Abstract
Background and Objectives: For the treatment of knee osteoarthritis (OA), intra-articular platelet-rich plasma (PRP) and novel crosslinked single-dose hyaluronic acid (HA) have both been reported to improve outcomes, but no study has compared them for the treatment of knee OA. We hypothesized patients with early-stage knee OA who received PRP injections would have more WOMAC score changes than those who received HA injections. This is the first prospective, double-blind, parallel, randomized controlled trial comparing the efficacy of intra-articular single-dose PRP versus novel crosslinked HA (HyajointPlus) for treating early-stage knee OA. Materials and Methods: This study analyzed 110 patients randomized into the PRP (n = 54) or HA (n = 56) groups. The primary outcome is the change of WOMAC score at 1-, 3-, and 6-month follow-ups compared to baseline. Results: The data revealed significant improvements in all WOMAC scores in the PRP group at 1-, 3-, and 6-month follow-up visits compared with the baseline level except for the WOMAC stiffness score at the 1-month follow up. In the HA group, significant improvements were observed only in the WOMAC pain score for all the follow-up visits and in WOMAC stiffness, function, and total scores at 6-month follow-up. When comparing the change of WOMAC score at 1-, 3-, and 6-month follow-ups, no significant differences were found between PRP and HA group. Conclusions: This study revealed that both PRP and HA can yield significant improvements in WOMAC scores at 6-month follow-up without any between-group differences at 1-, 3-, and 6-month follow-ups. Thus, both the single-injection regimens of PRP and HA can improve the functional outcomes for treating early-stage knee OA.
1. Introduction
Knee osteoarthritis (OA) is a common degenerative joint disease characterized by loss of articular cartilage, subchondral bone changes, and inflammation of synovial tissue [1,2,3]. The major symptoms of knee OA are pain, loss of function, and swelling [4]. It mostly affects older adults (age ≥ 65 years), and it affects women (incidence: 18%) more than men (incidence: 9.6%), according to a 2004 study involving patients ≥60 years of age [5]. An estimated 12.4 million (33.6%) older adults in the US have knee OA [4]. Risk factors related to knee OA include obesity, older age, previous knee trauma, hand OA, and female sex [6]. Surgical interventions including arthroscopic surgery, osteotomy, and arthroplasty are considered the next steps for treating knee OA, after other nonsurgical treatments have been attempted, such as physical therapy [7], oral nonsteroidal anti-inflammatory drugs (NSAIDs) [8], and intra-articular injections (of platelet-rich plasma [PRP], hyaluronic acid [HA], and corticosteroids) [8,9,10].
Platelet-rich plasma (PRP) is autologous conditioned plasma containing a high concentration of platelets [11]. Platelets are a natural source of growth factors, such as insulin-like growth factor, platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor beta, and platelet-derived angiogenic factor. Growth factors play a vital role in regenerative processes and wound healing [12]. The use of PRP in the treatment of knee OA has generated considerable attention [13].
Hyaluronic acid (HA) widely exists in human tissues, such as vitreous humor, heart valves, the umbilical cord, synovial fluid, skin, and skeletal tissues. Within the synovial fluid, HA provides joint lubrication, thus preventing the mechanical degradation of cartilage. In individuals with knee OA, HA synthesis and degradation are abnormal, resulting in reduced concentration and molecular weight of HA at the joint. These pathological changes reduce synovial fluid viscoelasticity, leading to cartilage damage; thus, intra-articular HA injection is a common therapy to treat knee OA. Some studies have mentioned better efficacy with a high molecular weight HA; however, some studies show no differences between high and low molecular weight HA. The optimal molecular weights, concentrations, and volumes of HA have not been established [14].
Several clinical trials and reviews have concluded that PRP injections have good clinical efficacy in the treatment of knee OA [15,16,17,18,19,20,21]. In addition, several trials and meta-analyses have reported that PRP injections are more effective than HA injections in terms of pain and physical function [22,23,24,25,26]. However, heterogeneity among studies was detected in their subgroup analyses, indicating large differences in clinical outcomes. Although the trials included in the meta-analysis were randomized controlled trials (RCTs), most of them (10 of 15) had a relatively small sample size (n < 50 for each group). Therefore, more high-level quality trials with a larger sample size are required to investigate the efficacy of PRP versus HA injections in the treatment of knee OA.
Conventional HA treatment for knee OA requires 3–5 intra-articular injections. However, newer HA products have longer-lasting activity, necessitating only one injection. An example of a new HA product is chemically crosslinked HA (e.g., HYAJOINT Plus), which results in increased viscoelasticity. It can significantly improve pain and functional results in knee OA [27,28,29].
