Value of the Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Predicting CPET Performance in Patients with Stable CAD and Recent Elective PCI
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Ethics Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowbar, A.N.; Howard, J.P.; Finegold, J.A.; Asaria, P.; Francis, D.P. 2014 Global Geographic Analysis of Mortality from Ischaemic Heart Disease by Country, Age and Income: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2014, 174, 293–298. [Google Scholar] [CrossRef]
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef]
- Goel, K.; Lennon, R.J.; Tilbury, R.T.; Squires, R.W.; Thomas, R.J. Impact of Cardiac Rehabilitation on Mortality and Cardiovascular Events after Percutaneous Coronary Intervention in the Community. Circulation 2011, 123, 2344–2352. [Google Scholar] [CrossRef]
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; Pedretti, R.F.; Schmid, J.-P.; Vigorito, C.; et al. Secondary Prevention through Comprehensive Cardiovascular Rehabilitation: From Knowledge to Implementation. 2020 Update. A Position Paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2020, 28, 460–495. [Google Scholar] [CrossRef]
- Price, K.J.; Gordon, B.A.; Bird, S.R.; Benson, A.C. A Review of Guidelines for Cardiac Rehabilitation Exercise Programmes: Is There an International Consensus? Eur. J. Prev. Cardiol. 2016, 23, 1715–1733. [Google Scholar] [CrossRef]
- Thomas, R.J.; Beatty, A.L.; Beckie, T.M.; Brewer, L.C.; Brown, T.M.; Forman, D.E.; Franklin, B.A.; Keteyian, S.J.; Kitzman, D.W.; Regensteiner, J.G.; et al. Home-Based Cardiac Rehabilitation: A Scientific Statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation 2019, 140, e69–e89. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Leducq Transatlantic Network on Atherothrombosis Inflammation in Atherosclerosis: From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Olsen, S.J.; Schirmer, H.; Bønaa, K.H.; Hanssen, T.A. Cardiac Rehabilitation after Percutaneous Coronary Intervention: Results from a Nationwide Survey. Eur. J. Cardiovasc. Nurs. 2018, 17, 273–279. [Google Scholar] [CrossRef]
- Long, L.; Anderson, L.; He, J.; Gandhi, M.; Dewhirst, A.; Bridges, C.; Taylor, R. Exercise-Based Cardiac Rehabilitation for Stable Angina: Systematic Review and Meta-Analysis. Open Heart 2019, 6, e000989. [Google Scholar] [CrossRef]
- Lala, A.; Shah, K.B.; Lanfear, D.E.; Thibodeau, J.T.; Palardy, M.; Ambardekar, A.V.; McNamara, D.M.; Taddei-Peters, W.C.; Baldwin, J.T.; Jeffries, N.; et al. Predictive Value of Cardiopulmonary Exercise Testing Parameters in Ambulatory Advanced Heart Failure. JACC Heart Fail. 2021, 9, 226–236. [Google Scholar] [CrossRef]
- Keteyian, S.J.; Brawner, C.A.; Savage, P.D.; Ehrman, J.K.; Schairer, J.; Divine, G.; Aldred, H.; Ophaug, K.; Ades, P.A. Peak Aerobic Capacity Predicts Prognosis in Patients with Coronary Heart Disease. Am. Heart J. 2008, 156, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Mezzani, A.; Hamm, L.F.; Jones, A.M.; McBride, P.E.; Moholdt, T.; Stone, J.A.; Urhausen, A.; Williams, M.A.; European Association for Cardiovascular Prevention and Rehabilitation; American Association of Cardiovascular and Pulmonary Rehabilitation; et al. Aerobic Exercise Intensity Assessment and Prescription in Cardiac Rehabilitation: A Joint Position Statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 2013, 20, 442–467. