Anti-Adherence and Antimicrobial Activities of Silver Nanoparticles against Serotypes C and K of Streptococcus mutans on Orthodontic Appliances
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of AgNP
2.2. Identification of S. mutans Serotypes by Polymerase Chain Reaction (PCR)
2.3. S. mutans Suspensions and Antibacterial Test
2.4. Preparation of Dental Samples
2.5. Adherence Testing
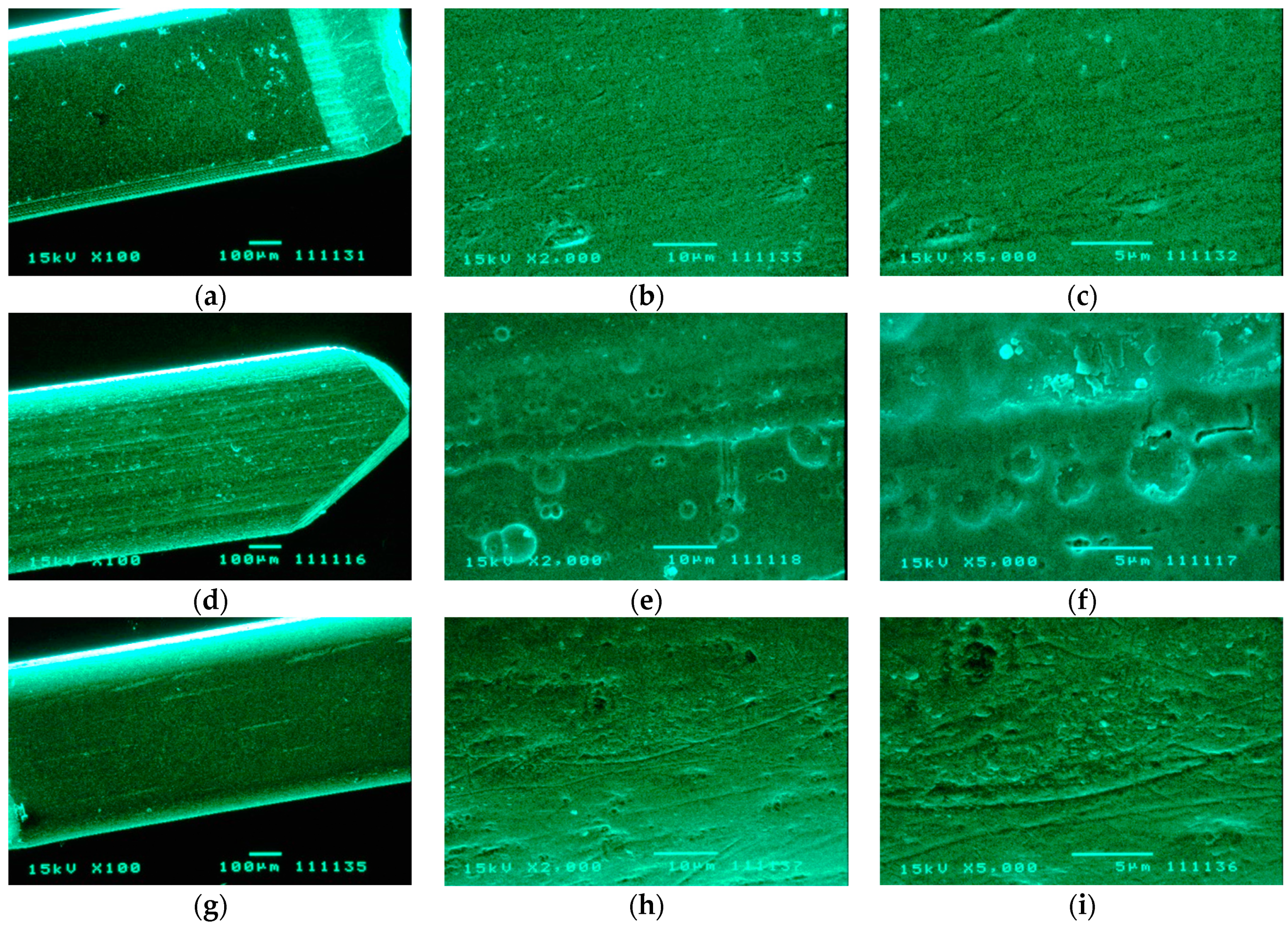
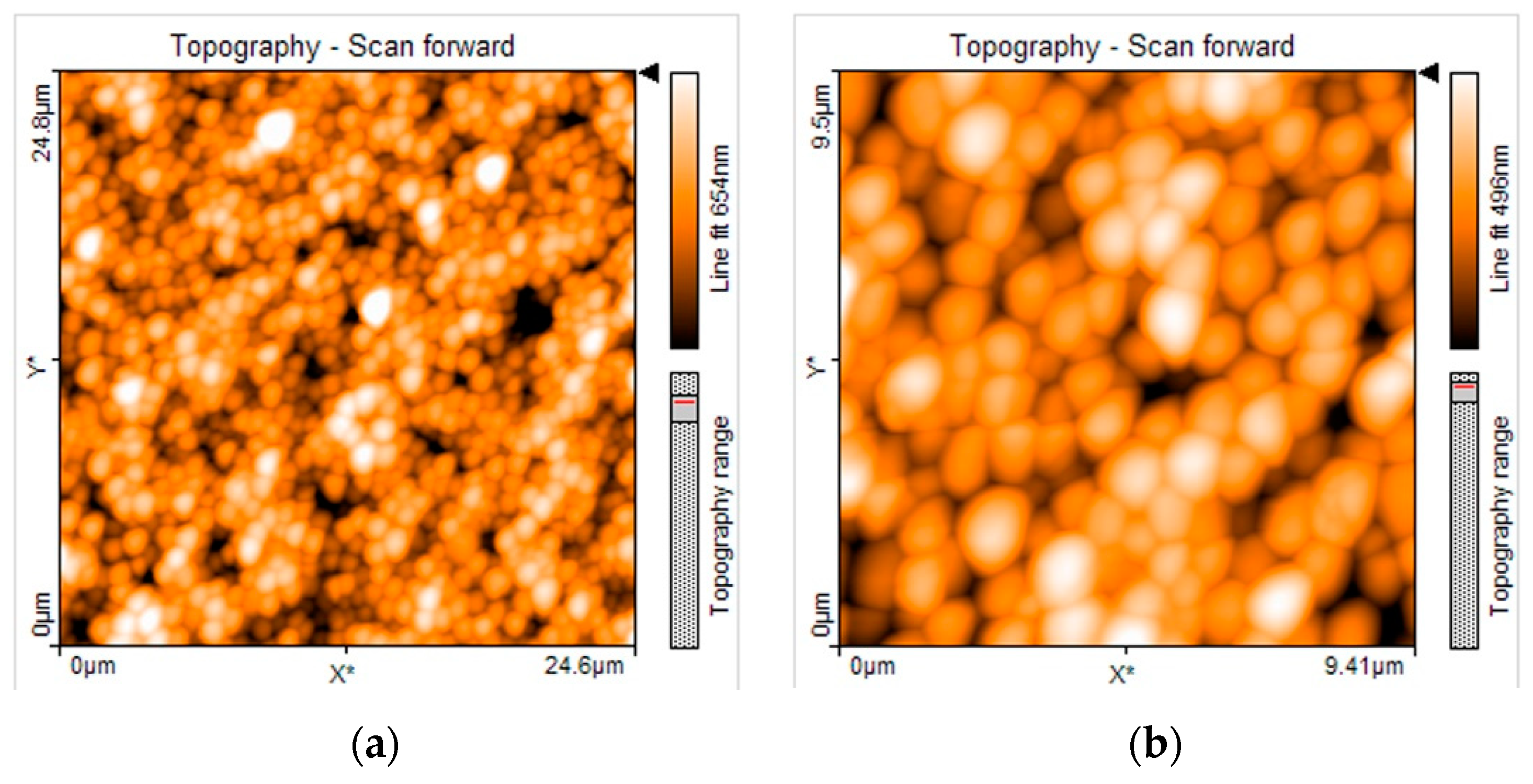
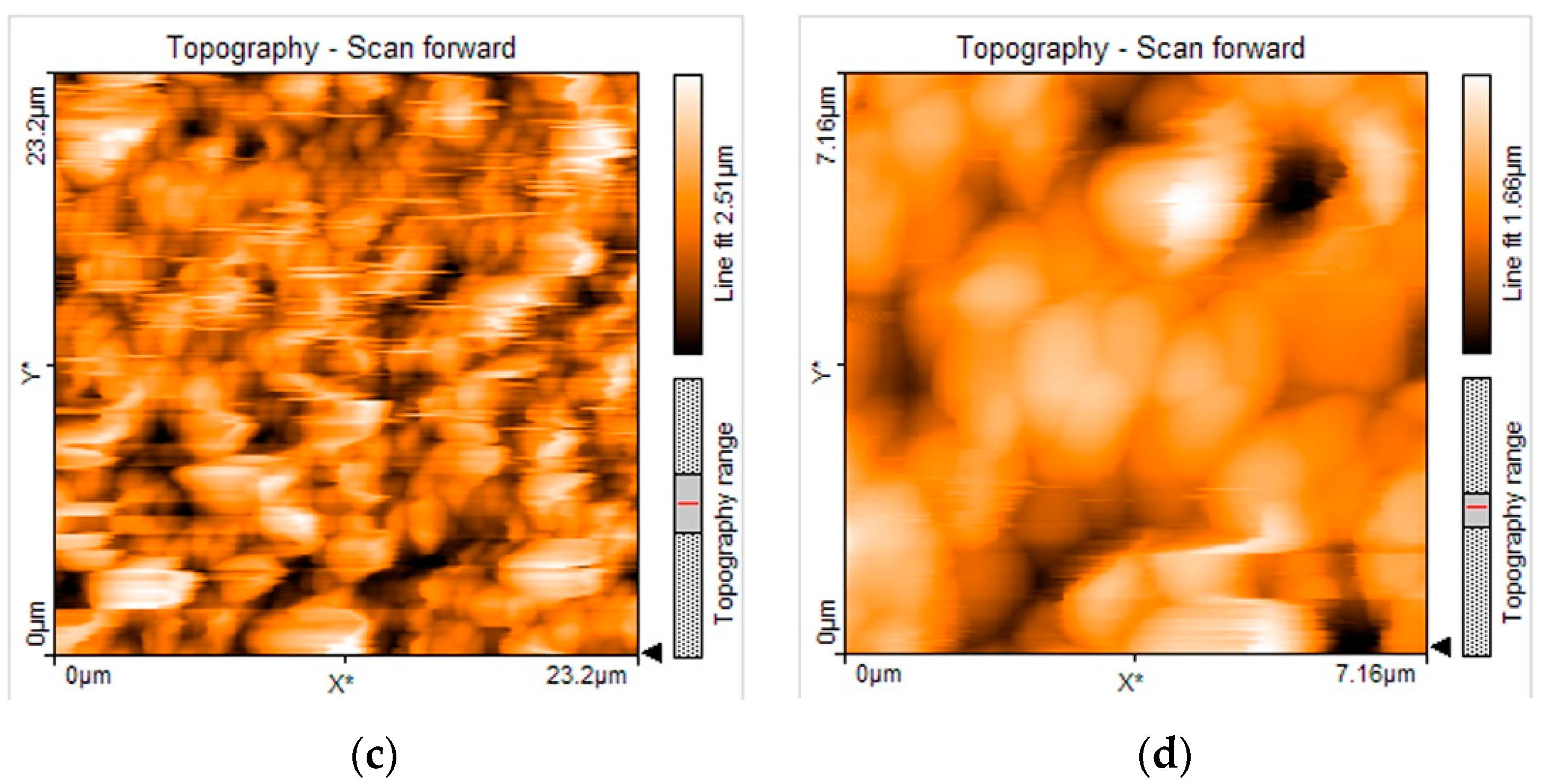
2.6. SEM and AFM Analysis
2.7. Statistical Analysis
3. Results
3.1. Characterization of AgNP
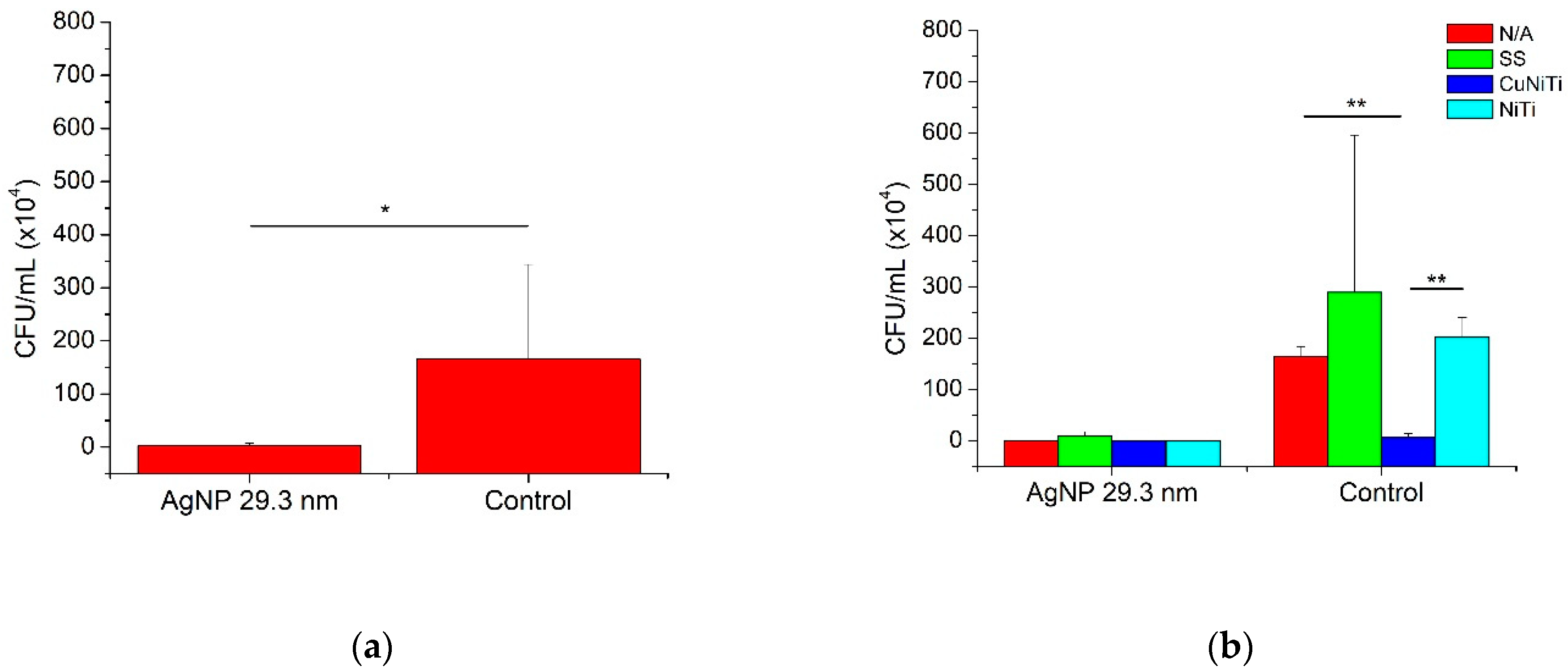
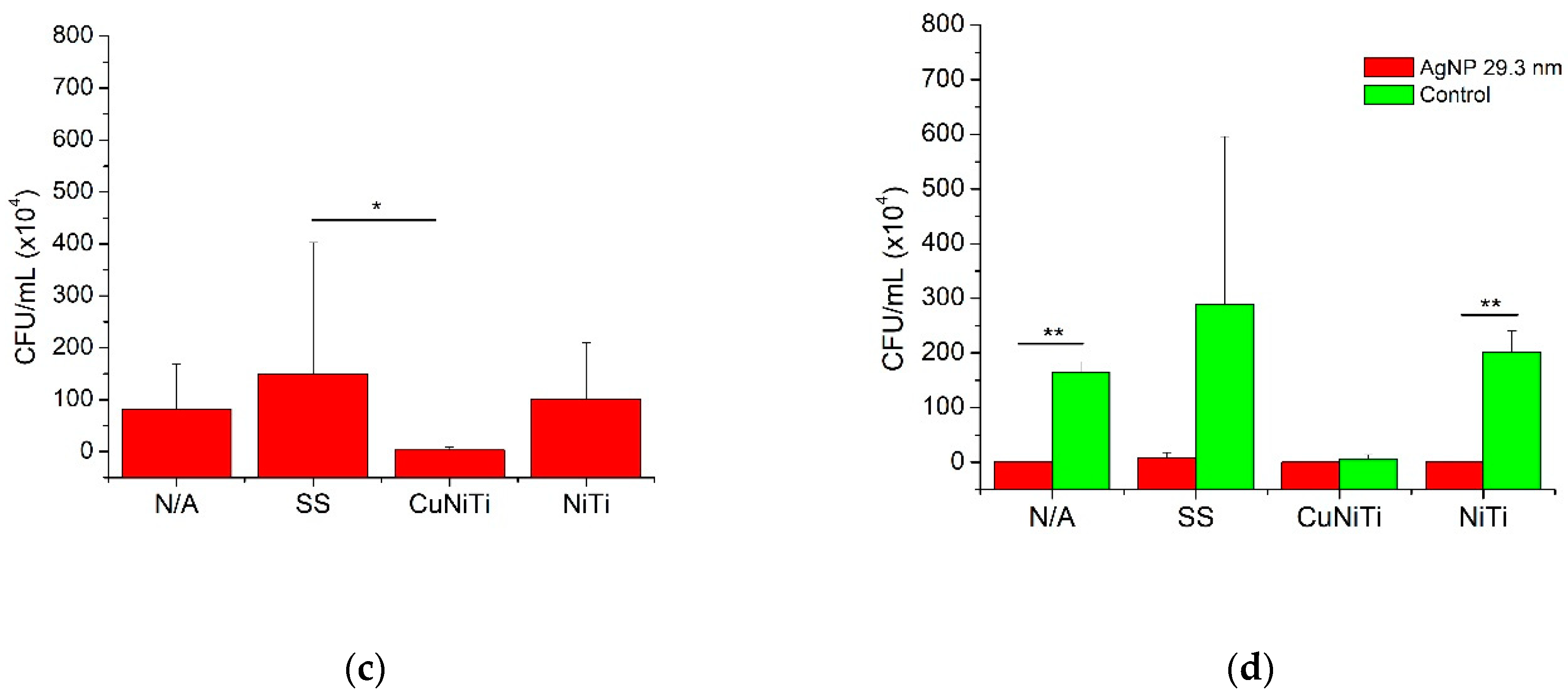
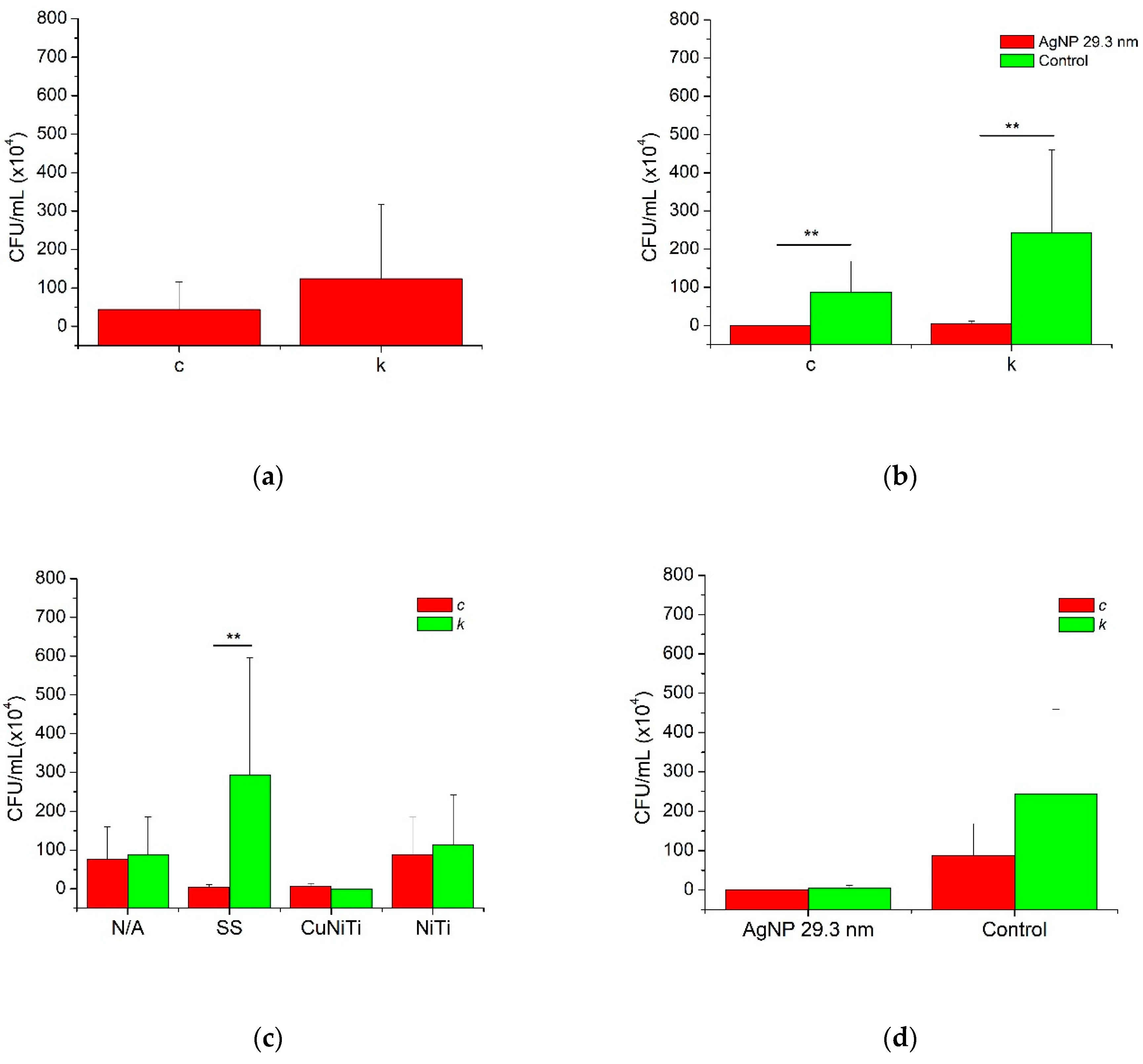
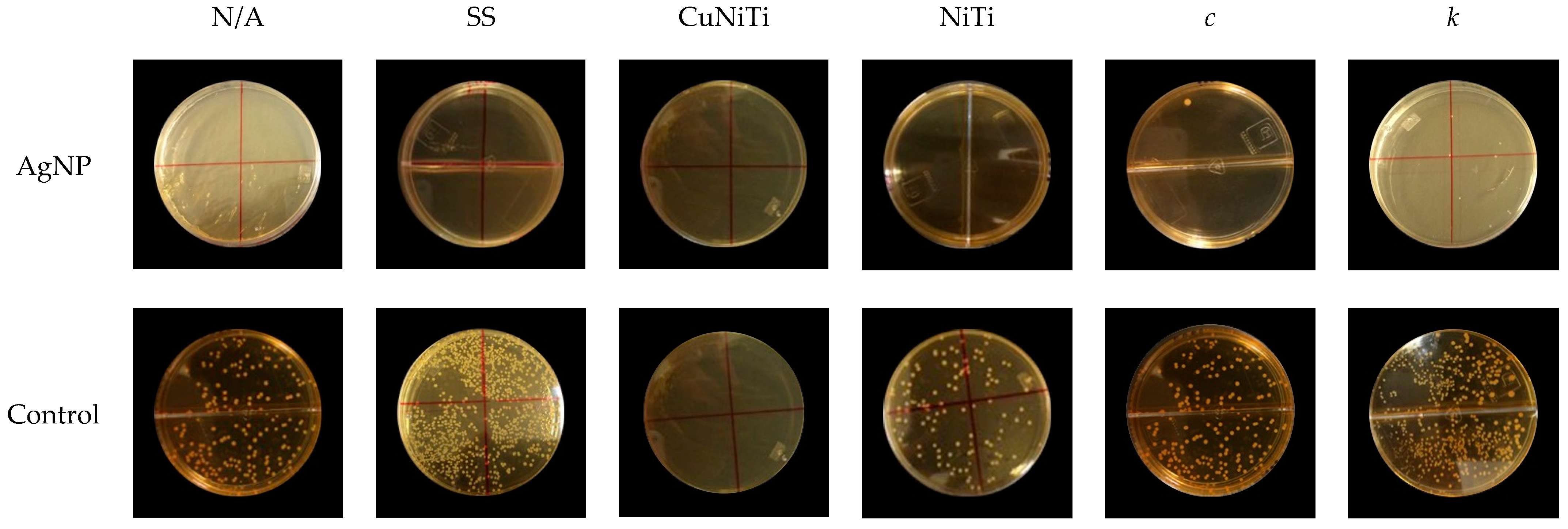
3.2. Adherence Testing
3.3. SEM and AFM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perkowski, K.; Baltaza, W.; Conn, D.B.; Marczyńska-Stolarek, M.; Chomicz, L. Examination of oral biofilm microbiota in patients using fixed orthodontic appliances in order to prevent risk factors for health complications. Ann. Agric. Environ. Med. 2019, 26, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, P.; Perkowski, K.; Starościak, B.; Baltaza, W.; Padzik, M.; Pionkowski, K.; Chomicz, L. Identification of infectious microbiota from oral cavity environment of various population group patients as a preventive approach to human health risk factors. Ann. Agric. Environ. Med. 2016, 23, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.; LeBlanc, D.J. Influences of orthodontic appliances on oral populations of mutans streptococci. Oral Microbiol. Immunol. 2002, 17, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Lin, Y.; Zheng, Y.; Li, W. The microbial changes in subgingival plaques of orthodontic patients: A systematic review and meta-analysis of clinical trials. BMC Oral Health 2017, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Andrucioli, M.C.D.; Nelson-Filho, P.; Matsumoto, M.A.N.; Saraiva, M.C.P.; Feres, M.; de Figueiredo, L.C.; Martins, L.P. Molecular detection of in-vivo microbial contamination of metallic orthodontic brackets by checkerboard DNA-DNA hybridization. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Lucchese, A.; Lajolo, C.; Rupe, C.; Di Stasio, D.; Romano, A.; Petruzzi, M.; Serpico, R. The Oral Microbiota Changes in Orthodontic Patients and Effects on Oral Health: An Overview. J. Clin. Med. 2021, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Bondemark, L.; Marcolina, M.; Manuelli, M. Changes in oral microbiota due to orthodontic appliances: A systematic review. J. Oral Microbiol. 2018, 10, 1476645. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Liu, Y.; Si, Y.; Zhang, Q.; Wang, L.; Liu, J.; Wang, C.; Xiao, S. Prevalence of fimA genotypes of Porphyromonas gingivalis in adolescent orthodontic patients. PLoS ONE 2017, 12, e0188420. [Google Scholar] [CrossRef]
