HPV and HIV Coinfection in Women from a Southeast Region of Romania—PICOPIV Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants, Data Collection and Ethical Statement
2.2. Specimen Collection, HIV Serology and Pap Tests
2.3. HPV Genotyping
2.4. Statistical Analyses
3. Results
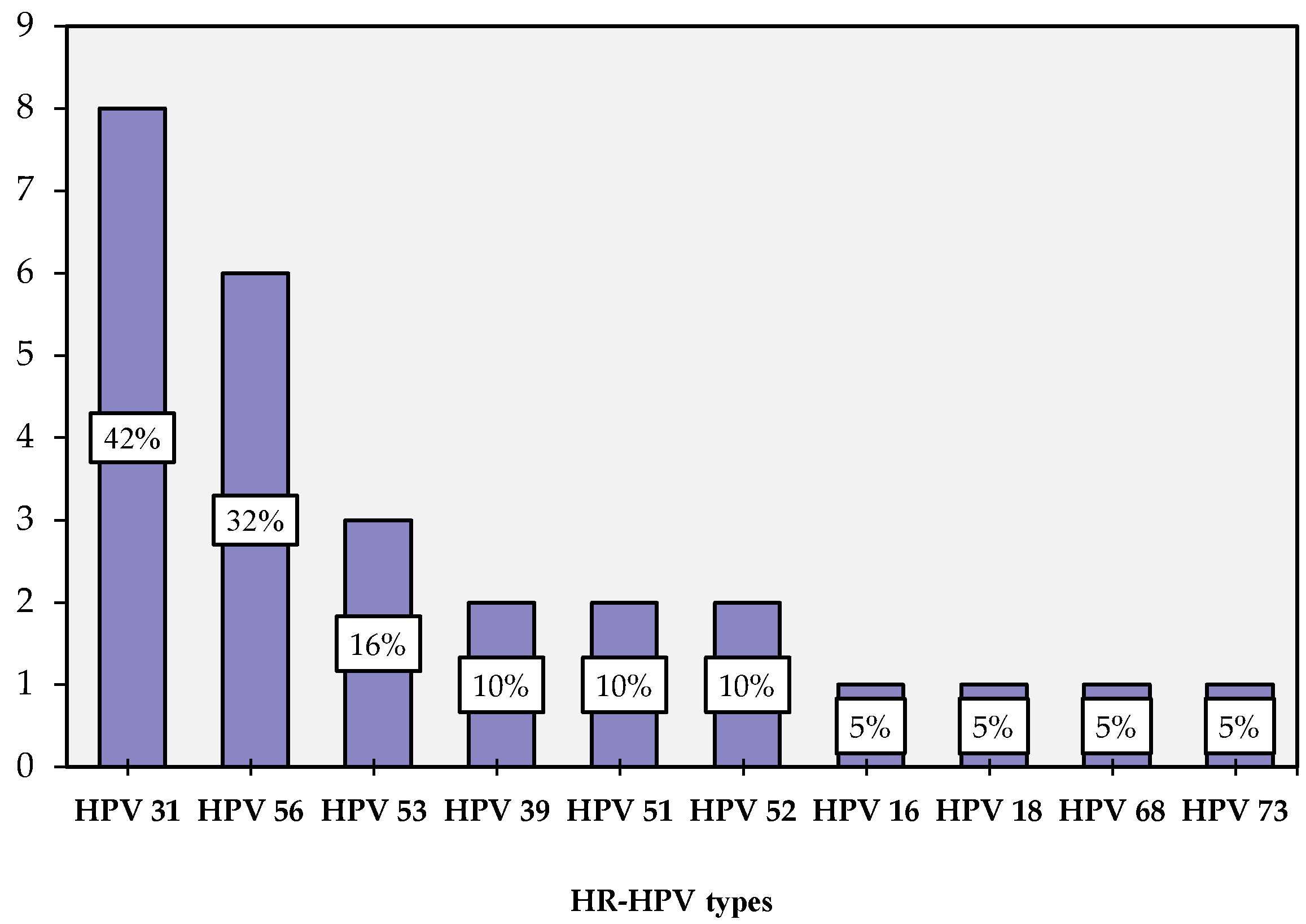
3.1. HPV Distribution