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.E.; Arena, R.; Boxer, R.; Dolansky, M.A.; Eng, J.J.; Fleg, J.L.; Haykowsky, M.; Jahangir, A.; Kaminsky, L.A.; Kitzman, D.W.; et al. Prioritizing Functional Capacity as a Principal End Point for Therapies Oriented to Older Adults with Cardiovascular Disease: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2017, 135, e894–e918. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.K.; Al-Mallah, M.H.; McEvoy, J.W.; Whelton, S.P.; Blumenthal, R.S.; Nasir, K.; Schairer, J.R.; Brawner, C.; Alam, M.; Keteyian, S.J.; et al. Prognostic Value of Exercise Capacity in Patients with Coronary Artery Disease: The FIT (Henry Ford Exercise Testing) Project. Mayo Clin. Proc. 2014, 89, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, N.; Cadarso-Suárez, C.; Lado-Baleato, O.; Díaz-Louzao, C.; Gil, C.P.; Reeh, J.; Rasmusen, H.; Prescott, E. Improvement in VO2peak Predicts Readmissions for Cardiovascular Disease and Mortality in Patients Undergoing Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 2020, 27, 811–819. [Google Scholar] [CrossRef]
- Yoshikane, H.; Yamamoto, T.; Ozaki, M.; Matsuzaki, M. Clinical significance of high-sensitivity C-reactive protein in lifestyle-related disease and metabolic syndrome. J. Cardiol. 2007, 50, 175–182. [Google Scholar]
- Erikssen, G.; Liestøl, K.; Bjørnholt, J.V.; Stormorken, H.; Thaulow, E.; Erikssen, J. Erythrocyte Sedimentation Rate: A Possible Marker of Atherosclerosis and a Strong Predictor of Coronary Heart Disease Mortality. Eur. Heart J. 2000, 21, 1614–1620. [Google Scholar] [CrossRef]
- Andresdottir, M.B.; Sigfusson, N.; Sigvaldason, H.; Gudnason, V. Erythrocyte Sedimentation Rate, an Independent Predictor of Coronary Heart Disease in Men and Women: The Reykjavik Study. Am. J. Epidemiol. 2003, 158, 844–851. [Google Scholar] [CrossRef]
- Strang, F.; Schunkert, H. C-Reactive Protein and Coronary Heart Disease: All Said—Is Not It? Mediat. Inflamm. 2014, 2014, 757123. [Google Scholar] [CrossRef]
- Colbert, L.H.; Visser, M.; Simonsick, E.M.; Tracy, R.P.; Newman, A.B.; Kritchevsky, S.B.; Pahor, M.; Taaffe, D.R.; Brach, J.; Rubin, S.; et al. Physical Activity, Exercise, and Inflammatory Markers in Older Adults: Findings from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2004, 52, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.; Ernst, E. Exercise and Thrombosis. Coron. Artery Dis. 2000, 11, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Schoene, N.; Gielen, S.; Hofer, J.; Erbs, S.; Schuler, G.; Hambrecht, R. Endothelial Dysfunction in Patients with Chronic Heart Failure: Systemic Effects of Lower-Limb Exercise Training. J. Am. Coll. Cardiol. 2001, 37, 392–397. [Google Scholar] [CrossRef]
- Krenn-Pilko, S.; Langsenlehner, U.; Thurner, E.-M.; Stojakovic, T.; Pichler, M.; Gerger, A.; Kapp, K.S.; Langsenlehner, T. The Elevated Preoperative Platelet-to-Lymphocyte Ratio Predicts Poor Prognosis in Breast Cancer Patients. Br. J. Cancer 2014, 110, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhu, G.-Q.; Xie, L.; Liu, W.-Y.; Shi, L.; Wang, O.-C.; Huang, Z.-H.; Braddock, M.; Guo, G.-L.; Zheng, M.-H. Preoperative Platelet to Lymphocyte Ratio Is a Valuable Prognostic Biomarker in Patients with Colorectal Cancer. Oncotarget 2016, 7, 25516–25527. [Google Scholar] [CrossRef] [PubMed]