- Oho, T.; Yamashita, Y.; Shimazaki, Y.; Kushiyama, M.; Koga, T. Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol. Immunol. 2000, 15, 258–262. [Google Scholar] [CrossRef]
- Mathur, V.P.; Dhillon, J.K. Dental Caries: A Disease Which Needs Attention. Indian J. Pediatr. 2018, 85, 202–206. [Google Scholar] [CrossRef]
- Maldupa, I.; Sopule, A.; Uribe, S.E.; Brinkmane, A.; Senakola, E. Caries Prevalence and Severity for 12-Year-Old Children in Latvia. Int. Dent. J. 2021, 71, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Banthia, R.; Chandki, R.; Banthia, P. Biofilms: A microbial home. J. Indian Soc. Periodontol. 2011, 15, 111. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Witkowska, E.; Kaveh, B.; Lif Holgerson, P.; Tanner, A.C.R. The Microbiome in Populations with a Low and High Prevalence of Caries. J. Dent. Res. 2016, 95, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. Ecological Hypothesis of Dentin and Root Caries. Caries Res. 2016, 50, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. The Role of Bacteria in the Caries Process. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- García-Godoy, F.; Hicks, M.J. Maintaining the integrity of the enamel surface. J. Am. Dent. Assoc. 2008, 139, 25S–34S. [Google Scholar] [CrossRef]
- Hicks, J.; Garcia-Godoy, F.; Flaitz, C. Biological factors in dental caries: Role of remineralization and fluoride in the dynamic process of demineralization and remineralization (part 3). J. Clin. Pediatr. Dent. 2004, 28, 203–214. [Google Scholar] [CrossRef]
- Rozen, R.; Bachrach, G.; Steinberg, D. Effect of carbohydrates on fructosyltransferase expression and distribution in Streptococcus mutans GS-5 biofilms. Carbohydr. Res. 2004, 339, 2883–2888. [Google Scholar] [CrossRef]
- Nakano, K.; Nomura, R.; Nakagawa, I.; Hamada, S.; Ooshima, T. Demonstration of Streptococcus mutans with a Cell Wall Polysaccharide Specific to a New Serotype, k, in the Human Oral Cavity. J. Clin. Microbiol. 2004, 42, 198–202. [Google Scholar] [CrossRef]
- Nakano, K.; Ooshima, T. Serotype classification of Streptococcus mutans and its detection outside the oral cavity. Future Microbiol. 2009, 4, 891–902. [Google Scholar] [CrossRef]
- Hirasawa, M.; Takada, K. A new selective medium for Streptococcus mutans and the distribution of S. mutans and S. sobrinus and their serotypes in dental plaque. Caries Res. 2003, 37, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Ozaki, K.; Seki, M.; Kawato, T.; Tanaka, H.; Nakano, Y.; Yamashita, Y. Analysis of loci required for determination of serotype antigenicity in Streptococcus mutans and its clinical utilization. J. Clin. Microbiol. 2003, 41, 4107–4112. [Google Scholar] [CrossRef] [PubMed]
