpoint Inhibitors: Recent Progress and Potential Biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havel, J.J.; Chowell, D.; Chan, T.A. The Evolving Landscape of Biomarkers for Checkpoint Inhibitor Immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Song, H.; Chen, Y.; Han, F.; Wang, Q.; Cui, Y. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Blood to Distinguish Lung Cancer Patients from Healthy Subjects. Dis. Markers 2020, 2020, 8844698. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, S.; Liu, Y.; Ma, L.; Zhu, J.; Xin, Y.; Wang, Y.; Yang, C.; Cheng, Y. Systemic Immune-Inflammation Index, Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio Can Predict Clinical Outcomes in Patients with Metastatic Non-Small-Cell Lung Cancer Treated with Nivolumab. J. Clin. Lab. Anal. 2019, 33, e22964. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, H.; Chen, B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J. Cancer 2017, 8, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Raedler, L.A. Keytruda (Pembrolizumab): First PD-1 Inhibitor Approved for Previously Treated Unresectable or Metastatic Melanoma. Am. Health Drug Benefits 2015, 8, 96–100. [Google Scholar]
- Brueckl, W.M.; Ficker, J.H.; Zeitler, G. Clinically Relevant Prognostic and Predictive Markers for Immune-Checkpoint-Inhibitor (ICI) Therapy in Non-Small Cell Lung Cancer (NSCLC). BMC Cancer 2020, 20, 1185. [Google Scholar] [CrossRef]
- Katayama, Y.; Yamada, T.; Chihara, Y.; Tanaka, S.; Tanimura, K.; Okura, N.; Hirose, K.; Uda, S.; Shiotsu, S.; Hirai, S.; et al. Significance of Inflammatory Indexes in Atezolizumab Monotherapy Outcomes in Previously Treated Non-Small-Cell Lung Cancer Patients. Sci. Rep. 2020, 10, 17495. [Google Scholar] [CrossRef]
- Zarogoulidis, K.; Zarogoulidis, P.; Darwiche, K.; Boutsikou, E.; Machairiotis, N.; Tsakiridis, K.; Katsikogiannis, N.; Kougioumtzi, I.; Karapantzos, I.; Huang, H.; et al. Treatment of Non-Small Cell Lung Cancer (NSCLC). J. Thorac. Dis. 2013, 5 (Suppl. S4), S389–S396. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; He, A.; Liu, A.; Tong, W.; Cao, D. Evaluation of the Prognostic Role of Platelet-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Int. Immunopharmacol. 2019, 77, 105957. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Prognostic Markers in Patients with Non-Small Cell Lung Cancer (NSCLC) Treated with Nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, S.; Liang, C.; Han, X.; Shi, Y. Pretreatment Hematological Markers Predict Clinical Outcome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Meta-Analysis. Thorac. Cancer 2018, 9, 1220–1230. [Google Scholar] [CrossRef]
- Lababede, O.; Meziane, M.A. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist 2018, 23, 844–848. [Google Scholar] [CrossRef] [Green Version]
- Zappa, C.; Mousa, S.A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Bironzo, P.; Di Maio, M. A Review of Guidelines for Lung Cancer. J. Thorac. Dis. 2018, 10 (Suppl. S13), S1556–S1563. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1–e34. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 19 October 2020).
- Suh, K.J.; Kim, S.H.; Kim, Y.J.; Kim, M.; Keam, B.; Kim, T.M.; Kim, D.-W.; Heo, D.S.; Lee, J.S. Post-Treatment Neutrophil-to-Lymphocyte Ratio at Week 6 Is Prognostic in Patients with Advanced Non-Small Cell Lung Cancers Treated with Anti-PD-1 Antibody. Cancer Immunol. Immunother. 2018, 67, 459–470. [Google Scholar] [CrossRef]
