Risk Factors of Early Atrial Fibrillation Recurrence Following Electrical Cardioversion When Left Ventricular Ejection Fraction Is Preserved
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Biochemical Analyses
2.3. Transthoracic Echocardiography
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations and Strengths
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibbs, H.; Freedman, B.; Rosenqvist, M.; Virdone, S.; Mahmeed, W.A.; Ambrosio, G.; Camm, A.J.; Jacobson, B.; Jerjes-Sanchez, C.; Kayani, G.; et al. Clinical outcomes in asymptomatic and symptomatic atrial fibrillation presentations in Garfield-AF: Implications for AF screening. Am. J. Med. 2021, 134, 893–901.e11. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Yin, Y.; Ling, Z.; Su, L.; Liu, Z.; Wu, J.; Du, H.; Lan, X.; Fan, J.; Chen, W.; et al. Predictors of late recurrence of atrial fibrillation after catheter ablation. Int. J. Cardiol. 2013, 164, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Schilling, R.J. Cardioversion of atrial fibrillation the use of Antiarrhythmic Drugs. Heart 2009, 96, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Dzeshka, M.S.; Lip, G.Y.H.; Snezhitskiy, V.; Shantsila, E. Cardiac fibrosis in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2015, 66, 943–959. [Google Scholar] [CrossRef] [Green Version]
- Allessie, M. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef]
- Habibi, M.; Lima, J.A.C.; Khurram, I.M.; Zimmerman, S.L.; Zipunnikov, V.; Fukumoto, K.; Spragg, D.; Ashikaga, H.; Rickard, J.; Marine, J.E.; et al. Association of left atrial function and left atrial enhancement in patients with atrial fibrillation. Circ. Cardiovasc. Imaging 2015, 8, e002769. [Google Scholar] [CrossRef] [Green Version]
- Kuppahally, S.S.; Akoum, N.; Burgon, N.S.; Badger, T.J.; Kholmovski, E.G.; Vijayakumar, S.; Rao, S.N.; Blauer, J.; Fish, E.N.; DiBella, E.V.R.; et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation. Circ. Cardiovasc. Imaging 2010, 3, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.J.; Choi, E.-Y.; Rhee, S.J.; Joung, B.; Lee, B.-H.; Lee, S.-H.; Kim, J.; Lee, M.-H.; Jang, Y.; Chung, N.; et al. Left atrial strain as predictor of successful outcomes in catheter ablation for atrial fibrillation: A two-dimensional myocardial imaging study. J. Interv. Card Electrophysiol. 2009, 26, 127–132. [Google Scholar] [CrossRef]
- Morris, D.A.; Parwani, A.; Huemer, M.; Wutzler, A.; Bekfani, T.; Attanasio, P.; Friedrich, K.; Kühnle, Y.; Haverkamp, W.; Boldt, L.H. Clinical significance of the assessment of the systolic and diastolic myocardial function of the left atrium in patients with paroxysmal atrial fibrillation and low CHADS2 index treated with catheter ablation therapy. Am. J. Cardiol. 2013, 111, 1002–1011. [Google Scholar] [CrossRef]