- Durmus, E.; Kivrak, T.; Gerin, F.; Sunbul, M.; Sari, I.; Erdogan, O. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Are Predictors of Heart Failure. Arq. Bras. De Cardiol. 2015, 105, 606–613. [Google Scholar] [CrossRef]
- Heidarpour, M.; Bashiri, S.; Vakhshoori, M.; Heshmat-Ghahdarijani, K.; Khanizadeh, F.; Ferdowsian, S.; Shafie, D. The Association between Platelet-to-Lymphocyte Ratio with Mortality among Patients Suffering from Acute Decompensated Heart Failure. BMC Cardiovasc. Disord. 2021, 21, 454. [Google Scholar] [CrossRef]
- Ye, G.; Chen, Q.; Chen, X.; Liu, Y.; Yin, T.; Meng, Q.; Liu, Y.; Wei, H.; Zhou, Q. The Prognostic Role of Platelet-to-Lymphocyte Ratio in Patients with Acute Heart Failure: A Cohort Study. Sci. Rep. 2019, 9, 10639. [Google Scholar] [CrossRef]
- Sun, X.-P.; Li, J.; Zhu, W.-W.; Li, D.-B.; Chen, H.; Li, H.-W.; Chen, W.-M.; Hua, Q. Impact of Platelet-to-Lymphocyte Ratio on Clinical Outcomes in Patients with ST-Segment Elevation Myocardial Infarction. Angiology 2017, 68, 346–353. [Google Scholar] [CrossRef]
- Dong, G.; Huang, A.; Liu, L. Platelet-to-lymphocyte Ratio and Prognosis in STEMI: A Meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13386. [Google Scholar] [CrossRef]
- Azab, B.; Shah, N.; Akerman, M.; McGinn, J.T. Value of Platelet/Lymphocyte Ratio as a Predictor of All-Cause Mortality after Non-ST-Elevation Myocardial Infarction. J. Thromb. Thrombolysis 2012, 34, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.; Gul, M.; Bozbay, M.; Cicek, G.; Uyarel, H.; Koroglu, B.; Uluganyan, M.; Aslan, S.; Tusun, E.; Surgit, O.; et al. The Relationship between Platelet to Lymphocyte Ratio and the Clinical Outcomes in ST Elevation Myocardial Infarction Underwent Primary Coronary Intervention. Blood Coagul. Fibrinolysis 2014, 25, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Willim, H.A.; Harianto, J.C.; Cipta, H. Platelet-to-Lymphocyte Ratio at Admission as a Predictor of In-Hospital and Long-Term Outcomes in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Cardiol. Res. 2021, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.; Yang, X. Decreased Platelet-to-Lymphocyte Ratio as Predictor of Thrombogenesis in Nonvalvular Atrial Fibrillation. Herz 2020, 45, 684–688. [Google Scholar] [CrossRef]
- Dereli, S.; Bayramoğlu, A.; Yontar, O.C. Usefulness of Platelet to Lymphocyte Ratio for Predicting Recurrence of Atrial Fibrillation after Direct Current Cardioversion. Ann. Noninvasive Electrocardiol. 2019, 24, e12616. [Google Scholar] [CrossRef]
- Velioğlu, Y.; Yüksel, A. Utility of Platelet-to-Lymphocyte Ratio to Support the Diagnosis of Acute Deep Vein Thrombosis. Turk. Gogus Kalp Damar Cerrahisi Derg. 2019, 27, 493–498. [Google Scholar] [CrossRef]
- Zhen, Y.; Chang, Z.; Liu, Z.; Zheng, J. Platelet to Lymphocyte Ratio Predicting 6-Month Primary Patency of Drug-Coated Balloon for Femoropopliteal Disease. BMC Cardiovasc. Disord. 2020, 20, 9. [Google Scholar] [CrossRef]