- Khunger, M.; Patil, P.D.; Khunger, A.; Li, M.; Hu, B.; Rakshit, S.; Basu, A.; Pennell, N.; Stevenson, J.P.; Elson, P.; et al. Post-Treatment Changes in Hematological Parameters Predict Response to Nivolumab Monotherapy in Non-Small Cell Lung Cancer Patients. PLoS ONE 2018, 13, e0197743. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, A.; Kurata, T.; Yoshioka, H.; Takeyasu, Y.; Niki, M.; Kibata, K.; Satsutani, N.; Ogata, M.; Miyara, T.; Nomura, S. Neutrophil-to-Lymphocyte Ratio as an Early Marker of Outcomes in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Nivolumab. Int. J. Clin. Oncol. 2018, 23, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Franchina, T.; Ricciardi, G.R.R.; Battaglia, A.; Scimone, A.; Berenato, R.; Giordano, A.; Adamo, V. Baseline Neutrophilia, Derived Neutrophil-to-Lymphocyte Ratio (DNLR), Platelet-to-Lymphocyte Ratio (PLR), and Outcome in Non Small Cell Lung Cancer (NSCLC) Treated with Nivolumab or Docetaxel. J. Cell Physiol. 2018, 233, 6337–6343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svaton, M.; Zemanova, M.; Skrickova, J.; Jakubikova, L.; Kolek, V.; Kultan, J.; Koubkova, L.; Bejckova, A.; Salajka, F.; Hrnciarik, M.; et al. Chronic Inflammation as a Potential Predictive Factor of Nivolumab Therapy in Non-Small Cell Lung Cancer. Anticancer Res. 2018, 38, 6771–6782. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Takeuchi, M.; Saitoh, M.; Takeda, S. Neutrophil-to-Lymphocyte Ratio after Four Weeks of Nivolumab Administration as a Predictive Marker in Patients with Pretreated Non-Small-Cell Lung Cancer. Thorac. Cancer 2018, 9, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Zer, A.; Sung, M.R.; Walia, P.; Khoja, L.; Maganti, M.; Labbe, C.; Shepherd, F.A.; Bradbury, P.A.; Feld, R.; Liu, G.; et al. Correlation of Neutrophil to Lymphocyte Ratio and Absolute Neutrophil Count with Outcomes With PD-1 Axis Inhibitors in Patients With Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, 426–434.e1. [Google Scholar] [CrossRef] [PubMed]
- Pavan, A.; Calvetti, L.; Dal Maso, A.; Attili, I.; Del Bianco, P.; Pasello, G.; Guarneri, V.; Aprile, G.; Conte, P.; Bonanno, L. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Oncologist 2019, 24, 1128–1136. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, T.; Takamori, S.; Haratake, N.; Toyozawa, R.; Miura, N.; Shimokawa, M.; Yamaguchi, M.; Seto, T.; Takenoyama, M. The Impact of Immune-Inflammation-Nutritional Parameters on the Prognosis of Non-Small Cell Lung Cancer Patients Treated with Atezolizumab. J. Thorac. Dis. 2020, 12, 1520–1528. [Google Scholar] [CrossRef]
- Prelaj, A.; Ferrara, R.; Rebuzzi, S.E.; Proto, C.; Signorelli, D.; Galli, G.; De Toma, A.; Randon, G.; Pagani, F.; Viscardi, G.; et al. EPSILoN: A Prognostic Score for Immunotherapy in Advanced Non-Small-Cell Lung Cancer: A Validation Cohort. Cancers 2019, 11, 1954. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Russano, M.; Franchina, T.; Migliorino, M.R.; Aprile, G.; Mansueto, G.; Berruti, A.; Falcone, A.; Aieta, M.; Gelibter, A.; et al. Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Outcomes with Nivolumab in Pretreated Non-Small Cell Lung Cancer (NSCLC): A Large Retrospective Multicenter Study. Adv. Ther. 2020, 37, 1145–1155. [Google Scholar] [CrossRef]
- Takada, K.; Takamori, S.; Yoneshima, Y.; Tanaka, K.; Okamoto, I.; Shimokawa, M.; Oba, T.; Osoegawa, A.; Tagawa, T.; Takenoyama, M.; et al. Serum Markers Associated with Treatment Response and Survival in Non-Small Cell Lung Cancer Patients Treated with Anti-PD-1 Therapy. Lung Cancer 2020, 145, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, H.; Yang, G.; Yang, L.; Li, J.; Wang, Y. The Value of Blood Biomarkers of Progression and Prognosis in ALK-Positive Patients with Non-Small Cell Lung Cancer Treated with Crizotinib. Asia Pac. J. Clin. Oncol. 2020, 16, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ksienski, D.; Wai, E.S.; Alex, D.; Croteau, N.S.; Freeman, A.T.; Chan, A.; Patterson, T.; Clarkson, M.; Fiorino, L.; Poonja, Z.; et al. Prognostic Significance of the Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio for Advanced Non-Small Cell Lung Cancer Patients with High PD-L1 Tumor Expression Receiving Pembrolizumab. Transl. Lung Cancer Res. 2021, 10, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Shi, L.; Wang, B.; Yang, J.; Xiao, Z.; Du, P.; Wang, Q.; Yang, W. Prognostic Role of Pretreatment Blood Neutrophil-to-Lymphocyte Ratio in Advanced Cancer Survivors: A Systematic Review and Meta-Analysis of 66 Cohort Studies. Cancer Treat Rev. 2017, 58, 1–13. [Google Scholar] [CrossRef]