- Moreno-Ruiz, L.A.; Madrid-Miller, A.; Martínez-Flores, J.E.; González-Hermosillo, J.A.; Arenas-Fonseca, J.; Zamorano-Velázquez, N.; Mendoza-Pérez, B. Left atrial longitudinal strain by speckle tracking as independent predictor of recurrence after electrical cardioversion in persistent and long standing persistent non-valvular atrial fibrillation. Int. J. Cardiovasc. Imaging 2019, 35, 1587–1596. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Li, W.; Wang, H.; Yin, L.; Ye, B.; Tang, Y.; Huang, C. CTRP9 ameliorates atrial inflammation, fibrosis, and vulnerability to atrial fibrillation in post--myocardial infarction rats. J. Am. Heart Assoc. 2019, 8, e013133. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takatsuki, S.; Inagawa, K.; Katsumata, Y.; Nishiyama, T.; Nishiyama, N.; Fukumoto, K.; Aizawa, Y.; Tanimoto, Y.; Tanimoto, K.; et al. Serum inflammation markers predicting successful initial catheter ablation for atrial fibrillation. Heart Lung Circ. 2014, 23, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, K.; Tada, H.; Hashimoto, T.; Ito, S.; Miyaji, K.; Naito, S.; Oshima, S.; Taniguchi, K. Plasma natriuretic peptide concentrations as a predictor for successful catheter ablation in patients with drug-refractory atrial fibrillation. Circ. J. 2007, 71, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillarisetti, J.; Reddy, N.; Biria, M.; Ryschon, K.; Nagarajan, D.; Murray, C.; Atkins, D.; Bommana, S.; Reddy, M.Y.; DiBiase, L.; et al. Elevated brain natriuretic peptide level in patients undergoing atrial fibrillation ablation: Is it a predictor of failed ablation or a mere function of atrial rhythm and rate at a point in time? J. Interv. Card Electrophysiol. 2014, 40, 161–168. [Google Scholar] [CrossRef]
- Nilsson, B.; Goetze, J.P.; Chen, X.; Pehrson, S.; Svendsen, J.H. Increased NT-pro-B-type natriuretic peptide independently predicts outcome following catheter ablation of atrial fibrillation. Scand. J. Clin. Lab. Investig. 2009, 69, 843–850. [Google Scholar] [CrossRef]
- Hwang, H.J.; Son, J.W.; Nam, B.-H.; Joung, B.; Lee, B.; Kim, J.B.; Lee, M.H.; Jang, Y.; Chung, N.; Shim, W.H.; et al. Incremental predictive value of pre-procedural N-terminal pro-B-type natriuretic peptide for short-term recurrence in atrial fibrillation ablation. Clin. Res. Cardiol. 2009, 98, 213–218. [Google Scholar] [CrossRef]
- Seewöster, T.; Büttner, P.; Zeynalova, S.; Hindricks, G.; Kornej, J. Are the atrial natriuretic peptides a missing link predicting low—Voltage areas in atrial fibrillation? Introducing the novel biomarker—Based Atrial Fibrillation substrate prediction (ANP) score. Clin. Cardiol. 2020, 43, 762–768. [Google Scholar] [CrossRef]
- Malouf, J.F.; Kanagala, R.; Al Atawi, F.O.; Rosales, A.G.; Davison, D.E.; Murali, N.S.; Tsang, T.S.M.; Chandrasekaran, K.; Ammash, N.M.; Friedman, P.A.; et al. High sensitivity C-reactive protein. J. Am. Coll. Cardiol. 2005, 46, 1284–1287. [Google Scholar] [CrossRef] [Green Version]