Considering the aforementioned context, this study compared the efficacy of intra-articular single PRP versus novel crosslinked HA for the treatment of early-stage knee OA. We hypothesized patients with early-stage knee OA who received PRP injections would have more WOMAC score changes than those who received HA injections. This study provided robust evidence with a large sample size to assess the efficacy of PRP versus HA in treating knee OA. To our knowledge, ours is the first prospective, double-blind, randomized controlled trial comparing the efficacy of intra-articular single PRP versus novel crosslinked HA (HyajointPlus) for the treatment of early-stage knee OA.
2. Materials and Methods
2.1. Patient Selection
This prospective, double-blind RCT recruited patients visiting the outpatient orthopedic clinic of Kaohsiung Medical University Hospital from November 2017 to April 2019 if they met the following criteria: (1) age > 50 years; (2) a diagnosis of primary knee osteoarthritis; and (3) Kellgren–Lawrence (K–L) grade < 3. Patients with any one of the following criteria were excluded: (1) age < 50 years; (2) K–L grade ≥ 3; (3) history or active presence of clinically significant inflammatory articular or rheumatic disease other than OA; (4) rapidly progressive OA before the start of the trial; (5) history of lower extremity surgery; (6) excessive mechanical axis deviation (varus > 5°, valgus > 5°); (7) body mass index > 30; (8) history or presence of malignant disorders; (9) systemic disorders, such as diabetes mellitus, severe cardiovascular diseases, hematologic diseases, immune-deficiencies, and infections; (10) systematic or intra-articular corticosteroid therapy in the previous 3 months; (11) prior treatment with HA in the past 6 months; (12) anticoagulants or antiaggregant therapy in the preceding 30 days; (13) nonsteroidal anti-inflammatory medications in the preceding 7 days; (14) platelet count < 150,000/mL; or (15) hemoglobin < 12 g/dL. Glucosamine, chondroitin, analgesics, or physical therapy were not permitted during the study period. Acetaminophen (500 mg; maximum daily dose, 4 g) can be used as a rescue medication [27].
All patients provided informed consent before participation in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Kaohsiung Medical University Hospital, Kaohsiung, Taiwan (IRB NO: KMUHIRB-F(II)-20170062). The study was registered at ClinicalTrials.gov (NCT04972383).
2.2. Randomization and Masking
This is a prospective, double-blind, parallel randomized controlled trial. The included patients were randomly allocated to the PRP or HA group at a 1:1 ratio by using permuted block randomization with block sizes of two, four, and six. Randomization was concealed through the use of opaque and sealed envelopes until the treatment was assigned. Both patients and physicians were blinded to the treatment allocation.
2.3. PRP and HA Preparation
A single unit of 10 mL of peripheral venous blood was harvested from all participants before injection.
In the PRP group, an Aeon Acti-PRP (Aeon Biotherapeutics Corporation, 7F., No.2, Ln.258, Ruiguang Rd., Neihu Dist., Taipei City, Taiwan) set was used, whole blood was centrifuged at 3200 rpm for 6 min, and an approximate volume of 4 mL PRP was obtained for use in a single-dose treatment using a leukocyte-poor technique.
In the HA group, HYAJOINT Plus (3 mL, SciVision Biotech, No.9, S.6th Rd., Qianzhen Dist., Kaohsiung City, Taiwan) containing 60 mg of purified sodium hyaluronate was used for single-dose treatment. HYAJOINT Plus is a novel HA product comprising chemically crosslinked HA, resulting in increased viscoelasticity and durable activity and requiring only one injection. HYAJOINT Plus is produced by microbial fermentation and synthesized by a novel cross-linking process by 1,4-butanediol diglycidyl ether (BDDE) to introduce an antidegradation feature. The carefully controlled cross-linking creates a viscous gel with an increased density of HA (2% of HA, 20 mg/mL) [27].
Both PRP and HA are injected from the anterolateral portal of the knee, which is 1 cm above the joint line and just lateral to the patellar tendon, with 5cc syringe and 21G needle. All PRP and HA injection syringes were covered with opaque envelopes, and the intra-articular injection procedure was performed by the same physician without the use of guidance from ultrasound or other imaging techniques.
2.4. Outcome Assessment
To screen the patients, blood analysis was applied to each patient 1 week before the treatment. The main characteristics were also recorded at baseline, including age, sex, BMI, and K–L grading. The Western Ontario and McMaster Universities Index (WOMAC) score was used to assess the patients’ clinical outcomes at baseline and 1-, 3-, and 6-month follow-ups [30]. The WOMAC is a self-reported measure and contains three subscales, including the pain (5 items), stiffness (2 items), and physical function (17 items) subscales. The primary outcome is the change of WOMAC score at 1-, 3-, and 6-month follow-ups compared to baseline.