- Gu, X.-B.; Tian, T.; Tian, X.-J.; Zhang, X.-J. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Non-Small Cell Lung Cancer: A Meta-Analysis. Sci. Rep. 2015, 5, 12493. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, G.; Sisi, M.; Andrini, E.; Palladini, A.; Giunchi, F.; Lollini, P.-L.; Ardizzoni, A.; Gelsomino, F. The Mechanisms of PD-L1 Regulation in Non-Small-Cell Lung Cancer (NSCLC): Which Are the Involved Players? Cancers 2020, 12, 3129. [Google Scholar] [CrossRef]
- Ren, F.; Zhao, T.; Liu, B.; Pan, L. Neutrophil-Lymphocyte Ratio (NLR) Predicted Prognosis for Advanced Non-Small-Cell Lung Cancer (NSCLC) Patients Who Received Immune Checkpoint Blockade (ICB). OncoTargets Ther. 2019, 12, 4235–4244. [Google Scholar] [CrossRef] [Green Version]
- Petrova, M.P.; Eneva, M.I.; Arabadjiev, J.I.; Conev, N.V.; Dimitrova, E.G.; Koynov, K.D.; Karanikolova, T.S.; Valev, S.S.; Gencheva, R.B.; Zhbantov, G.A.; et al. Neutrophil to Lymphocyte Ratio as a Potential Predictive Marker for Treatment with Pembrolizumab as a Second Line Treatment in Patients with Non-Small Cell Lung Cancer. Biosci Trends 2020, 14, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Erpenbeck, L.; Schön, M.P. Deadly Allies: The Fatal Interplay between Platelets and Metastasizing Cancer Cells. Blood 2010, 115, 3427–3436. [Google Scholar] [CrossRef]
- He, A.-D.; Xie, W.; Song, W.; Ma, Y.-Y.; Liu, G.; Liang, M.-L.; Da, X.-W.; Yao, G.-Q.; Zhang, B.-X.; Gao, C.-J.; et al. Platelet Releasates Promote the Proliferation of Hepatocellular Carcinoma Cells by Suppressing the Expression of KLF6. Sci. Rep. 2017, 7, 3989. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhan, P.; Lv, Y.; Shen, K.; Wei, Y.; Liu, H.; Song, Y. Prognostic Role of Pretreatment Neutrophil-to-Lymphocyte Ratio in Non-Small Cell Lung Cancer Patients Treated with Systemic Therapy: A Meta-Analysis. Transl. Lung Cancer Res. 2019, 8, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.D.; Hottenga, J.-J.; Abdellaoui, A.; Dolan, C.V.; de Geus, E.J.C.; Kluft, C.; Boomsma, D.I.; Willemsen, G. Causes of Variation in the Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios: A Twin-Family Study. Biomark Med. 2016, 10, 1061–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Sun, S.; Gao, X.-S.; Xiong, W.; Qin, S.; Qi, X.; Ma, M.; Li, X.; Zhou, D.; Wang, W.; et al. Prognostic Value of Platelet to Lymphocyte Ratio in Non-Small Cell Lung Cancer: Evidence from 3430 Patients. Sci. Rep. 2016, 6, 23893. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platini, H.; Ferdinand, E.; Kohar, K.; Prayogo, S.A.; Amirah, S.; Komariah, M.; Maulana, S. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Markers for Advanced Non-Small-Cell Lung Cancer Treated with Immunotherapy: A Systematic Review and Meta-Analysis. Medicina 2022, 58, 1069. https://doi.org/10.3390/medicina58081069
Platini H, Ferdinand E, Kohar K, Prayogo SA, Amirah S, Komariah M, Maulana S. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Markers for Advanced Non-Small-Cell Lung Cancer Treated with Immunotherapy: A Systematic Review and Meta-Analysis. Medicina. 2022; 58(8):1069. https://doi.org/10.3390/medicina58081069
Chicago/Turabian StylePlatini, Hesti, Eric Ferdinand, Kelvin Kohar, Stephanie Amabella Prayogo, Shakira Amirah, Maria Komariah, and Sidik Maulana. 2022. "Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Markers for Advanced Non-Small-Cell Lung Cancer Treated with Immunotherapy: A Systematic Review and Meta-Analysis" Medicina 58, no. 8: 1069. https://doi.org/10.3390/medicina58081069
APA StylePlatini, H., Ferdinand, E., Kohar, K., Prayogo, S. A., Amirah, S., Komariah, M., & Maulana, S. (2022). Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Markers for Advanced Non-Small-Cell Lung Cancer Treated with Immunotherapy: A Systematic Review and Meta-Analysis. Medicina, 58(8), 1069. https://doi.org/10.3390/medicina58081069