- Wazni, O. C reactive protein concentration and recurrence of atrial fibrillation after electrical cardioversion. Heart 2005, 91, 1303–1305. [Google Scholar] [CrossRef]
- Neuman, R.B.; Bloom, H.L.; Shukrullah, I.; Darrow, L.A.; Kleinbaum, D.; Jones, D.P.; Dudley, S.C. Oxidative stress markers are associated with persistent atrial fibrillation. Clin. Chem. 2007, 53, 1652–1657. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.; Wang, X.F.; Hou, Y.T.; Zhang, L. Correlation between atrial fibrillation, serum amyloid protein A and other inflammatory cytokines. Mol. Med. Rep. 2012, 6, 581–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. Corrigendum to: 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 4194. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.G. Quality specifications for imprecision of B-type natriuretic peptide assays. Clin. Chem. 2005, 51, 1307–1309. [Google Scholar] [CrossRef] [PubMed]
- Siersbæk-Nielsen, K.; Mølholm Hansen, J.; Kampmann, J.; Kristensen, M. Rapid evaluation of creatinine clearance. Lancet 1971, 297, 1133–1134. [Google Scholar] [CrossRef]
- Blume, G.G.; Mcleod, C.J.; Barnes, M.E.; Seward, J.B.; Pellikka, P.A.; Bastiansen, P.M.; Tsang, T.S. Left atrial function: Physiology, assessment, and clinical implications. Echocardiogr. Eur. Soc. Cardiol. 2011, 12, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nattel, S. Electrophysiologic remodeling: Are ion channels static players or dynamic movers? J. Cardiovasc. Electrophysiol. 1999, 10, 1553–1556. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Pluymaekers, N.; Dudink, E.; Luermans, J.; Meeder, J.G.; Lenderink, T.; Widdershoven, J.; Bucx, J.; Rienstra, M.; Kamp, O.; Van Opstal, J.M.; et al. Early or delayed cardioversion in recent-onset atrial fibrillation. N. Engl. J. Med. 2019, 380, 1499–1508. [Google Scholar] [CrossRef]
- Boriani, G.; Diemberger, I.; Biffi, M.; Camanini, C.; Valzania, C.; Corazza, I.; Martignani, C.; Zannoli, R.; Branzi, A. P wave dispersion and short-term vs. late atrial fibrillation recurrences after cardioversion. Int. J. Cardiol. 2005, 101, 355–361. [Google Scholar] [CrossRef]
- Casaclang-Verzosa, G.; Gersh, B.J.; Tsang, T.S.M. Structural and functional remodeling of the left atrium. J. Am. Coll. Cardiol. 2008, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Goette, A.; Honeycutt, C.; Langberg, J.J. Electrical remodeling in atrial fibrillation. Circulation 1996, 94, 2968–2974. [Google Scholar] [CrossRef] [PubMed]