- Lapirattanakul, J.; Nakano, K.; Nomura, R.; Nemoto, H.; Kojima, A.; Senawongse, P.; Srisatjaluk, R.; Ooshima, T. Detection of serotype k Streptococcus mutans in Thai subjects. Oral Microbiol. Immunol. 2009, 24, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Inaba, H.; Nomura, R.; Nemoto, H.; Takeda, M.; Yoshioka, H.; Matsue, H.; Takahashi, T.; Taniguchi, K.; Amano, A.; et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J. Clin. Microbiol. 2006, 44, 3313–3317. [Google Scholar] [CrossRef]
- Inaba, H. Roles of Oral Bacteria in Cardiovascular Diseases—From Molecular Mechanisms to Clinical Cases: Implications of Periodontal Diseases in Development of Systemic Diseases. J. Pharmacol. Sci. 2011, 113, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Nomura, R.; Shimizu, N.; Nakagawa, I.; Hamada, S.; Ooshima, T. Development of a PCR method for rapid identification of new Streptococcus mutans serotype k strains. J. Clin. Microbiol. 2004, 42, 4925–4930. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Otsugu, M.; Naka, S.; Teramoto, N.; Kojima, A.; Muranaka, Y.; Matsumoto-Nakano, M.; Ooshima, T.; Nakano, K. Contribution of the interaction of Streptococcus mutans serotype k strains with fibrinogen to the pathogenicity of infective endocarditis. Infect. Immun. 2014, 82, 5223–5234. [Google Scholar] [CrossRef]
- Naka, S.; Nomura, R.; Takashima, Y.; Okawa, R.; Ooshima, T.; Nakano, K. A specific Streptococcus mutans strain aggravates non-alcoholic fatty liver disease. Oral Dis. 2014, 20, 700–706. [Google Scholar] [CrossRef]
- Lara-Carrillo, E.; Montiel-Bastida, N.M.; Sánchez-Pérez, L.; Alanís-Tavira, J. Factors correlated with developing caries during orthodontic treatment: Changes in saliva and behavioral risks. J. Dent. Sci. 2012, 7, 218–223. [Google Scholar] [CrossRef][Green Version]
- Sonesson, M.; Bergstrand, F.; Gizani, S.; Twetman, S. Management of post-orthodontic white spot lesions: An updated systematic review. Eur. J. Orthod. 2017, 39, 116–121. [Google Scholar] [CrossRef]
- Heymann, G.C.; Grauer, D. A contemporary review of white spot lesions in orthodontics. J. Esthet. Restor. Dent. 2013, 25, 85–95. [Google Scholar] [CrossRef]
- Srivastava, K.; Tikku, T.; Khanna, R.; Sachan, K. Risk factors and management of white spot lesions in orthodontics. J. Orthod. Sci. 2013, 2, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Armstrong, S.; Chalmers, J.; Warren, J.J. White spot lesions: Prevention and treatment. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 690–696. [Google Scholar] [CrossRef]
- Marinho, V.C.C.; Hurst, D.; Baez, R.; Marthaler, T.M. Salt fluoridation for preventing dental caries. Cochrane Database Syst. Rev. 2016, 7, CD002284. [Google Scholar] [CrossRef]