- Karataş, M.B.; Çanga, Y.; İpek, G.; Özcan, K.S.; Güngör, B.; Durmuş, G.; Onuk, T.; Öz, A.; Şimşek, B.; Bolca, O. Association of Admission Serum Laboratory Parameters with New-Onset Atrial Fibrillation after a Primary Percutaneous Coronary Intervention. Coron Artery Dis. 2016, 27, 128–134. [Google Scholar] [CrossRef]
- Ayça, B.; Akin, F.; Çelik, Ö.; Yüksel, Y.; Öztürk, D.; Tekiner, F.; Çetin, Ş.; Okuyan, E.; Dinçkal, M.H. Platelet to Lymphocyte Ratio as a Prognostic Marker in Primary Percutaneous Coronary Intervention. Platelets 2015, 26, 638–644. [Google Scholar] [CrossRef]
- Meshaal, M.S.; Nagi, A.; Eldamaty, A.; Elnaggar, W.; Gaber, M.; Rizk, H. Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Independent Predictors of Outcome in Infective Endocarditis (IE). Egypt Heart J. 2019, 71, 13. [Google Scholar] [CrossRef]
- Tamhane, U.U.; Aneja, S.; Montgomery, D.; Rogers, E.-K.; Eagle, K.A.; Gurm, H.S. Association between Admission Neutrophil to Lymphocyte Ratio and Outcomes in Patients with Acute Coronary Syndrome. Am. J. Cardiol. 2008, 102, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Jang, H.-J.; Oh, I.-Y.; Yoon, C.-H.; Suh, J.-W.; Cho, Y.-S.; Youn, T.-J.; Cho, G.-Y.; Chae, I.-H.; Choi, D.-J. Prognostic Value of Neutrophil to Lymphocyte Ratio in Patients Presenting with ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2013, 111, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Akpek, M.; Kaya, M.G.; Lam, Y.Y.; Sahin, O.; Elcik, D.; Celik, T.; Ergin, A.; Gibson, C.M. Relation of Neutrophil/Lymphocyte Ratio to Coronary Flow to in-Hospital Major Adverse Cardiac Events in Patients with ST-Elevated Myocardial Infarction Undergoing Primary Coronary Intervention. Am. J. Cardiol. 2012, 110, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Emdin, M.; Passino, C.; Michelassi, C.; Battaglia, D.; Cocci, F. Predictive Value of Elevated Neutrophil-Lymphocyte Ratio on Cardiac Mortality in Patients with Stable Coronary Artery Disease. Clin. Chim. Acta 2008, 395, 27–31. [Google Scholar] [CrossRef]
- Kaya, H.; Ertaş, F.; İslamoğlu, Y.; Kaya, Z.; Atılgan, Z.A.; Çil, H.; Çalışkan, A.; Aydın, M.; Oylumlu, M.; Soydinç, M.S. Association between Neutrophil to Lymphocyte Ratio and Severity of Coronary Artery Disease. Clin. Appl. Thromb. Hemost. 2014, 20, 50–54. [Google Scholar] [CrossRef]
- Okan, S. The Relationship between Exercise Capacity and Neutrophil//Lymphocyte Ratio in Patients Taken to Cardiopulmonary Rehabilitation Program. Bratisl. Lek Listy 2020, 121, 206–210. [Google Scholar] [CrossRef]
- Scicali, R.; Mandraffino, G.; Di Pino, A.; Scuruchi, M.; Ferrara, V.; Squadrito, G.; Purrello, F.; Piro, S. Impact of High Neutrophil-to-Lymphocyte Ratio on the Cardiovascular Benefit of PCSK9 Inhibitors in Familial Hypercholesterolemia Subjects with Atherosclerotic Cardiovascular Disease: Real-World Data from Two Lipid Units. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3401–3406. [Google Scholar] [CrossRef]
- Mitu, M.; Suceveanu, M.; Mitu, F. Cardiovascular Rehabilitation in Romania. Rom. J. Cardiol. 2020, 30, 1–6. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; WHO: Geneva, Switzerland, 2006; ISBN 978-92-4-159493-6. [Google Scholar]
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Lancellotti, P.; Zamorano, J.L.; Habib, G.; Badano, L. The EACVI Textbook of Echocardiography; Oxford University Press: Oxford, UK, 2017; ISBN 978-0-19-103889-1. [Google Scholar]