- Ausma, J.; Litjens, N.; Lenders, M.H.; Duimel, H.; Mast, F.; Wouters, L.; Ramaeker, F.; Allessi, M.; Borgers, M. Time course of atrial fibrillation-induced cellular structural remodeling in Atria of the Goat. J. Mol. Cell. Cardiol. 2001, 33, 2083–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Fareh, S.; Leung, T.K.; Nattel, S. Promotion of atrial fibrillation by heart failure in dogs. Circulation 1999, 100, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, B.; Nattel, S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef] [Green Version]
- Duffy, H.S. Fibroblasts, myofibroblasts, and fibrosis: Fact, fiction, and the future. J. Cardiovasc. Pharmacol. 2011, 57, 373–375. [Google Scholar] [CrossRef]
- Jaakkola, S.; Lip, G.Y.H.; Biancari, F.; Nuotio, I.; Hartikainen, J.E.K.; Ylitalo, A.; Airaksinen, K.E.J. Predicting unsuccessful electrical cardioversion for acute atrial fibrillation (from the AF-CVS score). Am. J. Cardiol. 2017, 119, 749–752. [Google Scholar] [CrossRef] [Green Version]
- Kriz, R.; Freynhofer, M.K.; Weiss, T.W.; Egger, F.; Gruber, S.C.; Eisenburger, P.; Wojta, J.; Huber, K.; Koch, J. Safety and efficacy of pharmacological cardioversion of recent-onset atrial fibrillation: A single-center experience. Am. J. Emerg. Med. 2016, 34, 1486–1490. [Google Scholar] [CrossRef]
- Falsetti, L.; Viticchi, G.; Tarquinio, N.; Silvestrini, M.; Capeci, W.; Balloni, A.; Catozzo, V.; Gentile, A.; Pellegrini, F. CHA2DS2-vasc in the prediction of early atrial fibrillation relapses after electrical or pharmacological cardioversion. J. Cardiovasc. Med. 2014, 15, 636–641. [Google Scholar] [CrossRef]
- Mlodawska, E.; Tomaszuk-Kazberuk, A.; Lopatowska, P.; Kaminski, M.; Musial, W.J. CHA2DS2VASc score predicts unsuccessful electrical cardioversion in patients with persistent atrial fibrillation. Intern. Med. J. 2017, 47, 275–279. [Google Scholar] [CrossRef]
- Fornengo, C.; Antolini, M.; Frea, S.; Gallo, C.; Grosso Marra, W.; Morello, M.; Gaita, F. Prediction of atrial fibrillation recurrence after cardioversion in patients with left-atrial dilation. Eur. Heart J. Cardiovasc. Imaging 2014, 16, 335–341. [Google Scholar] [CrossRef]
- Saliba, W.; Gronich, N.; Barnett-Griness, O.; Rennert, G. Usefulness of CHADS2 and CHA2DS2-VASC scores in the prediction of new-onset atrial fibrillation: A population-based study. Am. J. Med. 2016, 129, 843–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranert, M.; Shchetynska-Marinova, T.; Liebe, V.; Doesch, C.; Papavassiliu, T.; Akin, I.; Borggrefe, M.; Honeck, A. Recurrence of atrial fibrillation in dependence of left atrial volume index. Vivo 2020, 34, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wałek, P.; Sielski, J.; Starzyk, K.; Gorczyca, I.; Roskal-Wałek, J.; Wożakowska-Kapłon, B. Echocardiographic assessment of left atrial morphology and function to predict maintenance of sinus rhythm after electrical cardioversion in patients with non-valvular persistent atrial fibrillation and normal function or mild dysfunction of left ventricle. Cardiol. J. 2020, 27, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, K.Z.; Yan, Q.D.; Huang, R.D.; Chen, J.H.; Chen, X.H.; Wang, W.W.; Xu, Z.; Chen, L.L.; Fan, L.; Zhang, F.L. The impact of echocardiographic parameter ratio of E/E’ on the late recurrence paroxysmal atrial fibrillation in patients accepted radiofrequency catheter ablation. Medicine 2020, 99, e19897. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Lisi, M.; Righini, F.M.; Massoni, A.; Natali, B.M.; Focardi, M.; Tacchini, D.; Geyer, A.; Curci, V.; Di Tommaso, C.; et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am. J. Cardiol. 2013, 111, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Wakami, K.; Ohte, N.; Asada, K.; Fukuta, H.; Goto, T.; Mukai, S.; Narita, H.; Kimura, G. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2009, 22, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Pellicori, P.; Zhang, J.; Lukaschuk, E.; Joseph, A.C.; Bourantas, C.V.; Loh, H.; Bragadeesh, T.; Clark, A.L.; Cleland, J.G. Left atrial function measured by cardiac magnetic resonance imaging in patients with heart failure: Clinical Associations and prognostic value. Eur. Heart J. 2015, 36, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, R.; Murata, M.; Roberts, R.; Tokuda, H.; Minakata, Y.; Suzuki, K.; Tsuruta, H.; Kimura, T.; Nishiyama, N.; Fukumoto, K.; et al. Left atrial strain is a powerful predictor of atrial fibrillation recurrence after catheter ablation: Study of a heterogeneous population with sinus rhythm or atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1008–1014. [Google Scholar] [CrossRef]
- Mirza, M.; Caracciolo, G.; Khan, U.; Mori, N.; Saha, S.K.; Srivathsan, K.; Altemose, G.; Scott, L.; Sengupta, P.; Jahangir, A. Left Atrial Reservoir function predicts atrial fibrillation recurrence after catheter ablation: A two-dimensional speckle strain study. J. Interv. Card. Electrophysiol. 2011, 31, 197–206. [Google Scholar] [CrossRef]
- Hammerstingl, C.; Schwekendiek, M.; Momcilovic, D.; Schuelers, R.; Sinning, J.; Schrickel, J.; Mittmann-Braun, E.; Nickeng, G.; Lickfett, L. Left atrial deformation imaging with ultrasound based two-dimensional speckle-tracking predicts the rate of recurrence of paroxysmal and persistent atrial fibrillation after successful ablation procedures. J. Cardiovasc. Electrophysiol. 2011, 23, 247–255. [Google Scholar] [CrossRef]
- Shaikh, A.Y.; Maan, A.; Khan, U.A.; Aurigemma, G.P.; Hill, J.C.; Kane, J.L.; Tighe, D.A.; Mick, E.; McManus, D.D. Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: A prospective study. Cardiovasc. Ultrasound 2012, 10, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Liu, F.; Ge, Z.X.; Li, J.M.; Xie, X.; Yang, J.H. Functional studies of left atrium and BNP in patients with paroxysmal atrial fibrillation and the prediction of recurrence after CPVA. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4997–5007. [Google Scholar] [CrossRef] [PubMed]