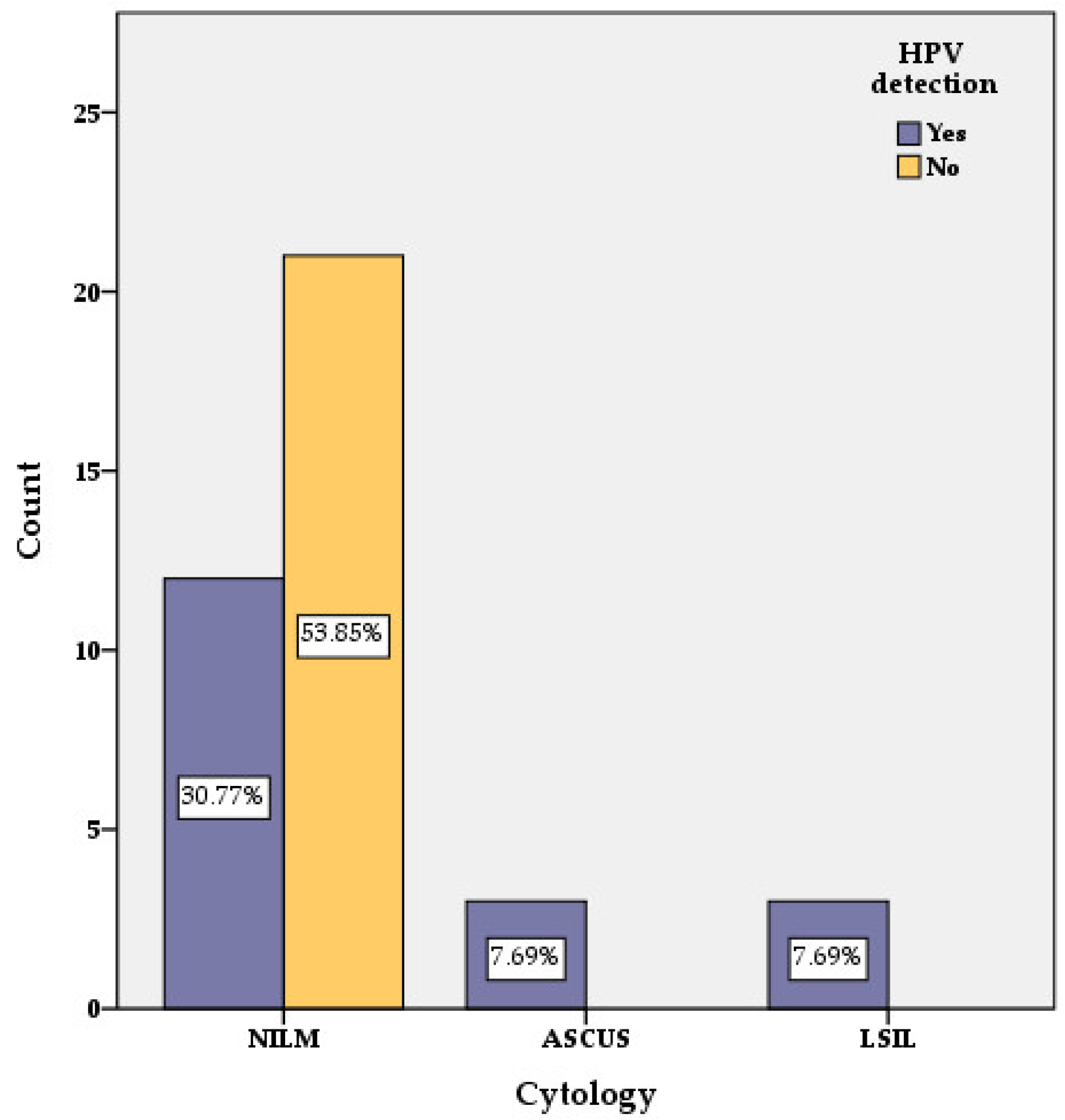
3.2. PAP Cytology
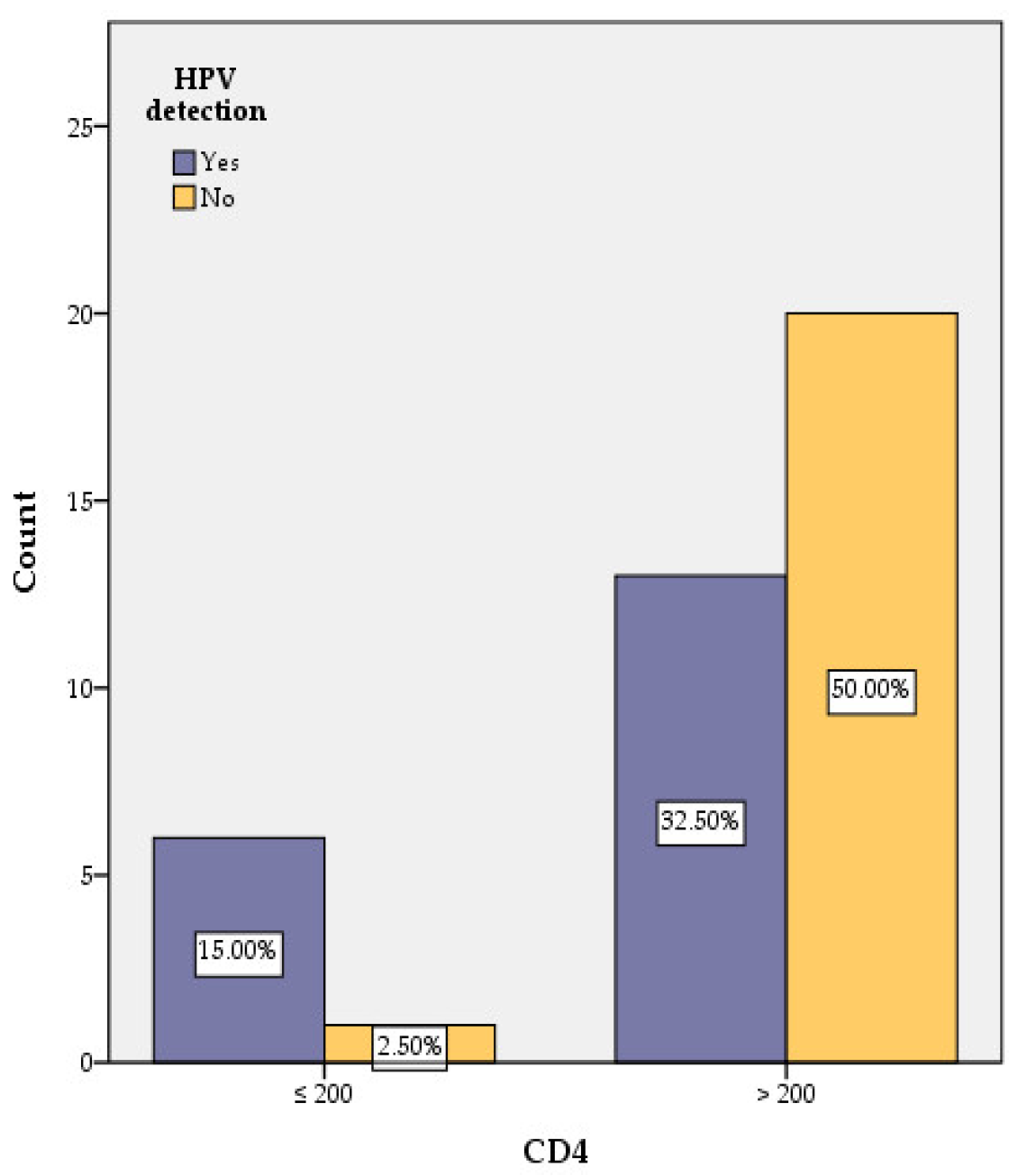
3.3. Correlation of HPV with CD4 Counts and HIV Viral Load
3.4. Correlation of HPV with Antiretroviral Therapy and Duration of HIV Detection
3.5. Correlation of HPV with Sexual Activity (Number of Sexual Partners and Age at First Sexual Intercourse)
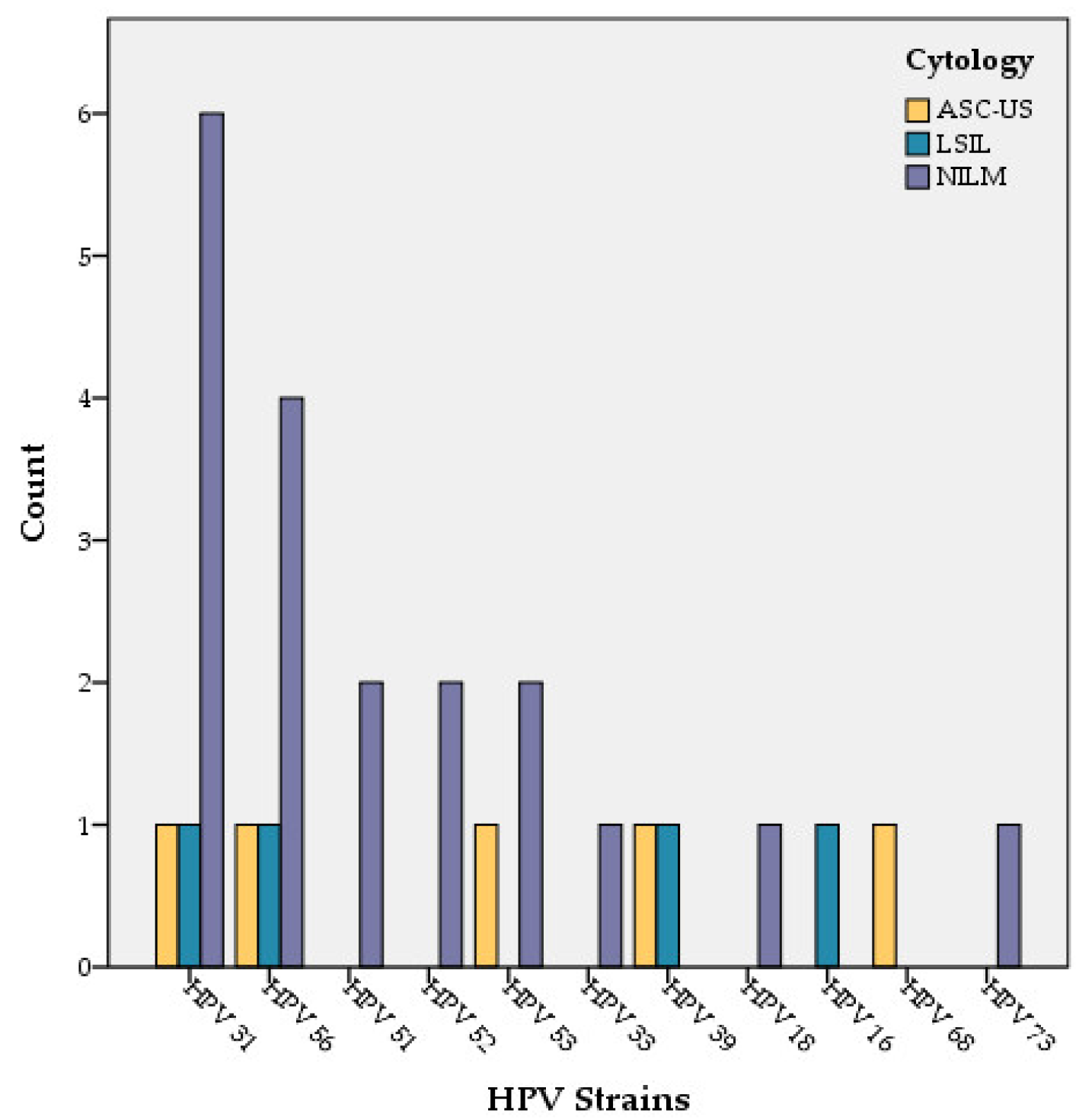
3.6. Correlation of HPV with Cytobacteriological Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, G.Y.; Bierman, R.; Beardsley, L.; Chang, C.J.; Burk, R.D. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998, 338, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.; Clifford, G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int. J. Cancer 2011, 128, 927–935. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human Papillomaviruses. Lyon (FR): International Agency for Research on Cancer. 2007. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 90.) 1, Human Papillomavirus (HPV) Infection. Available online: https://www.ncbi.nlm.nih.gov/books/NBK321770/ (accessed on 10 October 2021).