- Cooper, C.B.; Storer, T.W. Exercise Testing and Interpretation: A Practical Approach; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001; ISBN 978-0-521-64842-4. [Google Scholar]
- Walzik, D.; Joisten, N.; Zacher, J.; Zimmer, P. Transferring Clinically Established Immune Inflammation Markers into Exercise Physiology: Focus on Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio and Systemic Immune-Inflammation Index. Eur. J. Appl. Physiol. 2021, 121, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Fest, J.; Ruiter, R.; Ikram, M.A.; Voortman, T.; van Eijck, C.H.J.; Stricker, B.H. Reference Values for White Blood-Cell-Based Inflammatory Markers in the Rotterdam Study: A Population-Based Prospective Cohort Study. Sci. Rep. 2018, 8, 10566. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Williams, M.A.; Gulati, M.; Kligfield, P.; Balady, G.J.; Collins, E.; Fletcher, G. American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing Assessment of Functional Capacity in Clinical and Research Settings: A Scientific Statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007, 116, 329–343. [Google Scholar] [CrossRef]
- Vanhees, L.; Fagard, R.; Thijs, L.; Staessen, J.; Amery, A. Prognostic Significance of Peak Exercise Capacity in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 1994, 23, 358–363. [Google Scholar] [CrossRef]
- Coeckelberghs, E.; Buys, R.; Goetschalckx, K.; Cornelissen, V.A.; Vanhees, L. Prognostic Value of the Oxygen Uptake Efficiency Slope and Other Exercise Variables in Patients with Coronary Artery Disease. Eur. J. Prev. Cardiol. 2016, 23, 237–244. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Yayan, J. Emerging Families of Biomarkers for Coronary Artery Disease: Inflammatory Mediators. Vasc. Health Risk. Manag. 2013, 9, 435–456. [Google Scholar] [CrossRef]
- Gary, T.; Pichler, M.; Belaj, K.; Hafner, F.; Gerger, A.; Froehlich, H.; Eller, P.; Rief, P.; Hackl, G.; Pilger, E.; et al. Platelet-to-Lymphocyte Ratio: A Novel Marker for Critical Limb Ischemia in Peripheral Arterial Occlusive Disease Patients. PLoS ONE 2013, 8, e67688. [Google Scholar] [CrossRef]
- Afari, M.E.; Bhat, T. Neutrophil to Lymphocyte Ratio (NLR) and Cardiovascular Diseases: An Update. Expert Rev. Cardiovasc. 2016, 14, 573–577. [Google Scholar] [CrossRef]
- Canpolat, U.; Aytemir, K.; Yorgun, H.; Şahiner, L.; Kaya, E.B.; Kabakçı, G.; Tokgözoğlu, L.; Oto, A. Role of Preablation Neutrophil/Lymphocyte Ratio on Outcomes of Cryoballoon-Based Atrial Fibrillation Ablation. Am. J. Cardiol. 2013, 112, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Im, S.I.; Shin, S.Y.; Na, J.O.; Kim, Y.H.; Choi, C.U.; Kim, S.H.; Kim, J.W.; Kim, E.J.; Han, S.W.; Rha, S.-W.; et al. Usefulness of Neutrophil/Lymphocyte Ratio in Predicting Early Recurrence after Radiofrequency Catheter Ablation in Patients with Atrial Fibrillation. Int. J. Cardiol. 2013, 168, 4398–4400. [Google Scholar] [CrossRef] [PubMed]