- Katbeh, A.; De Potter, T.; Geelen, P.; Di Gioia, G.; Kodeboina, M.; Balogh, Z.; Albano, M.; Vanderheyden, M.; Bartunek, J.; Barbato, E.; et al. Heart failure with preserved ejection fraction or non-cardiac dyspnea in paroxysmal atrial fibrillation: The role of left atrial strain. Int. J. Cardiol. 2021, 323, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kurt, M.; Tanboga, I.H.; Aksakal, E.; Kaya, A.; Isik, T.; Ekinci, M.; Bilen, E. Relation of left ventricular end-diastolic pressure and N-terminal pro-brain natriuretic peptide level with left atrial deformation parameters. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 524–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagueh, S.F. Non-invasive assessment of left ventricular filling pressure. Eur. J. Heart Fail. 2018, 20, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Kebed, K.Y.; Addetia, K.; Lang, R.M. Importance of the left atrium. Heart Fail. Clin. 2019, 15, 191–204. [Google Scholar] [CrossRef]
- Rosenberg, J.H.; Werner, J.H.; Plitt, G.D.; Noble, V.V.; Spring, J.T.; Stephens, B.A.; Siddique, A.; Merritt-Genore, H.L.; Moulton, M.J.; Agrawal, D.K. Immunopathogenesis and biomarkers of recurrent atrial fibrillation following ablation therapy in patients with preexisting atrial fibrillation. Expert Rev. Cardiovasc. 2019, 17, 193–207. [Google Scholar] [CrossRef]
- Michniewicz, E.; Mlodawska, E.; Lopatowska, P.; Tomaszuk-Kazberuk, A.; Malyszko, J. Patients with atrial fibrillation and coronary artery disease—Double trouble. Adv. Med. Sci. 2018, 63, 30–35. [Google Scholar] [CrossRef]
- Liu, C.L.; Shen, D.L.; Zhu, K.; Tang, J.N.; Hai, Q.M.; Zhang, J.Y. Levels of interleukin-33 and interleukin-6 in patients with acute coronary syndrome or stable angina. Clin. Investig. Med. 2013, 36, 234. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.C.; Du, Y.M.; Wu, S.L.; Chen, Q.X.; Wu, H.L.; Zhou, S.F. Activated nuclear factor-ΚB and increased tumor necrosis factor-α in atrial tissue of atrial fibrillation. Scand Cardiovasc J. 2009, 43, 292–297. [Google Scholar] [CrossRef]
- Harada, M.; Van Wagoner, D.R.; Nattel, S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015, 79, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquissi, F.C. The predictive role of inflammatory biomarkers in atrial fibrillation as seen through Neutrophil-lymphocyte ratio mirror. J. Biomark. 2016, 2016, 8160393. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Egami, Y.; Ukita, K.; Kawamura, A.; Nakamura, H.; Matsuhiro, Y.; Yasumoto, K.; Tsuda, M.; Okamoto, N.; Tanaka, A.; et al. Atrial fibrillation type modulates the clinical predictive value of neutrophil-to-lymphocyte ratio for atrial fibrillation recurrence after catheter ablation. Int. J. Cardiol. Heart Vasc. 2020, 31, 100664. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Li, X.; Hao, L.; Zhong, J. Role of tumor necrosis factor alpha in the pathogenesis of Atrial Fibrillation: A novel potential therapeutic target? Ann. Med. 2015, 47, 316–324. [Google Scholar] [CrossRef] [PubMed]