2.5. Statistical Analysis
The minimum requisite sample size was calculated using G*Power software (v.3.0.10, a free to use software from Heinrich-Heine-University Düsseldorf, Germany). The statistical tests included a between-group F-test and repeated-measures tests. The minimum sample size was calculated to be 31 participants per group (α = 0.05; power = 0.8; number of groups = 2; number of measurements = 4; because no preliminary data were available, we used moderate values of 0.25 for Cohen’s effect size f and 0.3 for Cohen’s effect size r of correlation among repeated measures). Assuming a 20% dropout rate, we estimated the minimum number of participants to be 38 per group. Categorical variables were expressed in terms of frequency or percentage, and their difference between the two groups at baseline were compared using an χ2 test. Continuous variables of age and BMI were expressed in terms of the mean ± standard deviation, and the differences between the two groups were compared using a two-sample t test. Continuous variables of WOMAC scores were expressed in terms of the mean ± standard error of the mean, and the differences between the two groups at baseline and follow-up visits were compared using a two-sample t test. The changes between baseline and follow-up visits and the changes between the two groups were assessed using generalized estimating equations. Statistical analyses were performed using IBM SPSS software (v.19; IBM, Armonk, NY, USA). Statistical significance was indicated at p < 0.05.
3. Results
The 116 included patients were randomly assigned to PRP and HA groups (n = 58 each). Among these patients, one patient did not receive the allocated treatment (the PRP group). Four patients dropped out of the trial (three from the PRP group and one from the HA group), and one was lost to follow-up (the HA group). Eventually, the data of 110 patients (54 in the PRP group and 56 in the HA group) were analyzed (Figure 1).
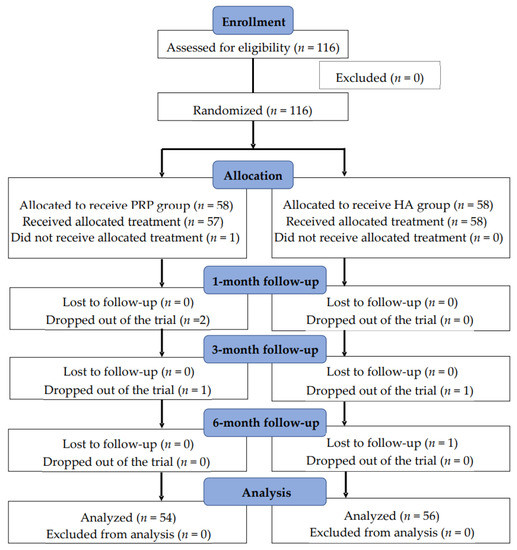
Figure 1.
Flow chart of this study.
The mean ages in the PRP and HA groups were 61.87 ± 5.46 and 63.00 ± 5.33 years, respectively. Both groups had more women than men (77.8% and 71.4% women in the PRP and HA groups, respectively). Both groups did not differ with respect to age, sex, BMI, K–L grade, treatment side, and WOMAC scores at baseline (Table 1).

Table 1.
Main characteristics of the included patients in the two treatment groups.
3.1. PRP Group
The mean WOMAC total score was 18.74 ± 1.85 at baseline and 14.96 ± 1.60, 13.43 ± 1.42, and 14.39 ± 1.56 at 1-, 3-, and 6-month follow-ups, respectively (Table 2). Relative to the baseline, the WOMAC total score significantly improved at the 1-month (−3.78 ± 1.20, p = 0.001), 3-month (−5.31 ± 1.48, p < 0.001), and 6-month follow-ups (−4.35 ± 1.61, p = 0.006). The WOMAC total score improved continually from baseline at 1-month and 3-month follow-ups but not at 6-month follow-up (Table 3 and Figure 2).

Table 2.
Mean WOMAC scores at different follow-up visits in the two treatment groups.

Table 3.
Changes in WOMAC scores from baseline to follow-up visits in the two treatment groups.
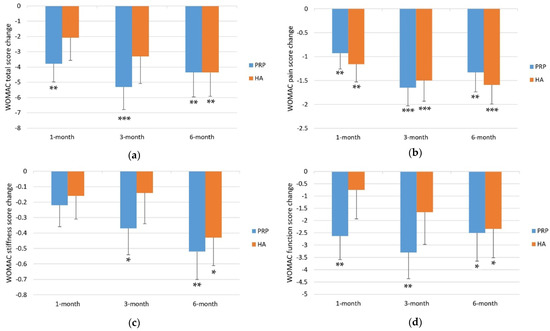
Figure 2.