- Aribas, A.; Akilli, H.; Gul, E.E.; Kayrak, M.; Demir, K.; Duman, C.; Alibasic, H.; Yazici, M.; Ozdemir, K.; Gok, H. Can Neutrophil/Lymphocyte Ratio Predict Recurrence after Electrical Cardioversion in Non-Valvular Atrial Fibrillation? Anadolu Kardiyol. Derg. 2012, 13, 123–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chatterjee, S.; Chandra, P.; Guha, G.; Kalra, V.; Chakraborty, A.; Frankel, R.; Shani, J. Pre-Procedural Elevated White Blood Cell Count and Neutrophil-Lymphocyte (N/L) Ratio Are Predictors of Ventricular Arrhythmias During Percutaneous Coronary Intervention. Cardiovasc. Haematol. Disord.-Drug Targets 2011, 11, 58–60. [Google Scholar] [CrossRef]
- Uthamalingam, S.; Patvardhan, E.A.; Subramanian, S.; Ahmed, W.; Martin, W.; Daley, M.; Capodilupo, R. Utility of the Neutrophil to Lymphocyte Ratio in Predicting Long-Term Outcomes in Acute Decompensated Heart Failure. Am. J. Cardiol. 2011, 107, 433–438. [Google Scholar] [CrossRef]
- Guasti, L.; Dentali, F.; Castiglioni, L.; Maroni, L.; Marino, F.; Squizzato, A.; Ageno, W.; Gianni, M.; Gaudio, G.; Grandi, A.; et al. Neutrophils and Clinical Outcomes in Patients with Acute Coronary Syndromes and/or Cardiac Revascularisation: A Systematic Review on More than 34,000 Subjects. Thromb. Haemost. 2011, 106, 591–599. [Google Scholar] [CrossRef]
- Ntalouka, M.P.; Nana, P.; Kouvelos, G.N.; Stamoulis, K.; Spanos, K.; Giannoukas, A.; Matsagkas, M.; Arnaoutoglou, E. Association of Neutrophil–Lymphocyte and Platelet–Lymphocyte Ratio with Adverse Events in Endovascular Repair for Abdominal Aortic Aneurysm. J. Clin. Med. 2021, 10, 1083. [Google Scholar] [CrossRef]
- Russu, E.; Mureșan, A.V.; Arbănași, E.M.; Kaller, R.; Hosu, I.; Voidăzan, S.; Arbănași, E.M.; Coșarcă, C.M. The Predictive Role of NLR and PLR in Outcome and Patency of Lower Limb Revascularization in Patients with Femoropopliteal Disease. J. Clin. Med. 2022, 11, 2620. [Google Scholar] [CrossRef]
- Garagoli, F.; Fiorini, N.; Pérez, M.N.; Rabellino, J.M.; Valle Raleigh, J.; Chas, J.G.; DI Caro, V.; Pizarro, R.; Bluro, I.M. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Predict in-Hospital Mortality in Symptomatic but Unruptured Abdominal Aortic Aneurysm Patients. Int. Angiol. 2022, 5, 149–156. [Google Scholar] [CrossRef]
- Arbănași, E.M.; Mureșan, A.V.; Coșarcă, C.M.; Kaller, R.; Bud, T.I.; Hosu, I.; Voidăzan, S.T.; Arbănași, E.M.; Russu, E. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Impact on Predicting Outcomes in Patients with Acute Limb Ischemia. Life 2022, 12, 822. [Google Scholar] [CrossRef]
- Pasqui, E.; de Donato, G.; Giannace, G.; Panzano, C.; Alba, G.; Cappelli, A.; Setacci, C.; Palasciano, G. The Relation between Neutrophil/Lymphocyte and Platelet/Lymphocyte Ratios with Mortality and Limb Amputation after Acute Limb Ischaemia. Vascular 2022, 30, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hoberstorfer, T.; Wadowski, P.P.; Kopp, C.W.; Panzer, S.; Gremmel, T. Platelet-to-Lymphocyte and Neutrophil-to-Lymphocyte Ratios Predict Target Vessel Restenosis after Infrainguinal Angioplasty with Stent Implantation. J. Clin. Med. 2020, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Efros, O.; Beit Halevi, T.; Meisel, E.; Soffer, S.; Barda, N.; Cohen, O.; Kenet, G.; Lubetsky, A. The Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Patients Hospitalized with Acute Pulmonary Embolism. J. Clin. Med. 2021, 10, 4058. [Google Scholar] [CrossRef] [PubMed]