- Hausen, H.Z. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 2000, 92, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D., Jr.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef]
- Tate, D.R.; Anderson, R.J. Recrudescence of cervical dysplasia among women who are infected with the human immunodeficiency virus: A case-control analysis. Am. J. Obstet. Gynecol. 2002, 186, 880–882. [Google Scholar] [CrossRef]
- Choudhury, S.A.; Choudhury, N.A.; Humphrey, A.D.; Berthaud, V.; Ladson, G.; Tucker, V.A.; Vermund, S.H. Higher Prevalence of Human Papillomavirus-Related Cervical Precancerous Abnormalities in HIV-Infected Compared to HIV-Uninfected Women. J. Natl. Med. Assoc. 2016, 108, 19–23. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Duerr, A.; Burk, R.; Klein, R.S.; Paramsothy, P.; Schuman, P.; Cu-Uvin, S.; Shah, K. Characterization of genital human papillomavirus infection in women who have or who are at risk of having HIV infection. Am. J. Obstet. Gynecol. 2002, 186, 21–27. [Google Scholar] [CrossRef]
- HPV, HIV and Cervical Cancer. Available online: https://www.unaids.org/sites/default/files/media_asset/JC2851_HPV-HIV-cervicalcancer_en.pdf (accessed on 10 October 2021).
- Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb. Mortal. Wkly. Rep. 1992, 41, 1–19. [Google Scholar]
- The Nobel Prize in Physiology or Medicine 2008. NobelPrize.org. Nobel Prize Outreach AB 2022. Wed. 4 May 2022. Available online: https://www.nobelprize.org/prizes/medicine/2008/summary/ (accessed on 17 April 2022).
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Romania. Summary Report. 22 October 2021. Available online: https://hpvcentre.net/statistics/reports/ROU.pdf (accessed on 15 November 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ellerbrock, T.V.; Chiasson, M.A.; Bush, T.J.; Sun, X.-W.; Sawo, D.; Brudney, K.; Wright, J.T.C. Incidence of Cervical Squamous Intraepithelial Lesions in HIV-Infected Women. JAMA 2000, 283, 1031–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strickler, H.D.; Burk, R.D.; Fazzari, M.; Anastos, K.; Minkoff, H.; Massad, L.S.; Hall, C.; Bacon, M.; Levine, A.M.; Watts, D.H.; et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J. Natl. Cancer Inst. 2005, 97, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palefsky, J.M. Cervical human papillomavirus infection and cervical intraepithelial neoplasia in women positive for human immunodeficiency virus in the era of highly active antiretroviral therapy. Curr. Opin. Oncol. 2003, 15, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Heard, I.; Palefsky, J.M.; Kazatchkine, M.D. The impact of HIV antiviral therapy on human papillomavirus (HPV) infections and HPV-related diseases. Antivir. Ther. 2004, 9, 13–22. [Google Scholar] [CrossRef]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/health-topics/cervical-cancer#tab=tab_1 (accessed on 20 September 2021).