Mean changes in WOMAC scores from baseline to follow-up visits. (a) WOMAC total score change; (b) WOMAC pain score change; (c) WOMAC stiffness score change; (d) WOMAC function score change. Error bars indicate one standard error of the mean. Asterisks indicate p-values for the difference from baseline to follow up visits. * p-value < 0.05. ** p-value < 0.01. *** p-value < 0.001.
The mean WOMAC pain score was 4.20 ± 0.42 at baseline and 3.28 ± 0.37, 2.56 ± 0.32, and 2.87 ± 0.35 at 1-, 3-, and 6-month follow-ups, respectively (Table 2). Compared with the baseline, the WOMAC pain score significantly improved at the 1-month (−0.93 ± 0.33, p = 0.005), 3-month (−1.65 ± 0.38, p < 0.001), and 6-month (−1.33 ± 0.41, p = 0.001) follow-ups. The WOMAC pain score improved continually from baseline at 1-month and 3-month follow-ups but not at 6-month follow-up (Table 3 and Figure 2).
The mean WOMAC stiffness score was 1.89 ± 0.19 at baseline and 1.67 ± 0.19, 1.52 ± 0.16, and 1.37 ± 0.17 at the 1-, 3-, and 6-month follow-ups, respectively (Table 2). Compared with the baseline, the WOMAC stiffness score did not significantly improve at the 1-month follow-up (−0.22 ± 0.14, p = 0.107) but significantly improved at the 3-month follow-up (−0.37 ± 0.17, p = 0.026) and 6-month follow-ups (−0.52 ± 0.18, p = 0.004) (Table 3 and Figure 2).
The mean WOMAC function score was 12.65 ± 1.38 at baseline and 10.02 ± 1.15, 9.35 ± 1.03, and 10.15 ± 1.12 at the 1-, 3-, and 6-month follow-ups, respectively (Table 2). Compared with the baseline, the WOMAC function score significantly improved at the 1-month (−2.63 ± 0.96, p = 0.006), 3-month (−3.30 ± 1.07, p = 0.002), and 6-month (−2.50 ± 1.15, p = 0.029) follow-ups. The WOMAC function score improved continually from baseline at 1-month and 3-month follow-ups but not at 6-month follow-up (Table 3 and Figure 2).
3.2. HA Group
The mean WOMAC total score was 18.23 ± 1.80 at baseline and 16.16 ± 2.04, 14.93 ± 2.17, and 13.88 ± 1.91 at the 1-, 3-, and 6-month follow-ups, respectively (Table 2). Compared with the baseline, the WOMAC total score did not significantly improve at the 1- (−2.07 ± 1.50, p = 0.163) and 3-month follow-ups (−3.30 ± 1.78, p = 0.061) but significantly improved at the 6-month follow-up (−4.36 ± 1.56, p = 0.005) (Table 3 and Figure 2).
The mean WOMAC pain score was 4.38 ± 0.43 at baseline and 3.21 ± 0.39, 2.88 ± 0.41, and 2.79 ± 0.38, respectively, at the 1-, 3-, and 6-month follow-ups, respectively (Table 2). Compared with the baseline, the WOMAC pain score significantly improved at the 1-month (−1.16 ± 0.37, p = 0.002), 3-month (−1.50 ± 0.43, p < 0.001), and 6-month (−1.59 ± 0.40, p < 0.001) follow-ups (Table 3 and Figure 2).
The mean the WOMAC stiffness score was 1.73 ± 0.21 at baseline and 1.57 ± 0.19, 1.59 ± 0.21, and 1.30 ± 0.16 at the 1-, 3-, and 6-month follow-ups, respectively (Table 2). Compared with the baseline, the WOMAC stiffness score did not significantly improve at the 1-month (−0.16 ± 0.15, p = 0.281) and 3-month (−0.14 ± 0.20, p = 0.467) follow-ups but significantly improved at the 6-month (−0.43 ± 0.18, p = 0.013) follow-up (Table 3 and Figure 2).
The mean WOMAC function score was 12.13 ± 1.27 at baseline and 11.38 ± 1.54, 10.46 ± 1.59, and 9.79 ± 1.44 at the 1-, 3-, and 6-month follow-ups, respectively (Table 2). Compared with the baseline, the WOMAC function score did not significantly improve at the 1-month (−0.75 ± 1.18, p = 0.523) and 3-month (−1.66 ± 1.31, p = 0.200) follow-ups but significantly improved at the 6-month (−2.34 ± 1.17, p = 0.044) follow-up (Table 3 and Figure 2).