- Marinho, V.C.; Higgins, J.P.; Sheiham, A.; Logan, S. Combinations of topical fluoride (toothpastes, mouthrinses, gels, varnishes) versus single topical fluoride for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2004, 2004, CD002781. [Google Scholar] [CrossRef]
- Marinho, V.C.; Higgins, J.P.; Logan, S.; Sheiham, A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003, 2003, CD002782. [Google Scholar] [CrossRef]
- Cossellu, G.; Lanteri, V.; Butera, A.; Laffi, N.; Merlini, A.; Farronato, G. Timing considerations on the shear bond strength of orthodontic brackets after topical fluoride varnish applications. J. Orthod. Sci. 2017, 6, 11. [Google Scholar] [CrossRef]
- Benson, P.E.; Parkin, N.; Dyer, F.; Millett, D.T.; Furness, S.; Germain, P. Fluorides for the prevention of early tooth decay (demineralised white lesions) during fixed brace treatment. Cochrane Database Syst. Rev. 2013, 2013, CD003809. [Google Scholar] [CrossRef]
- Stecksén-Blicks, C.; Renfors, G.; Oscarson, N.D.; Bergstrand, F.; Twetman, S. Caries-Preventive Effectiveness of a Fluoride Varnish: A Randomized Controlled Trial in Adolescents with Fixed Orthodontic Appliances. Caries Res. 2007, 41, 455–459. [Google Scholar] [CrossRef]
- Yates, R.; Jenkins, S.; Newcombe, R.; Wade, W.; Moran, J.; Addy, M. A 6-month home usage trial of a 1% chlorhexidine toothpaste: (I). Effects on plaque, gingivitis, calculus and toothstaining. J. Clin. Periodontol. 1993, 20, 130–138. [Google Scholar] [CrossRef]
- Marghalani, A.A.; Guinto, E.; Phan, M.; Dhar, V.; Tinanoff, N. Effectiveness of Xylitol in Reducing Dental Caries in Children. Pediatr. Dent. 2017, 39, 103–110. [Google Scholar]
- Worthington, H.V.; MacDonald, L.; Poklepovic Pericic, T.; Sambunjak, D.; Johnson, T.M.; Imai, P.; Clarkson, J.E. Home use of interdental cleaning devices, in addition to toothbrushing, for preventing and controlling periodontal diseases and dental caries. Cochrane Database Syst. Rev. 2019, 2020, CD012018. [Google Scholar] [CrossRef]
- Kale, S.; Kakodkar, P.; Shetiya, S.; Abdulkader, R. Prevalence of dental caries among children aged 5-15 years from 9 countries in the Eastern Mediterranean Region: A meta-analysis. East. Mediterr. Health J. 2020, 26, 726–735. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, K.; Xie, X.; Dai, Z.; Zhao, Z.; Imazato, S.; Al-Dulaijan, Y.; Al-Qarni, F.; Weir, M.; Reynolds, M.; et al. Nanostructured Polymeric Materials with Protein-Repellent and Anti-Caries Properties for Dental Applications. Nanomaterials 2018, 8, 393. [Google Scholar] [CrossRef]