- Nayar, R.; Wilbur, D.C. The Pap Test and Bethesda 2014. Acta Cytol. 2015, 59, 121–132. [Google Scholar] [CrossRef]
- Halichidis, S.; Cambrea, S.C.; Resul, G.; Mocanu, L.; Costandache, I.; Ceamitru, N.; Arghir, O.C. Correlations between Babes-Papanicolaou’s findings and immunosuppression in Human Immunodeficiency Virus infected women. Gyneco.eu 2013, 9, 122–127. [Google Scholar] [CrossRef]
- Boşoteanu, M.; Boşoteanu, C.; Deacu, M.; Aşchie, M. The importance of monitoring protocols in cervical carcinoma screening. Rom. J. Morphol. Embryol. 2011, 52 (Suppl. 1), 297–302. [Google Scholar]
- Stolnicu, S.; Musca, S.; Micu, D.; Micu, L.; Moldovan, C.; Puscasiu, L. Prevalence of abnormal PAP smears in a consecutive and previously unscreened population in Romania. Int. J. Gynecol. Obstet. 2014, 124, 156–159. [Google Scholar] [CrossRef]
- Massad, L.S.; Ahdieh, L.; Benning, L.; Minkoff, H.; Greenblatt, R.M.; Watts, H.; Miotti, P.; Anastos, K.; Moxley, M.; Muderspach, L.I.; et al. Evolution of cervical abnormalities among women with HIV-1: Evidence from surveillance cytology in the women’s interagency HIV study. J. Acquir. Immune Defic. Syndr. 2001, 27, 432–442. [Google Scholar] [CrossRef]
- Ene, L.; Voinea, C.; Stefanescu, C.; Sima, D.; Duiculescu, D.; Mehta, S.R. Cervical HPV infection in Romanian women infected with HIV during early childhood. Int. J. STD AIDS 2016, 27, 1079–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursu, R.G.; Onofriescu, M.; Nemescu, D.; Iancu, L.S. HPV prevalence and type distribution in women with or without cervical lesions in the Northeast region of Romania. Virol. J. 2011, 8, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feticu, L.; Bocşan, I.S.; Bondor, C.; Boboş, C. Prevalenţa virusurilor papilloma umane izolate din leziunile colului uterin la o populaţie feminină din Transilvania (Prevalence of human papilloma virus isolated from cervix lesions in a female population from Transilvania). Rev. Med. Chir. Soc. Med. Nat. Iasi. 2012, 116, 567–574. (In Romanian) [Google Scholar] [PubMed]