3.3. PRP vs. HA
When the improvements in WOMAC scores from baseline to 1-month follow-up between groups were compared, no significant differences in WOMAC total (p = 0.369), pain (p = 0.634), stiffness (p = 0.762), and function (p = 0.214) scores were found. However, the improvements in WOMAC function and total score in the PRP group were better than those in the HA group (Table 3 and Figure 2).
When the improvements in WOMAC scores from baseline to 3-month follow-up between the groups were compared, no significant differences in WOMAC total (p = 0.380), pain (p = 0.793), stiffness (p = 0.376), and function (p = 0.329) scores were observed. However, the improvements in WOMAC stiffness, function, and total score in the PRP group were better than those in the HA group (Table 3 and Figure 2).
When the improvements in WOMAC scores from baseline to 6-month follow-up between groups were compared, no significant differences were observed in WOMAC total (p = 0.998), pain (p = 0.651), stiffness (p = 0.719), or function (p = 0.921) scores, and the improvements in all of these scores in the PRP group were similar to those in the HA group (Table 3 and Figure 2).
4. Discussion
This is the first prospective, double-blind, randomized controlled trial comparing the efficacy of intra-articular single PRP versus novel crosslinked HA (HyajointPlus) for the treatment of early-stage knee OA with robust evidence and a large sample size. The results revealed that the PRP group had significant improvements in WOMAC pain, stiffness, function, and total scores at all follow-up visits, except for the WOMAC stiffness score at 1-month follow-up relative to baseline. The HA group exhibited significant improvement in the WOMAC pain score at all follow-up visits and had a significant improvement in WOMAC stiffness, function, and total scores at the 6-month follow-up relative to baseline. For the PRP group, the improvements in WOMAC pain, function, and total scores improved from baseline to the 1- and 3-month follow-up but did not continually improve at the 6-month follow-up. The WOMAC stiffness continually improved from baseline to 1-, 3-, and 6-month follow-ups. For the HA group, the improvements in WOMAC pain, stiffness, function, and total scores were continually increased from baseline to 1-, 3-, and 6-month follow-ups. However, no significant between-group differences were observed in WOMAC pain, stiffness, function, and total scores at any follow-up visit. Thus, both PRP and HA significantly improved all WOMAC scores at the 6-month follow-up with comparable efficacy.
The severity of knee OA may affect the efficacy of intra-articular HA. A meta-analysis reported that intra-articular HA therapy was associated with significant improvement in pain relief among patients with early–moderate knee OA (K–L grades 1–3) but not patients with end-stage knee OA (K–L grade 4) [31]. This indicates that patients with end-stage knee OA do not respond to HA therapy. Accordingly, we only included patients with early-stage knee OA (K–L grade 1–2).
Patients with knee OA are primarily concerned with pain [32]. Improvement in physical activity has been associated with pain relief [33]. We observed that WOMAC pain scores significantly improved at all follow-up visits in both the PRP and HA groups. The accepted threshold for a minimum clinically important difference (MCID) in the WOMAC score for OA of the lower limbs is a 12% improvement from baseline [34]. In our study, improvements in the WOMAC total score from baseline to the 6-month follow-up were 23.21% and 23.92% in the PRP and HA groups, respectively, both being greater than the MCID. Thus, our results indicated good pain and functional results in both the PRP and the HA groups.
The PRP preparation method may affect the efficacy of intra-articular PRP therapy for knee OA. Several RCTs preparing PRP using a leukocyte-rich technique reported no significant difference in self-reported pain and symptoms between PRP and HA groups [17,35,36]. By contrast, several meta-analyses have reported that PRP prepared using a leukocyte-poor technique was associated with significantly better improvement in self-reported symptoms relative to a HA group, indicating that it is a better choice for treating knee OA (although more evidence is required) [24,37]. PRP injections yield favorable outcomes for knee OA, but the optimal injection number and frequency remain unclear. A meta-analysis reported that multiple injections of PRP for treating knee OA were more effective in improving joint functionality relative to a single injection at 6 months [38]. A study using three injections for treating knee OA reported that the improvement in the WOMAC pain score in the PRP group was significantly better than that in the HA group [39]. However, one injection of PRP is also safe and effective for treating knee OA [40,41,42]. In this study, we adopted single-injection therapy using a leukocyte-poor technique, and we observed that the improvements in WOMAC pain, stiffness, function, and total scores in the PRP group were significant at all follow-up visits. However, the optimal injection regimen remains undetermined and thus a topic for further investigation.