- Baysal, E.; Burak, C.; Cay, S.; Aksu, T.; Altintas, B.; Yaylak, B.; Sevük, U.; Bilge, Ö. The Neutrophil to Lymphocyte Ratio Is Associated with Severity of Rheumatic Mitral Valve Stenosis. J. Blood Med. 2015, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Polat, N.; Yildiz, A.; Yuksel, M.; Bilik, M.Z.; Aydin, M.; Acet, H.; Akil, M.A.; Oylumlu, M.; Kaya, H.; Ertas, F.; et al. Association of Neutrophil–Lymphocyte Ratio with the Presence and Severity of Rheumatic Mitral Valve Stenosis. Clin. Appl. Thromb. Hemost. 2014, 20, 793–798. [Google Scholar] [CrossRef]
- Cakici, M. Neutrophil to Lymphocyte Ratio Predicts Poor Functional Capacity in Patients with Heart Failure. Arch. Turk. Soc. Cardiol. 2014, 42, 612–620. [Google Scholar] [CrossRef]
- Yıldız, A.; Yüksel, M.; Oylumlu, M.; Polat, N.; Akıl, M.A.; Acet, H. The Association between the Neutrophil/Lymphocyte Ratio and Functional Capacity in Patients with Idiopathic Dilated Cardiomyopathy. Anatol. J. Cardiol. 2015, 15, 13–17. [Google Scholar] [CrossRef]
- Furman, M.I.; Benoit, S.E.; Barnard, M.R.; Valeri, C.R.; Borbone, M.L.; Becker, R.C.; Hechtman, H.B.; Michelson, A.D. Increased Platelet Reactivity and Circulating Monocyte-Platelet Aggregates in Patients with Stable Coronary Artery Disease. J. Am. Coll. Cardiol. 1998, 31, 352–358. [Google Scholar] [CrossRef]
- Zhang, S.-Z.; Jin, Y.-P.; Qin, G.-M.; Wang, J.-H. Association of Platelet-Monocyte Aggregates with Platelet Activation, Systemic Inflammation, and Myocardial Injury in Patients with Non-St Elevation Acute Coronary Syndromes. Clin. Cardiol. 2007, 30, 26–31. [Google Scholar] [CrossRef]
- Church, T.S.; Lavie, C.J.; Milani, R.V.; Kirby, G.S. Improvements in Blood Rheology after Cardiac Rehabilitation and Exercise Training in Patients with Coronary Heart Disease. Am. Heart J. 2002, 143, 349–355. [Google Scholar] [CrossRef]
- Sun, X.-P.; Li, J.; Zhu, W.-W.; Li, D.-B.; Chen, H.; Li, H.-W.; Chen, W.-M.; Hua, Q. Platelet to Lymphocyte Ratio Predicts Contrast-Induced Nephropathy in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Angiology 2018, 69, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.C.C.; Gonzalez, M.C.; Avesani, C.M.; Prado, C.M.; Peixoto, M.D.R.G.; Mota, J.F. Low Hand Grip Strength Is Associated with Worse Functional Capacity and Higher Inflammation in People Receiving Maintenance Hemodialysis. Nutrition 2022, 93, 111469. [Google Scholar] [CrossRef] [PubMed]
- Szortyka, M.F.V.; Cristiano, V.B.; Ceresér, K.M.; Francesconi, L.P.; Lobato, M.I.; Gama, C.; Belmonte-de-Abreu, P. Physical Functional Capacity and C-Reactive Protein in Schizophrenia. Front. Psychiatry 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Kerget, B.; Aksakal, A.; Kerget, F. Evaluation of the Relationship between Laboratory Parameters and Pulmonary Function Tests in COVID-19 Patients. Int. J. Clin. Pract. 2021, 75, e14237. [Google Scholar] [CrossRef]
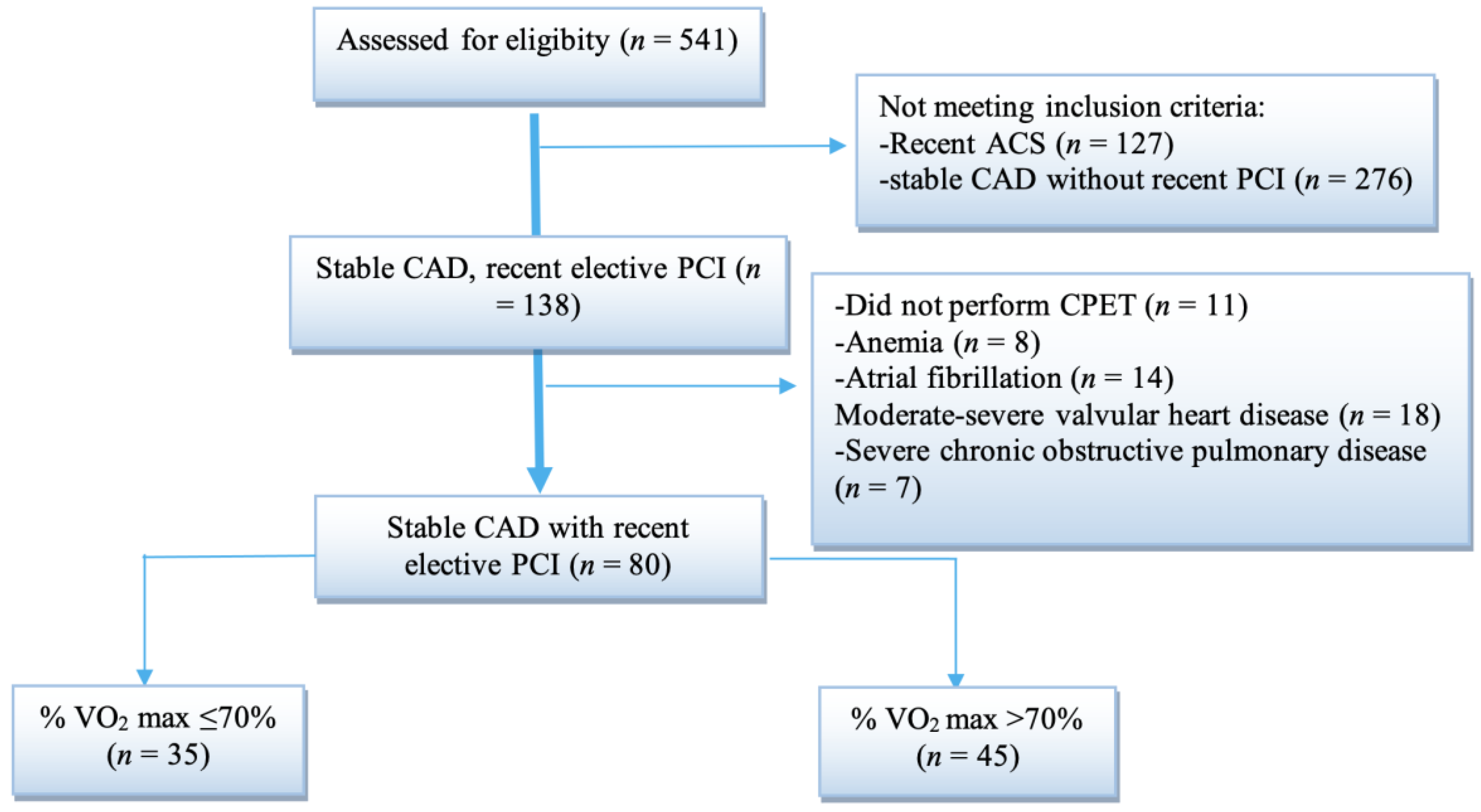
| Parameters | All Patients (n = 80) | % VO2 Max >70 (n = 45) | % VO2 Max ≤70 (n = 35) | p Value * |
|---|---|---|---|---|
| Age (years) × | 55.51 ± 11.83 | 57.02 ± 12.08 | 53.57 ± 11.38 | 0.19 |
| NLR × | 1.97 ± 0.80 | 1.83 ± 0.65 | 2.15 ± 0.93 | 0.07 |
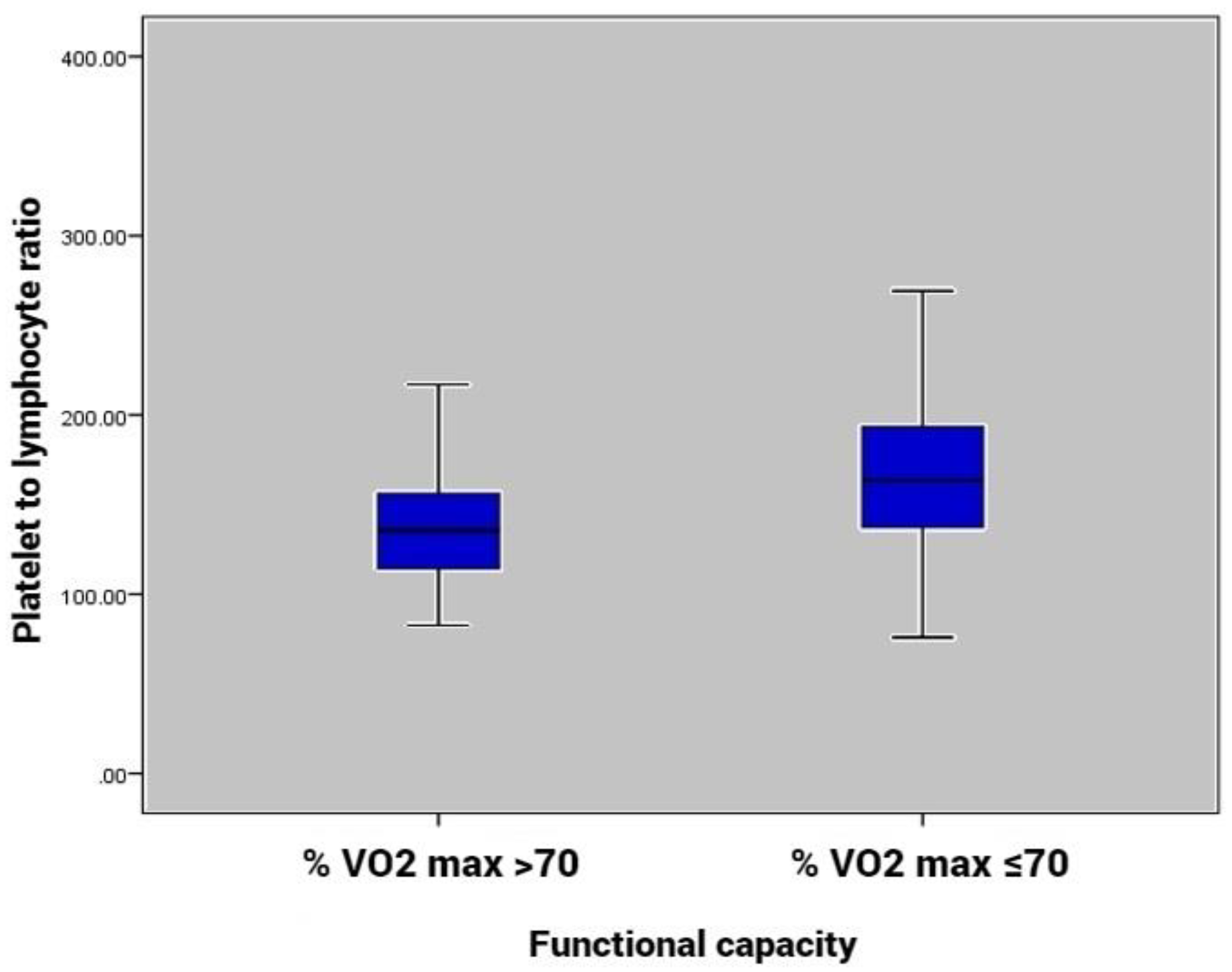
| PLR × | 155.6 ± 52.7 | 137.4 ± 35.9 | 169.8 ± 59.3 | 0.003 |
| Platelet count, ×103/μL × | 256 ± 60 | 244.4 ± 56.1 | 266.3 ± 56.1 | 0.11 |