- Boda, D.; Neagu, M.; Constantin, C.; Voinescu, R.N.; Caruntu, C.; Zurac, S.; Spandidos, D.A.; Drakoulis, N.; Tsoukalas, D.; Tsatsakis, A.M. HPV strain distribution in patients with genital warts in a female population sample. Oncol. Lett. 2016, 12, 1779–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. 5), F12–F23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmas, M.C.; Larsen, C.; van Benthem, B.; Hamers, F.F.; Bergeron, C.; Poveda, J.D.; Anzén, B.; van den Hoek, A.; Meier, F.; Peña, J.M.; et al. Cervical squamous intraepithelial lesions in HIV-infected women: Prevalence, incidence and regression. European Study Group on Natural History of HIV Infection in Women. AIDS 2000, 14, 1775–1784. [Google Scholar] [CrossRef] [Green Version]
- Clifford, G.M.; Tully, S.; Franceschi, S. Carcinogenicity of Human Papillomavirus (HPV) Types in HIV-Positive Women: A Meta-Analysis from HPV Infection to Cervical Cancer. Clin. Infect. Dis. 2017, 64, 1228–1235. [Google Scholar] [CrossRef]
- Palefsky, J. Biology of HPV in HIV infection. Adv. Dent. Res. 2006, 19, 99–105. [Google Scholar] [CrossRef]
- Johnson, J.C.; Burnett, A.F.; Willet, G.D.; Young, M.A.; Doniger, J. High frequency of latent and clinical human papillomavirus cervical infections in immunocompromised human immunodeficiency virus-infected women. Obstet. Gynecol. 1992, 79, 321–327. [Google Scholar] [CrossRef]
- Vermund, S.H.; Kelley, K.F.; Klein, R.S.; Feingold, A.R.; Schreiber, K.; Munk, G.; Burk, R.D. High risk of human papillomavirus infection and cervical squamous intraepithelial lesions among women with symptomatic human immunodeficiency virus infection. Am. J. Obstet. Gynecol. 1991, 165, 392–400. [Google Scholar] [CrossRef]
- Minkoff, H.L.; Eisenberger-Matityahu, D.; Feldman, J.; Burk, R.; Clarke, L. Prevalence and incidence of gynecologic disorders among women infected with human immunodeficiency virus. Am. J. Obstet. Gynecol. 1999, 180, 824–836. [Google Scholar] [CrossRef]
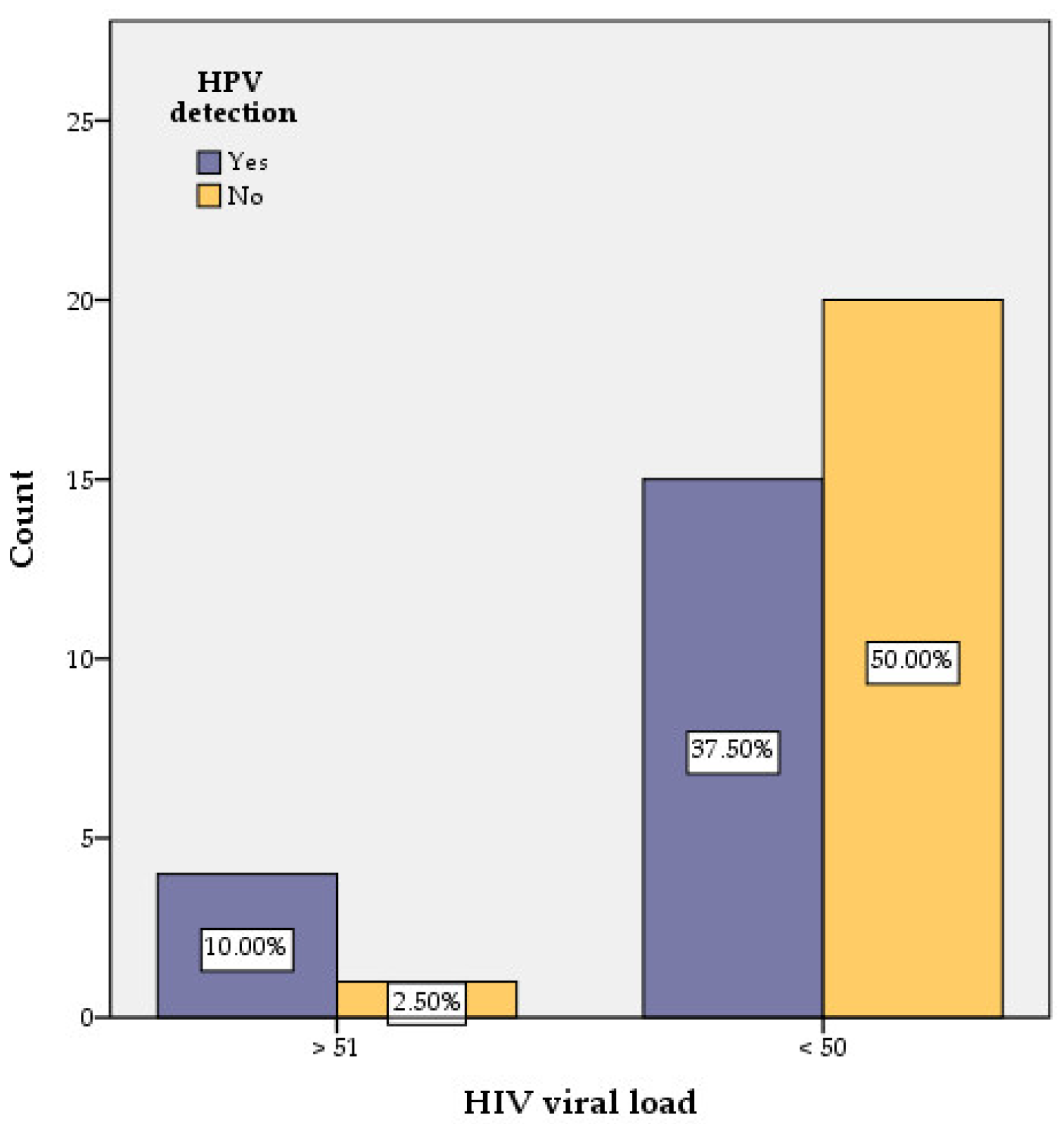
| Influencing Factors | Number of Women with HPV Detection | χ2calc | Odds Ratio | 95% Confidence Interval | Significance (Two-Sided) | |||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Lower | Upper | |||||
| CD4 | ≤200 cells/μL | 6 | 1 | 4.969 | 9.231 | 1.093 | 85.775 | 0.026 |
| >200 cells/μL | 13 | 20 | ||||||
| HIV viral load | >51 copies/mL | 4 | 1 | 2.420 | 5.333 | 0.539 | 52.734 | 0.120 |
| <50 copies/mL | 15 | 20 | ||||||