Intra-articular HA injection has long been used to treat knee OA, and most HA preparations require multiple injections. However, differences in HA formulations, origins, concentrations, and dosing regimens mean that the present body of clinical evidence is inconclusive. In our study, we used the novel crosslinked HA (HYAJOINT Plus) as a single-injection therapy. To date, only a few studies have reported the efficacy of HYAJOINT Plus for treating knee OA. Sun et al. indicated that a single injection of HYAJOINT Plus is safe and effective for 6 months in patients with K–L 2 or 3 knee OA [27]. Tuan et al. also concluded that a single injection of HYAJOINT Plus is effective with respect to both subjective and objective indicators for 6 months in patients with K–L 2 or 3 knee OA [28]. A recent study by Sun et al. demonstrated that combined injections of HYAJOINT Plus and PRP are safe and effective for 6 months in patients with K–L 2 knee OA [29]. In this study, we observed that the improvements in WOMAC pain, stiffness, function, and total scores were significant at the 6-month follow-up. The single-injection regimen could reduce the time spent on and the discomfort associated with the injection process, thus offering potential benefits to patients with knee OA.
Four patients in the HA group developed mild joint swelling, with pain after injection, but it resolved spontaneously within 1–3 days. No infection, allergy, or other severe adverse events were noted during the study.
This study has some limitations. First, the efficacy of PRP versus HA was unclear after 6 months due to our relatively short follow-up period of up to 6 months. A study revealed that PRP and HA treatments yielded significant improvements at a 24-month follow-up [18]. Second, we did not use real-time image guidance during the injection procedure to ensure that the location of the injection site was accurate; this may affect the efficacy of PRP or HA treatment and possibly induce bias in our comparison between the two groups. Third, pain, stiffness, and functional outcomes were assessed using self-reported questionnaires, and our results lacked objective data, such as those from magnetic resonance imaging. Fourth, we did not include a placebo group. Although a previous meta-analysis revealed a 40–50% reduction in pain with the use of HA compared with a placebo [43], the placebo effect cannot be identified. Fifth, the standard deviation(SD) value of the WOMAC score is relatively large, which shows the disadvantage that the discreteness of the WOMAC score value is not concentrated in our study. More accurate questionnaires or data collection methods may be needed to improve this shortcoming.
5. Conclusions
This double-blind RCT provided robust evidence on the efficacy of intra-articular PRP versus HA in the treatment of early-stage knee OA. The results revealed that both PRP and HA can yield significant improvements in WOMAC scores at 6-month follow-up and WOMAC pain scores at 1-, 3-, and 6-month follow-ups without any between-group differences. However, PRP tended to yield greater improvement in WOMAC stiffness, function, and total scores relative to the HA group at 3-month follow-up.
Author Contributions
Conceptualization, Y.-C.W., C.-L.L., Y.-J.C., Y.-C.T., S.-Y.L. and C.-H.C.; data curation, Y.-C.W., C.-L.L. and Y.-J.C.; Formal analysis, Y.-J.C. and Y.-C.T.; Funding acquisition, Y.-C.T. and H.-T.H.; Investigation, Y.-C.T. and C.-H.C.; Methodology, C.-L.L., Y.-C.T. and S.-Y.L.; Project administration, P.P.-H.C. and H.-T.H.; Resources, Y.-C.W., C.-L.L. and Y.-J.C.; Software, Y.-C.W.; Supervision, Y.-C.T., P.P.-H.C. and H.-T.H.; Validation, Y.-C.W., C.-L.L., Y.-J.C. and Y.-C.T.; Writing—original draft, Y.-C.W. and C.-L.L.; Writing—review & editing, Y.-C.T. and H.-T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Health and Welfare of Taiwan (MOHW106-TDU-B-212-113006), the Ministry of Science and Technology of Taiwan (MOST 106-2314-B-037-089-), Kaohsiung Municipal Ta-Tung Hospital (KMTTH-108-005), and Kaohsiung Municipal Hsiao-Kang Hospital (KMHK-107-012).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and it was approved by the Institutional Review Board of Kaohsiung Medical University Hospital, Kaohsiung, Taiwan (IRB NO: KMUHIRB-F(II)-20170062, date of approval: 23 May 2017). The study was registered at ClinicalTrials.gov (NCT04972383).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because of confidentiality issues.