- Jasso-Ruiz, I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Morales-Luckie, R.A.; Sawada, T.; Yamaguchi, R. Silver nanoparticles in orthodontics, a new alternative in bacterial inhibition: In vitro study. Prog. Orthod. 2020, 21, 24. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Holguín-Meráz, C.; Zaragoza-Contreras, E.A.; Martínez-Martínez, R.E.; Donohue-Cornejo, A.; Loyola-Rodríguez, J.P.; Cuevas-González, J.C.; Reyes-López, S.Y. Antimicrobial and Substantivity Properties of Silver Nanoparticles against Oral Microbiomes Clinically Isolated from Young and Young-Adult Patients. J. Nanomater. 2019, 2019, 3205971. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Martínez-Castañón, G.A.; Loyola-Rodríguez, J.P.; Niño-Martínez, N.; Ruiz, F.; Zavala-Alonso, N.V.; Lara, R.H.; Reyes-López, S.Y. Bovine Serum Albumin and Chitosan Coated Silver Nanoparticles and Its Antimicrobial Activity against Oral and Nonoral Bacteria. J. Nanomater. 2015, 2015, 420853. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Boegli, L.; James, G.; Velasquillo, C.; Sánchez-Sánchez, R.; Martínez-Martínez, R.E.; Martínez-Castañón, G.A.; Martinez-Gutierrez, F. Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Mater. Sci. Eng. C 2015, 55, 360–366. [Google Scholar] [CrossRef]
- Martínez-Robles, Á.; Loyola-Rodríguez, J.; Zavala-Alonso, N.; Martinez-Martinez, R.; Ruiz, F.; Lara-Castro, R.; Donohué-Cornejo, A.; Reyes-López, S.; Espinosa-Cristóbal, L. Antimicrobial Properties of Biofunctionalized Silver Nanoparticles on Clinical Isolates of Streptococcus mutans and Its Serotypes. Nanomaterials 2016, 6, 136. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions onEscherichia coli andStaphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Treuel, L.; Brandholt, S.; Maffre, P.; Wiegele, S.; Shang, L.; Nienhaus, G.U. Impact of Protein Modification on the Protein Corona on Nanoparticles and Nanoparticle–Cell Interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef]
- Lee, W.; Kim, K.-J.; Lee, D.G. A novel mechanism for the antibacterial effect of silver nanoparticles on Escherichia coli. BioMetals 2014, 27, 1191–1201. [Google Scholar] [CrossRef]
- Jiménez-Ramírez, A.J.; Martínez-Martínez, R.E.; Ayala-Herrera, J.L.; Zaragoza-Contreras, E.A.; Domínguez-Pérez, R.A.; Reyes-López, S.Y.; Donohue-Cornejo, A.; Cuevas-González, J.C.; Silva-Benítez, E.L.; Espinosa-Cristóbal, L.F. Antimicrobial Activity of Silver Nanoparticles against Clinical Biofilms from Patients with and without Dental Caries. J. Nanomater. 2021, 2021, 5587455. [Google Scholar] [CrossRef]
- Tran, Q.T.; Nguyen, V.S.; Hoang, T.K.D.; Nguyen, H.L.; Bui, T.T.; Nguyen, T.V.A.; Nguyen, D.H.; Nguyen, H.H. Preparation and properties of silver nanoparticles loaded in activated carbon for biological and environmental applications. J. Hazard. Mater. 2011, 192, 1321–1329. [Google Scholar] [CrossRef]
- Vanitha, G.; Rajavel, K.; Boopathy, G.; Veeravazhuthi, V.; Neelamegam, P. Physiochemical charge stabilization of silver nanoparticles and its antibacterial applications. Chem. Phys. Lett. 2017, 669, 71–79. [Google Scholar] [CrossRef]