| First sexual intercourse | ≤18 years | 14 | 8 | 5.105 | 4.550 | 1.181 | 17.524 | 0.024 |
| >18 years | 5 | 13 | ||||||
| No. of sexual partners | >2 partners | 15 | 10 | 4.177 | 4.125 | 1.102 | 16.667 | 0.041 |
| ≤2 partners | 4 | 11 | ||||||
| Vaginal candidiasis | Yes | 9 | 3 | 5.199 | 5.4 | 1.183 | 24.645 | 0.023 |
| No | 10 | 18 | ||||||
| Gardnerella | Yes | 6 | 1 | 4.969 | 9.231 | 0.993 | 85.775 | 0.026 |
| No | 13 | 20 | ||||||
| Case Number | HPV Types | PAP Cytology |
|---|---|---|
| 04 | 56 | LSIL |
| 05 | 56 | NILM |
| 06 | 56 | NILM |
| 07 | 16, 31 | LSIL |
| 08 | 51 | NILM |
| 09 | 31 | NILM |
| 10 | 31, 56 | ASCUS |
| 11 | 18, 33 | NILM |
| 16 | 51, 52, 53, 56 | NILM |
| 17 | 31 | NILM |
| 18 | 31 | NILM |
| 24 | 31 | NILM |
| 25 | 56 | NILM |
| 27 | 68 | ASCUS |
| 33 | 39 | LSIL |
| 36 | 39, 53 | ASCUS |
| 35 | 31 | NILM |
| 37 | 53, 73 | NILM |
| 39 | 31, 52 | NILM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cambrea, S.C.; Aschie, M.; Resul, G.; Mitroi, A.F.; Chisoi, A.; Nicolau, A.A.; Baltatescu, G.I.; Cretu, A.M.; Lupasteanu, G.; Serbanescu, L.; et al. HPV and HIV Coinfection in Women from a Southeast Region of Romania—PICOPIV Study. Medicina 2022, 58, 760. https://doi.org/10.3390/medicina58060760
Cambrea SC, Aschie M, Resul G, Mitroi AF, Chisoi A, Nicolau AA, Baltatescu GI, Cretu AM, Lupasteanu G, Serbanescu L, et al. HPV and HIV Coinfection in Women from a Southeast Region of Romania—PICOPIV Study. Medicina. 2022; 58(6):760. https://doi.org/10.3390/medicina58060760
Chicago/Turabian StyleCambrea, Simona Claudia, Mariana Aschie, Ghiulendan Resul, Anca Florentina Mitroi, Anca Chisoi, Antonela Anca Nicolau, Gabriela Izabela Baltatescu, Ana Maria Cretu, Gabriela Lupasteanu, Lucian Serbanescu, and et al. 2022. "HPV and HIV Coinfection in Women from a Southeast Region of Romania—PICOPIV Study" Medicina 58, no. 6: 760. https://doi.org/10.3390/medicina58060760
APA StyleCambrea, S. C., Aschie, M., Resul, G., Mitroi, A. F., Chisoi, A., Nicolau, A. A., Baltatescu, G. I., Cretu, A. M., Lupasteanu, G., Serbanescu, L., Manea, M., Topliceanu, S. T., Petcu, L. C., Pazara, L., & Cozaru, G. C. (2022). HPV and HIV Coinfection in Women from a Southeast Region of Romania—PICOPIV Study. Medicina, 58(6), 760. https://doi.org/10.3390/medicina58060760