| Risk Factors for AF Recurrence | Total (n = 92) | Sinus Rhythm (n = 51) | AF Recurrence (n = 41) | p Value |
|---|---|---|---|---|
| Age, years | 63.30 ± 9.89 | 61.98 ± 9.24 | 64.95 ± 10.52 | NS |
| Male gender, n (%) | 57 (62.0%) | 34 (66.7%) | 23 (56.1%) | NS |
| Overweight (BMI ≥ 30 kg/m2), n (%) | 35 (38.0%) | 15 (29.4%) | 20 (48.8%) | NS |
| Arterial hypertension, n (%) | 71 (78.9%) | 38 (76.0%) | 33 (82.5%) | NS |
| AH duration, years | 5 (4–10) | 5 (4–9) | 7 (3–15) | 0.035 |
| Diabetes mellitus, n (%) | 13 (14.4%) | 6 (12.0%) | 7 (17.5%) | NS |
| Smoking, n (%) | 21 (22.8%) | 9 (19.1%) | 12 (31.6%) | NS |
| Dyslipidaemia, n (%) | 26 (45.6%) | 15 (44.1%) | 11 (47.8%) | NS |
| Duration of AF from the first episode, months | 13 (3–47) | 7 (2–38) | 24 (6–54) | 0.008 |
| The duration of persistent | ||||
| AF, n (%) | ||||
| 1–3 months | 41 (46.6%) | 24 (51.1%) | 17 (41.5%) | |
| 3–6 months | 23 (26.1%) | 11 (23.4%) | 12 (29.3%) | NS |
| 6–12 months | 24 (27.3%) | 12 (25.5%) | 12 (29.3%) | |
| First AF episode, n (%) | 54 (58.7%) | 35 (68.6%) | 19 (46.3%) | 0.036 |
| Beta-blockers, n (%) | 82 (93.2%) | 43 (89.6%) | 39 (97.5%) | NS |
| Class IC antiarrhythmic drugs, n (%) | 21 (22.8%) | 6 (11.8%) | 15 (36.6%) | NS |
| Class III antiarrhythmic drugs (Amiodarone), n (%) | 42 (45.7%) | 27 (52.9%) | 15 (36.6%) | NS |
| ACE inhibitors/ARB, n (%) | 66 (71.7%) | 37 (72.5%) | 29 (70.7%) | NS |
| MRA, n (%) | 15 (16.3%) | 11 (21.6%) | 4 (9.8%) | NS |
| Statins, n (%) | 18 (19.6%) | 11 (21.6%) | 7 (17.1%) | NS |
| CHA2DS2-VASc score ≥ 2, n (%) | 65 (70.7%) | 36 (70.6%) | 29 (70.7%) | NS |
| Echocardiographic Parameters | Total (n = 92) | Sinus Rhythm (n = 51) | AF Recurrence (n = 41) | p Value |
|---|---|---|---|---|
| LVEDD, mm | 50.12 ± 3.86 | 50.29 ± 3.99 | 49.91 ± 3.75 | 0.655 |
| MMI, g/m2 | 101.36 ± 22.04 | 98.50 ± 20.27 | 104.86 ± 23.78 | 0.187 |
| LV EF, % | 54.68 ± 7.92 | 54.43 ± 6.55 | 54.96 ± 9.29 | 0.767 |
| LV GLS, % | −15.60 ± 2.89 | −16.57 ± 2.59 | −14.58 ± 2.86 | 0.001 |
| LA diameter, mm | 48.20 ± 4.83 | 47.61 ± 5.54 | 48.97 ± 3.63 | 0.208 |
| LA volume index, mL/m2 | 50.27 ± 12.76 | 48.24 ± 14.41 | 52.96 ± 9.74 | 0.104 |
| LA EF, % | 27.95 ± 9.80 | 30.29 ± 11.03 | 24.85 ± 6.89 | 0.009 |
| LA strain, % | 14.07 ± 5.88 | 17.49 ± 5.51 | 10.65 ± 3.96 | <0.0001 |
| E wave, m/s | 94.69 ± 17.58 | 88.08 ± 16.17 | 103.33 ± 15.62 | <0.0001 |
| e‘ average, cm/s | 10.28 ± 1.26 | 10.55 ± 1.28 | 9.96 ± 1.17 | 0.026 |
| E/e‘ | 9.39 ± 2.09 | 8.53 ± 1.98 | 10.47 ± 1.70 | <0.0001 |