- Loyola-Rodriguez, J.P.; Martinez-Martinez, R.E.; Flores-Ferreyra, B.I.; Patiño-Marin, N.; Alpuche-Solis, A.G.; Reyes-Macias, J.F. Distribution of Streptococcus mutans and Streptococcus sobrinus in Saliva of Mexican Preschool Caries-free and Caries-active Children by Microbial and Molecular (PCR) Assays. J. Clin. Pediatr. Dent. 2007, 32, 121–126. [Google Scholar] [CrossRef]
- Hoshino, T.; Kawaguchi, M.; Shimizu, N.; Hoshino, N.; Ooshima, T.; Fujiwara, T. PCR detection and identification of oral streptococci in saliva samples using gtf genes. Diagn. Microbiol. Infect. Dis. 2004, 48, 195–199. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Loyola-Rodríguez, J.P.; Patiño-Marín, N.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis of silver particles with different sizes and morphologies. Mater. Lett. 2009, 63, 1266–1268. [Google Scholar] [CrossRef]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. Inhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentine. Nanotoxicology 2014, 8, 745–754. [Google Scholar] [CrossRef]
- Bürgers, R.; Eidt, A.; Frankenberger, R.; Rosentritt, M.; Schweikl, H.; Handel, G.; Hahnel, S. The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch. Oral Biol. 2009, 54, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B. Metal-Based Nanoparticles for the Treatment of Infectious Diseases. Molecules 2017, 22, 1370. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cristóbal, L.F.; Martínez-Castañón, G.A.; Téllez-Déctor, E.J.; Niño-Martínez, N.; Zavala-Alonso, N.V.; Loyola-Rodríguez, J.P. Adherence inhibition of Streptococcus mutans on dental enamel surface using silver nanoparticles. Mater. Sci. Eng. C 2013, 33, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Moolya, N.; Sharma, R.; Shetty, A.; Gupta, N.; Gupta, A.; Jalan, V. Orthodontic bracket designs and their impact on microbial profile and periodontal disease: A clinical trial. J. Orthod. Sci. 2014, 3, 125. [Google Scholar] [CrossRef]
- Allaker, R.P. The Use of Nanoparticles to Control Oral Biofilm Formation. J. Dent. Res. 2010, 89, 1175–1186. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ohashi, S.; Aono, M.; Kokubo, T.; Yamada, I.; Yamauchi, J. Antibacterial activity of silver ions implanted in SiO2 filler on oral streptococci. Dent. Mater. 1996, 12, 227–229. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A review of shape memory polymer composites and blends. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1661–1672. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Dobrzynski, W.; Zawadzka-Knefel, A.; Janecki, M.; Kurek, K.; Lubojanski, A.; Szymonowicz, M.; Rybak, Z.; Wiglusz, R.J. Nanomaterials Application in Orthodontics. Nanomaterials 2021, 11, 337. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; López-Ruiz, N.; Cabada-Tarín, D.; Reyes-López, S.Y.; Zaragoza-Contreras, A.; Constandse-Cortéz, D.; Donohué-Cornejo, A.; Tovar-Carrillo, K.; Cuevas-González, J.C.; Kobayashi, T. Antiadherence and Antimicrobial Properties of Silver Nanoparticles against Streptococcus mutans on Brackets and Wires Used for Orthodontic Treatments. J. Nanomater. 2018, 2018, 9248527. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Martínez-Castañón, G.A.; Martínez-Martínez, R.E.; Loyola-Rodríguez, J.P.; Patiño-Marín, N.; Reyes-Macías, J.F.; Ruiz, F. Antimicrobial sensibility of Streptococcus mutans serotypes to silver nanoparticles. Mater. Sci. Eng. C 2012, 32, 896–901. [Google Scholar] [CrossRef]
- Hernández-Sierra, J.F.; Ruiz, F.; Cruz Pena, D.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; de Jesús Pozos Guillén, A.; Tapia-Pérez, H.; Martínez Castañón, G. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 237–240. [Google Scholar] [CrossRef]
- Eliades, T.; Eliades, G.; Brantley, W.A. Microbial attachment on orthodontic appliances: I. Wettability and early pellicle formation on bracket materials. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 351–360. [Google Scholar] [CrossRef]
- Lee, H.J.; Kwon, T.Y.; Kim, K.H.; Hong, S.H. Soybean extracts facilitate bacterial agglutination and prevent biofilm formation on orthodontic wire. J. Med. Food 2014, 17, 135–141. [Google Scholar] [CrossRef]
- Fournier, A.; Payant, L.; Bouclin, R. Adherence of Streptococcus mutans to orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 1998, 114, 414–417. [Google Scholar] [CrossRef]
- Abraham, K.S.; Jagdish, N.; Kailasam, V.; Padmanabhan, S. Streptococcus mutans adhesion on nickel titanium (NiTi) and copper-NiTi archwires: A comparative prospective clinical study. Angle Orthod. 2017, 87, 448–454. [Google Scholar] [CrossRef]
- Kim, I.H.; Park, H.S.; Kim, Y.K.; Kim, K.H.; Kwon, T.Y. Comparative short-term in vitro analysis of mutans streptococci adhesion on esthetic, nickel-titanium, and stainless-steel arch wires. Angle Orthod. 2014, 84, 680–686. [Google Scholar] [CrossRef]
- Hepyukselen, B.G.; Cesur, M.G. Comparison of the microbial flora from different orthodontic archwires using a cultivation method and PCR: A prospective study. Orthod. Craniofacial Res. 2019, 22, 354–360. [Google Scholar] [CrossRef]
- Yousif, A.; Abd El-Karim, U. Microscopic study of surface roughness of four orthodontic arch wires. Tanta Dent. J. 2016, 13, 199. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Arendsen, L.P.; Thakar, R.; Sultan, A.H. The Use of Copper as an Antimicrobial Agent in Health Care, Including Obstetrics and Gynecology. Clin. Microbiol. Rev. 2019, 32, e00125-18. [Google Scholar] [CrossRef]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez y Baena, R.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. Biomed Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef] [PubMed]