Acknowledgments
We would like to thank Yi-Chun Hung and Chia-Lung Shih for statistical contributions to this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Malemud, C.J. Biologic basis of osteoarthritis: State of the evidence. Curr. Opin. Rheumatol. 2015, 27, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef]
- Jüni, P.; Hari, R.; Rutjes, A.W.; Fischer, R.; Silletta, M.G.; Reichenbach, S.; da Costa, B.R. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 2015, Cd005328. [Google Scholar] [CrossRef] [Green Version]
- Blagojevic, M.; Jinks, C.; Jeffery, A.; Jordan, K.P. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2010, 18, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Aziem, A.A.; Soliman, E.S.; Mosaad, D.M.; Draz, A.H. Effect of a physiotherapy rehabilitation program on knee osteoarthritis in patients with different pain intensities. J. Phys. Ther. Sci. 2018, 30, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.R.; Deshpande, B.R.; Collins, J.E.; Katz, J.N.; Losina, E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: Systematic analytic review. Osteoarthr. Cartil. 2016, 24, 962–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southworth, T.M.; Naveen, N.B.; Tauro, T.M.; Leong, N.L.; Cole, B.J. The Use of Platelet-Rich Plasma in Symptomatic Knee Osteoarthritis. J. Knee Surg. 2019, 32, 37–45. [Google Scholar] [CrossRef]
- Zeng, C.; Lane, N.E.; Hunter, D.J.; Wei, J.; Choi, H.K.; McAlindon, T.E.; Li, H.; Lu, N.; Lei, G.; Zhang, Y. Intra-articular corticosteroids and the risk of knee osteoarthritis progression: Results from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2019, 27, 855–862. [Google Scholar] [CrossRef]
- Mishra, A.; Harmon, K.; Woodall, J.; Vieira, A. Sports medicine applications of platelet rich plasma. Curr. Pharm. Biotechnol. 2012, 13, 1185–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, N.T.; Schultz, G.S. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am. J. Surg. 1993, 165, 728–737. [Google Scholar] [CrossRef]
- Cugat, R.; Cuscó, X.; Seijas, R.; Álvarez, P.; Steinbacher, G.; Ares, O.; Wang-Saegusa, A.; García-Balletbó, M. Biologic enhancement of cartilage repair: The role of platelet-rich plasma and other commercially available growth factors. Arthroscopy 2015, 31, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Hackel, J.; Niazi, F.; Shaw, P.; Nicholls, M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: A systematic review. Semin. Arthritis Rheum. 2018, 48, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rayegani, S.M.; Raeissadat, S.A.; Taheri, M.S.; Babaee, M.; Bahrami, M.H.; Eliaspour, D.; Ghorbani, E. Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop. Rev. 2014, 6, 5405. [Google Scholar] [CrossRef] [Green Version]
- Vaquerizo, V.; Plasencia, M.; Arribas, I.; Seijas, R.; Padilla, S.; Orive, G.; Anitua, E. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: A randomized controlled trial. Arthroscopy 2013, 29, 1635–1643. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Di Martino, A.; Di Matteo, B.; Merli, M.L.; Cenacchi, A.; Fornasari, P.M.; Marcacci, M. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: Study design and preliminary results of a randomized controlled trial. BMC Musculoskelet. Disord. 2012, 13, 229. [Google Scholar] [CrossRef] [Green Version]
- Di Martino, A.; Di Matteo, B.; Papio, T.; Tentoni, F.; Selleri, F.; Cenacchi, A.; Kon, E.; Filardo, G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am. J. Sports Med. 2019, 47, 347–354. [Google Scholar] [CrossRef]
- Sánchez, M.; Anitua, E.; Azofra, J.; Aguirre, J.J.; Andia, I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: A retrospective cohort study. Clin. Exp. Rheumatol. 2008, 26, 910–913. [Google Scholar]
- Sánchez, M.; Jorquera, C.; Sánchez, P.; Beitia, M.; García-Cano, B.; Guadilla, J.; Delgado, D. Platelet-rich plasma injections delay the need for knee arthroplasty: A retrospective study and survival analysis. Int. Orthop. 2021, 45, 401–410. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Aguirre, J.J.; Prado, R.; Padilla, S.; Orive, G. Efficacy and safety of plasma rich in growth factors intra-articular infiltrations in the treatment of knee osteoarthritis. Arthroscopy 2014, 30, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, H.; Pan, J.; Lin, J.; Zeng, L.; Liang, G.; Yang, W.; Liu, J. Meta-analysis Comparing Platelet-Rich Plasma vs Hyaluronic Acid Injection in Patients with Knee Osteoarthritis. Pain Med. 2019, 20, 1418–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wang, C.; You, D.; Zhao, S.; Zhu, Z.; Xu, M. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. Medicine 2020, 99, e19388. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2021, 49, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Chen, H.; Zhao, L.; Huang, W. Platelet-Rich Plasma Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-analysis of 26 Randomized Controlled Trials. Arthroscopy 2021, 37, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Fiz, N.; Azofra, J.; Usabiaga, J.; Aduriz Recalde, E.; Garcia Gutierrez, A.; Albillos, J.; Gárate, R.; Aguirre, J.J.; Padilla, S.; et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy 2012, 28, 1070–1078. [Google Scholar] [CrossRef]