| Neutrophil count, ×103/μL × | 3.32 ± 1.25 | 2.92 ± 0.90 | 3.83 ± 1.45 | 0.001 |
| Lymphocyte count, ×103/μL † | 1.72 (1.44–1.99) | 1.45 (1.31–2.43) | 1.86 (1.65–1.88) | 0.06 |
| CRP (mg/dl) † | 0.41 (0.24–1.04) | 0.28 (0.15–1.26) | 0.54 (0.26–0.89) | 0.82 |
| LVEF × | 51.31 ± 11.04 | 55.67 ± 9.26 | 48.71 ± 10.93 | 0.003 |
| BMI (kg/m2) † | 28.7 (27.4–33) | 28.4 (27.4–32.4) | 30.15 (25.82–33.17) | 0.68 |
| Hypertension □ | 66 (82.5) | 38 (84.4) | 28 (80) | 0.76 |
| Diabetes □ | 22 (27.5) | 14 (31,1) | 8 (22.9) | 0.45 |
| HbA1c (%) × | 7.11 ± 1.47 | 6.58 ± 1.10 | 7.67 ± 1.66 | 0.05 |
| LDL (mg/dl) † | 84 (69.8–108) | 73(69.8-104) | 100.8 (56.6–124) | 0.57 |
| Resting HR × | 81.9 ± 15.69 | 84.00 ± 17.25 | 77.57 ± 12.76 | 0.05 |
| % peak HR × | 77.98 ± 12.25 | 82.38 ± 11.21 | 72.31 ± 11.27 | 0.001 |
| Resting SBP (mmHg) × | 127.3 ± 12.65 | 130 ± 13.39 | 125.3 ± 11.79 | 0.1 |
| Resting DBP (mmHg) × | 81.5 ± 7.52 | 80.78 ± 7.305 | 82.43 ± 7.8 | 0.33 |
| Parameters | NLR | PLR | Resting HR | % Peak HR | % Peak WR | % VO2 Max |
|---|---|---|---|---|---|---|
| NLR | 1 | 0.369 * | −0.087 | −0.043 | −0.104 | −0.133 |
| PLR | 0.369 * | 1 | 0.207 | 0.172 | 0.105 | 0.249 * |
| Resting HR | −0.087 | 0.207 | 1 | 0.594 * | −0.053 | 0.144 |
| % peak HR | −0.043 | 0.172 | 0.594 * | 1 | 360 * | 0.448 * |
| % peak WR | −0.104 | 0.105 | −0.053 | 0.360 * | 1 | 0.705 * |
| % VO2 max | −0.133 | 0.249 * | 0.144 | 0.448 * | 0.705 * | 1 |
| Variables | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Neutrophil count, ×103/μL | 1.00 | 0.999–1.002 | 0.523 |
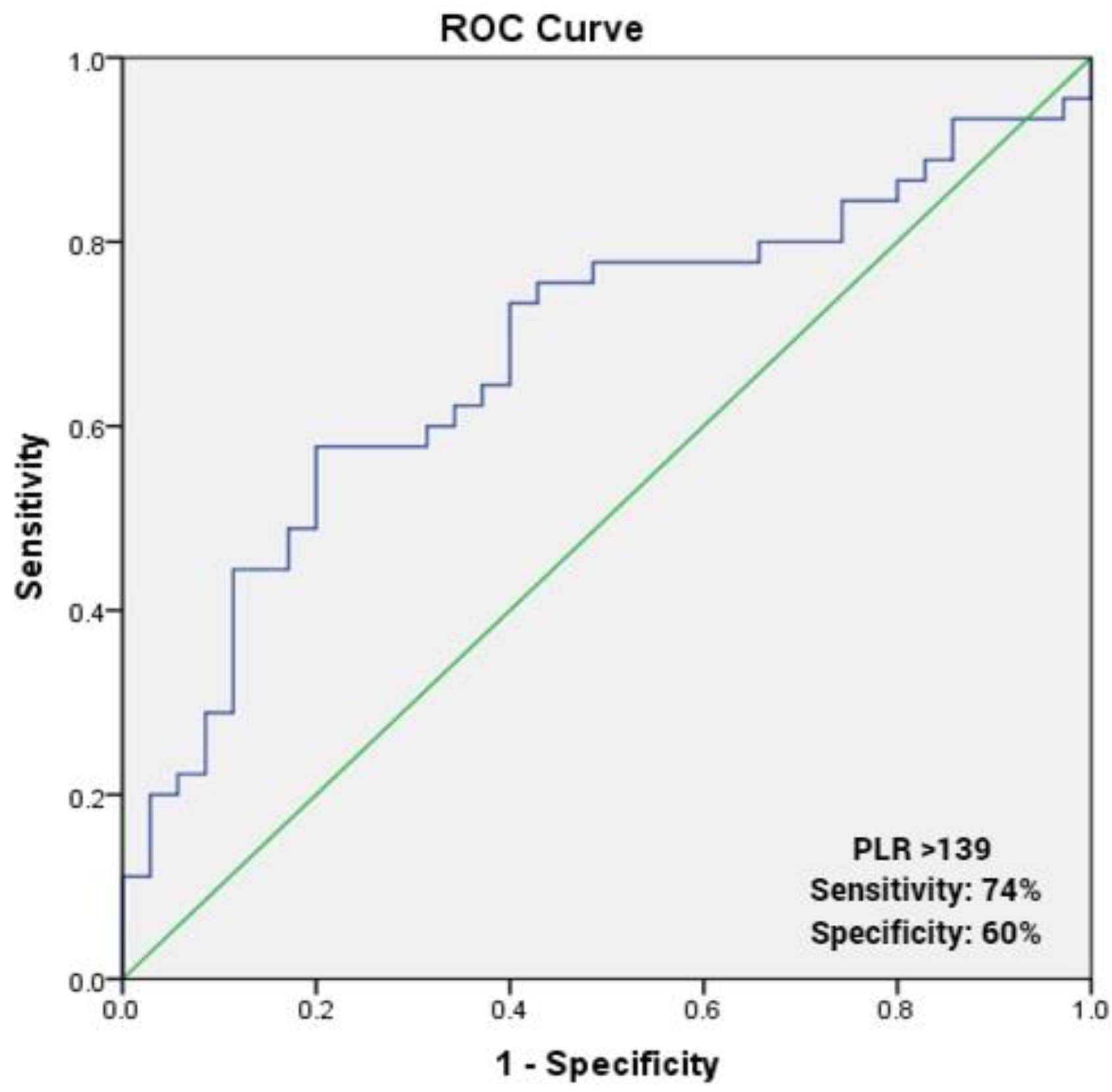
| PLR | 1.015 | 1.004–1.027 | 0.009 |
| LVEF | 1.07 | 1.003–1.141 | 0.042 |
| % peak HR | 1.088 | 1.029–1.151 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drugescu, A.; Roca, M.; Zota, I.M.; Costache, A.-D.; Gavril, O.I.; Gavril, R.S.; Vasilcu, T.F.; Mitu, O.; Esanu, I.M.; Roca, I.-C.; et al. Value of the Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Predicting CPET Performance in Patients with Stable CAD and Recent Elective PCI. Medicina 2022, 58, 814. https://doi.org/10.3390/medicina58060814
Drugescu A, Roca M, Zota IM, Costache A-D, Gavril OI, Gavril RS, Vasilcu TF, Mitu O, Esanu IM, Roca I-C, et al. Value of the Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Predicting CPET Performance in Patients with Stable CAD and Recent Elective PCI. Medicina. 2022; 58(6):814. https://doi.org/10.3390/medicina58060814
Chicago/Turabian StyleDrugescu, Andrei, Mihai Roca, Ioana Mădălina Zota, Alexandru-Dan Costache, Oana Irina Gavril, Radu Sebastian Gavril, Teodor Flaviu Vasilcu, Ovidiu Mitu, Irina Mihaela Esanu, Iulia-Cristina Roca, and et al. 2022. "Value of the Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Predicting CPET Performance in Patients with Stable CAD and Recent Elective PCI" Medicina 58, no. 6: 814. https://doi.org/10.3390/medicina58060814
APA StyleDrugescu, A., Roca, M., Zota, I. M., Costache, A.-D., Gavril, O. I., Gavril, R. S., Vasilcu, T. F., Mitu, O., Esanu, I. M., Roca, I.-C., Ghiciuc, C. M., & Mitu, F. (2022). Value of the Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Predicting CPET Performance in Patients with Stable CAD and Recent Elective PCI. Medicina, 58(6), 814. https://doi.org/10.3390/medicina58060814