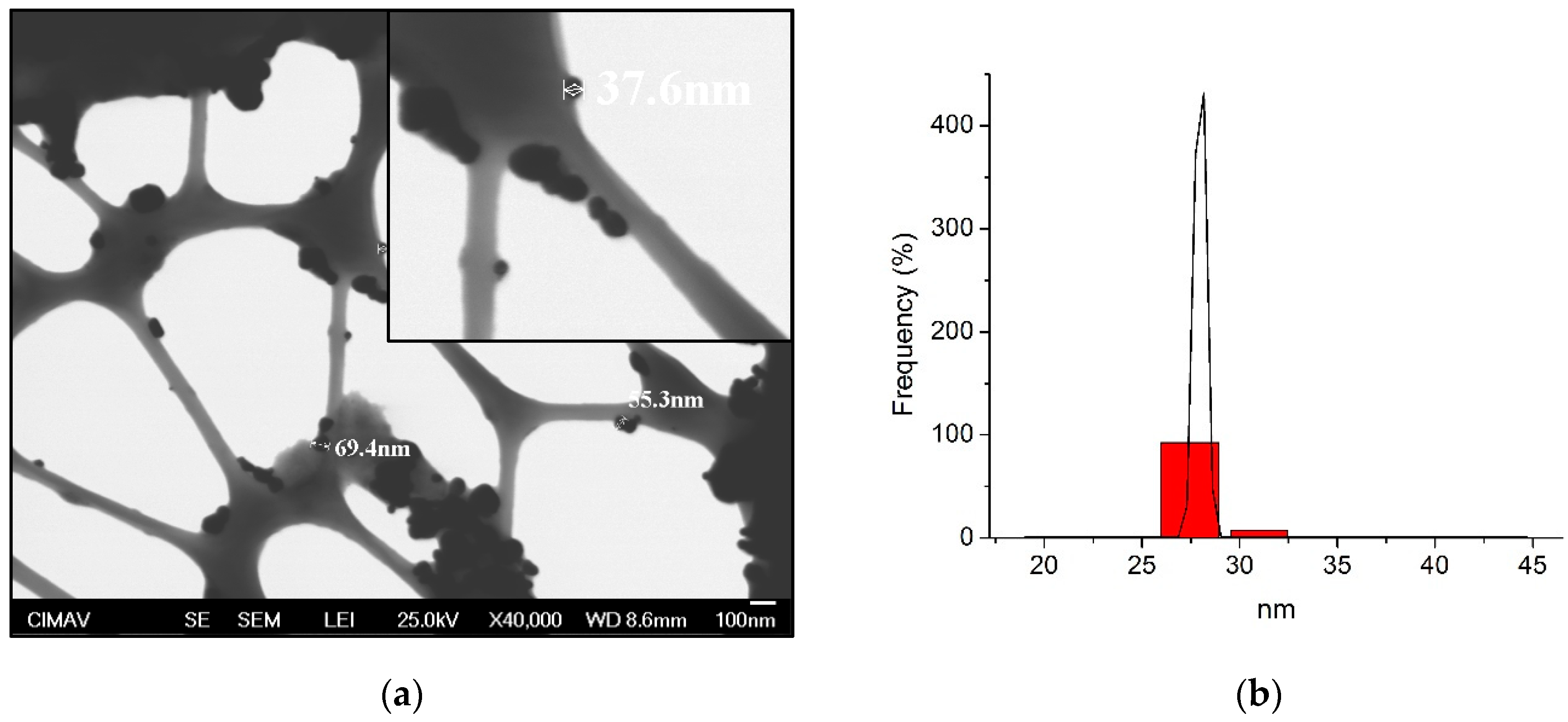
| AgNP (nm) | DLS Diameter (nm) | Shape | Initial Concentration (µg/mL) | Zeta Potential (mV) | MIC in Serotype c (µg/mL) | MIC in Serotype k (µg/mL) |
|---|---|---|---|---|---|---|
| 29.3 | 29.3 ± 0.7 | Spherical | 1070 | −36.5 ± 5.7 | 55.7 ± 19.3 | 111.4 ± 38.6 |
| AgNP 29.3 nm (CFU/mL × 104) | Control (CFU/mL × 104) | Total (CFU/mL × 104) | |
|---|---|---|---|
| Serotype | |||
| c | 0.5 ± 0.7 * | 87.5 ± 80.9 | 44.0 ± 71.4 |
| k | 4.2 ± 7.7 * | 243.5 ± 216.1 | 123.9 ± 193.1 |
| Archwire | |||
| N/A | 0.3 ± 0.8 * | 164.5 ± 19.2 a | 82.4 ± 86.7 |
| SS | 8.6 ± 9.1 | 289.3 ± 306.8 | 149.0 ± 253.6 a |
| CuNiTi | 0.1 ± 0.4 | 6.5 ± 7.5 a,b | 3.3 ± 6.0 a |
| NiTi | 0.3 ± 0.8 * | 202.0 ± 38.7 b | 101.1 ± 108.5 |
| Total | 2.3 ± 5.6 * | 165.5 ± 178.4 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nafarrate-Valdez, R.A.; Martínez-Martínez, R.E.; Zaragoza-Contreras, E.A.; Áyala-Herrera, J.L.; Domínguez-Pérez, R.A.; Reyes-López, S.Y.; Donohue-Cornejo, A.; Cuevas-González, J.C.; Loyola-Rodríguez, J.P.; Espinosa-Cristóbal, L.F. Anti-Adherence and Antimicrobial Activities of Silver Nanoparticles against Serotypes C and K of Streptococcus mutans on Orthodontic Appliances. Medicina 2022, 58, 877. https://doi.org/10.3390/medicina58070877
Nafarrate-Valdez RA, Martínez-Martínez RE, Zaragoza-Contreras EA, Áyala-Herrera JL, Domínguez-Pérez RA, Reyes-López SY, Donohue-Cornejo A, Cuevas-González JC, Loyola-Rodríguez JP, Espinosa-Cristóbal LF. Anti-Adherence and Antimicrobial Activities of Silver Nanoparticles against Serotypes C and K of Streptococcus mutans on Orthodontic Appliances. Medicina. 2022; 58(7):877. https://doi.org/10.3390/medicina58070877
Chicago/Turabian StyleNafarrate-Valdez, Rosa Amalia, Rita Elizabeth Martínez-Martínez, Erasto Armando Zaragoza-Contreras, José Luis Áyala-Herrera, Rubén Abraham Domínguez-Pérez, Simón Yobanny Reyes-López, Alejandro Donohue-Cornejo, Juan Carlos Cuevas-González, Juan Pablo Loyola-Rodríguez, and León Francisco Espinosa-Cristóbal. 2022. "Anti-Adherence and Antimicrobial Activities of Silver Nanoparticles against Serotypes C and K of Streptococcus mutans on Orthodontic Appliances" Medicina 58, no. 7: 877. https://doi.org/10.3390/medicina58070877
APA StyleNafarrate-Valdez, R. A., Martínez-Martínez, R. E., Zaragoza-Contreras, E. A., Áyala-Herrera, J. L., Domínguez-Pérez, R. A., Reyes-López, S. Y., Donohue-Cornejo, A., Cuevas-González, J. C., Loyola-Rodríguez, J. P., & Espinosa-Cristóbal, L. F. (2022). Anti-Adherence and Antimicrobial Activities of Silver Nanoparticles against Serotypes C and K of Streptococcus mutans on Orthodontic Appliances. Medicina, 58(7), 877. https://doi.org/10.3390/medicina58070877