- Sun, S.F.; Hsu, C.W.; Lin, H.S.; Liou, I.H.; Chen, Y.H.; Hung, C.L. Comparison of Single Intra-Articular Injection of Novel Hyaluronan (HYA-JOINT Plus) with Synvisc-One for Knee Osteoarthritis: A Randomized, Controlled, Double-Blind Trial of Efficacy and Safety. J. Bone Jt. Surg. Am. 2017, 99, 462–471. [Google Scholar] [CrossRef]
- Tuan, S.; Liou, I.; Su, H.; Tsai, Y.; Chen, G.; Sun, S. Improvement of self-reported functional scores and thickening of quadriceps and femoral intercondylar cartilage under ultrasonography after single intra-articular injection of a novel cross-linked hyaluronic acid in the treatment of knee osteoarthritis. J. Back Musculoskelet. Rehabil. 2018, 31, 709–718. [Google Scholar] [CrossRef]
- Sun, S.F.; Lin, G.C.; Hsu, C.W.; Lin, H.S.; Liou, I.S.; Wu, S.Y. Comparing efficacy of intraarticular single crosslinked Hyaluronan (HYAJOINT Plus) and platelet-rich plasma (PRP) versus PRP alone for treating knee osteoarthritis. Sci. Rep. 2021, 11, 140. [Google Scholar] [CrossRef]
- Thumboo, J.; Chew, L.H.; Soh, C.H. Validation of the Western Ontario and Mcmaster University osteoarthritis index in Asians with osteoarthritis in Singapore. Osteoarthr. Cartil. 2001, 9, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, M.; Shaw, P.; Niazi, F.; Bhandari, M.; Bedi, A. The Impact of Excluding Patients with End-Stage Knee Disease in Intra-Articular Hyaluronic Acid Trials: A Systematic Review and Meta-Analysis. Adv. Ther. 2019, 36, 147–161. [Google Scholar] [CrossRef] [Green Version]
- Hawker, G.A. The Challenge of Pain for Patients with OA. HSS J 2012, 8, 42–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettinger, W.H., Jr.; Burns, R.; Messier, S.P.; Applegate, W.; Rejeski, W.J.; Morgan, T.; Shumaker, S.; Berry, M.J.; O’Toole, M.; Monu, J.; et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA 1997, 277, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Angst, F.; Aeschlimann, A.; Stucki, G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheumatol. 2001, 45, 384–391. [Google Scholar] [CrossRef]
- Filardo, G.; Di Matteo, B.; Di Martino, A.; Merli, M.L.; Cenacchi, A.; Fornasari, P.; Marcacci, M.; Kon, E. Platelet-Rich Plasma Intra-articular Knee Injections Show No Superiority Versus Viscosupplementation: A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 1575–1582. [Google Scholar] [CrossRef]
- Paterson, K.L.; Nicholls, M.; Bennell, K.L.; Bates, D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: A double-blind, randomized controlled pilot study. BMC Musculoskelet. Disord. 2016, 17, 67. [Google Scholar] [CrossRef] [Green Version]
- Riboh, J.C.; Saltzman, B.M.; Yanke, A.B.; Fortier, L.; Cole, B.J. Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2016, 44, 792–800. [Google Scholar] [CrossRef]
- Vilchez-Cavazos, F.; Millán-Alanís, J.M.; Blázquez-Saldaña, J.; Álvarez-Villalobos, N.; Peña-Martínez, V.M.; Acosta-Olivo, C.A.; Simental-Mendía, M. Comparison of the Clinical Effectiveness of Single Versus Multiple Injections of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2019, 7, 2325967119887116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaková, T.; Rosocha, J.; Lacko, M.; Harvanová, D.; Gharaibeh, A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am. J. Phys. Med. Rehabil. 2012, 91, 411–417. [Google Scholar] [CrossRef]
- Patel, S.; Dhillon, M.S.; Aggarwal, S.; Marwaha, N.; Jain, A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: A prospective, double-blind, randomized trial. Am. J. Sports Med. 2013, 41, 356–364. [Google Scholar] [CrossRef]
- Martini, L.I.; Via, A.G.; Fossati, C.; Randelli, F.; Randelli, P.; Cucchi, D. Single Platelet-Rich Plasma Injection for Early Stage of Osteoarthritis of the Knee. Joints 2017, 5, 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buendía-López, D.; Medina-Quirós, M.; Fernández-Villacañas Marín, M. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: A randomized controlled trial. J. Orthop. Traumatol. 2018, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Colen, S.; van den Bekerom, M.P.; Mulier, M.; Haverkamp, D. Hyaluronic acid in the treatment of knee osteoarthritis: A systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs 2012, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).