| DT, ms | 175.85 ± 20.24 | 178.10 ± 22.02 | 173.00 ± 17.65 | 0.275 |
| Diastolic dysfunction (≥3 criteria) | 25 (30.9%) | 11 (23.9%) | 14 (40.0%) | 0.121 |
| Biomarkers | Total (n = 92) | Sinus Rhythm (n = 51) | AF Recurrence (n = 41) | p Value |
|---|---|---|---|---|
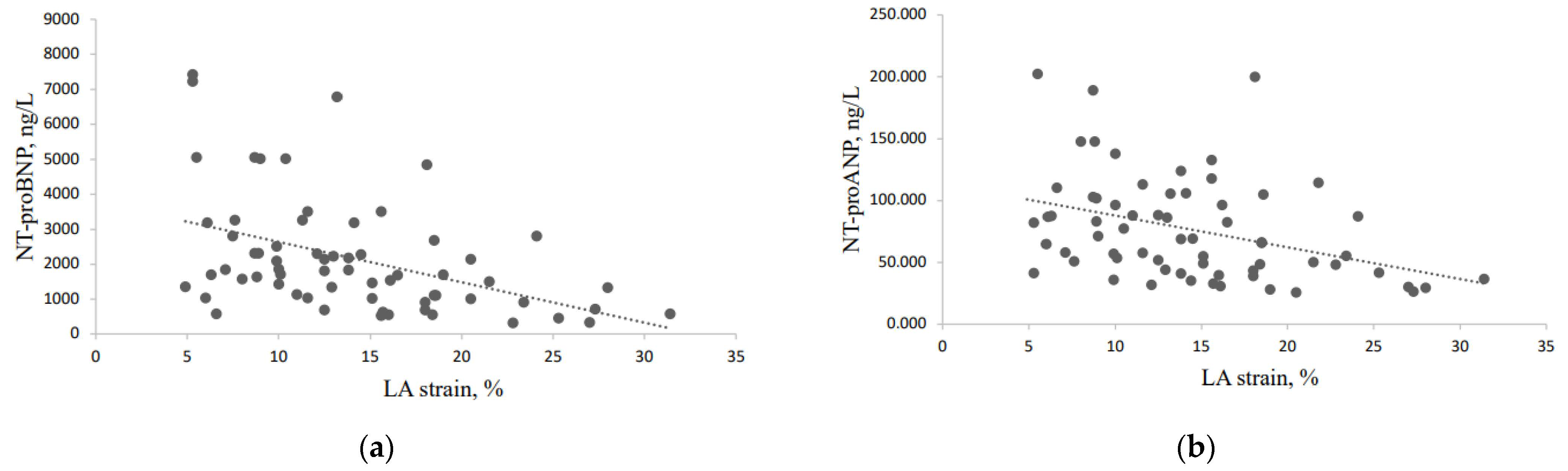
| NT-proBNP, ng/L | 2148.26 ± 1714.70 | 1940.33 ± 1675.78 | 2421.16 ± 1753.31 | 0.235 |
| NT-proANP, ng/L | 75,898.38 ± 40,536.58 | 76,042.76 ± 41,615.74 | 75,696.23 ± 39,679.52 | 0.972 |
| IL-6, pg/mL | 9.95 (1.96–152.05) | 8.94 (1.96–152.05) | 8.76 (2.57–152.05) | 0.772 |
| TNF-α, pg/mL | 4.36 ± 1.99 | 3.96 ± 1.45 | 4.67 ± 2.30 | 0.136 |
| hs-CRP, pg/mL | 1.53 (0.46–4.09) | 1.53 (0.47–5.35) | 1.53 (0.43- 3.47) | 0.487 |
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age, years | 1.0 | 0.9–1.0 | 0.153 | |||
| AF history from first episode > 12 months | 2.6 | 1.1–6.1 | 0.028 | |||
| LV GLS (%) | 1.3 | 1.1–1.6 | 0.004 | |||
| E, cm/s | 1.1 | 1.0–1.1 | <0.0001 | |||
| E/e′ ratio | 1.8 | 1.3–2.4 | <0.0001 | |||
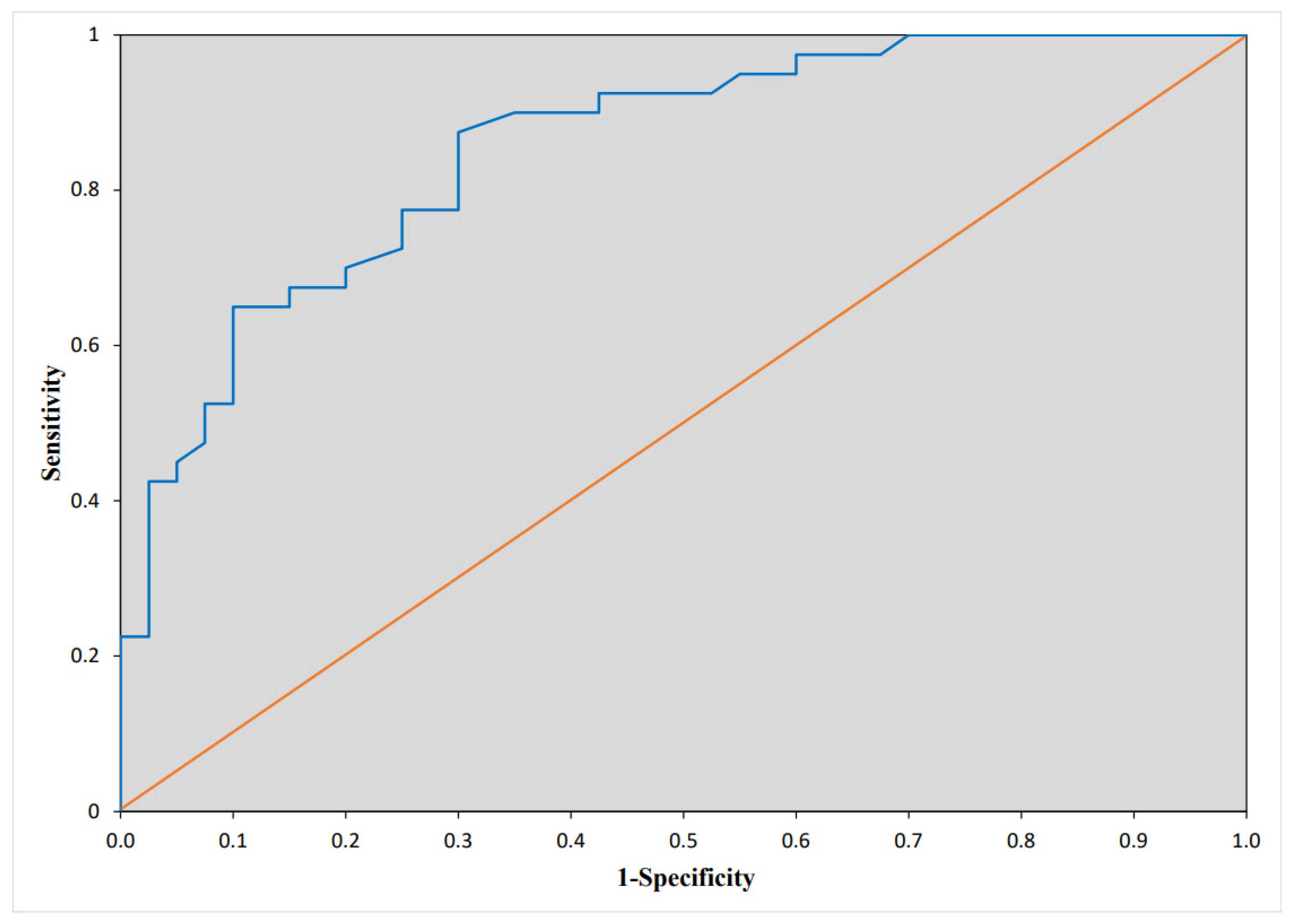
| LA strain (%) | 0.7 | 0.6–0.8 | <0.0001 | 0.65 | 0.5–0.9 | 0.004 |
| LA EF (%) | 0.9 | 0.9–1.0 | 0.018 | |||
| NT-proBNP > 1335 ng/L | 3.5 | 1.1–10.4 | 0.026 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaliūtė, R.; Leleika, A.; Apanavičiūtė, I.; Kazakevičius, T.; Mizarienė, V.; Zabiela, V.; Kavoliūnienė, A.; Ragaišytė, N.; Urbonienė, D.; Šakalytė, G. Risk Factors of Early Atrial Fibrillation Recurrence Following Electrical Cardioversion When Left Ventricular Ejection Fraction Is Preserved. Medicina 2022, 58, 1053. https://doi.org/10.3390/medicina58081053
Karaliūtė R, Leleika A, Apanavičiūtė I, Kazakevičius T, Mizarienė V, Zabiela V, Kavoliūnienė A, Ragaišytė N, Urbonienė D, Šakalytė G. Risk Factors of Early Atrial Fibrillation Recurrence Following Electrical Cardioversion When Left Ventricular Ejection Fraction Is Preserved. Medicina. 2022; 58(8):1053. https://doi.org/10.3390/medicina58081053
Chicago/Turabian StyleKaraliūtė, Rasa, Arnoldas Leleika, Ieva Apanavičiūtė, Tomas Kazakevičius, Vaida Mizarienė, Vytautas Zabiela, Aušra Kavoliūnienė, Nijolė Ragaišytė, Daiva Urbonienė, and Gintarė Šakalytė. 2022. "Risk Factors of Early Atrial Fibrillation Recurrence Following Electrical Cardioversion When Left Ventricular Ejection Fraction Is Preserved" Medicina 58, no. 8: 1053. https://doi.org/10.3390/medicina58081053
APA StyleKaraliūtė, R., Leleika, A., Apanavičiūtė, I., Kazakevičius, T., Mizarienė, V., Zabiela, V., Kavoliūnienė, A., Ragaišytė, N., Urbonienė, D., & Šakalytė, G. (2022). Risk Factors of Early Atrial Fibrillation Recurrence Following Electrical Cardioversion When Left Ventricular Ejection Fraction Is Preserved. Medicina, 58(8), 1053. https://doi.org/10.3390/medicina58081053






