Minimally Invasive Spine Stabilization for Pyogenic Spondylodiscitis: A 23-Case Series and Review of Literature
Abstract
1. Introduction
2. Materials and Methods
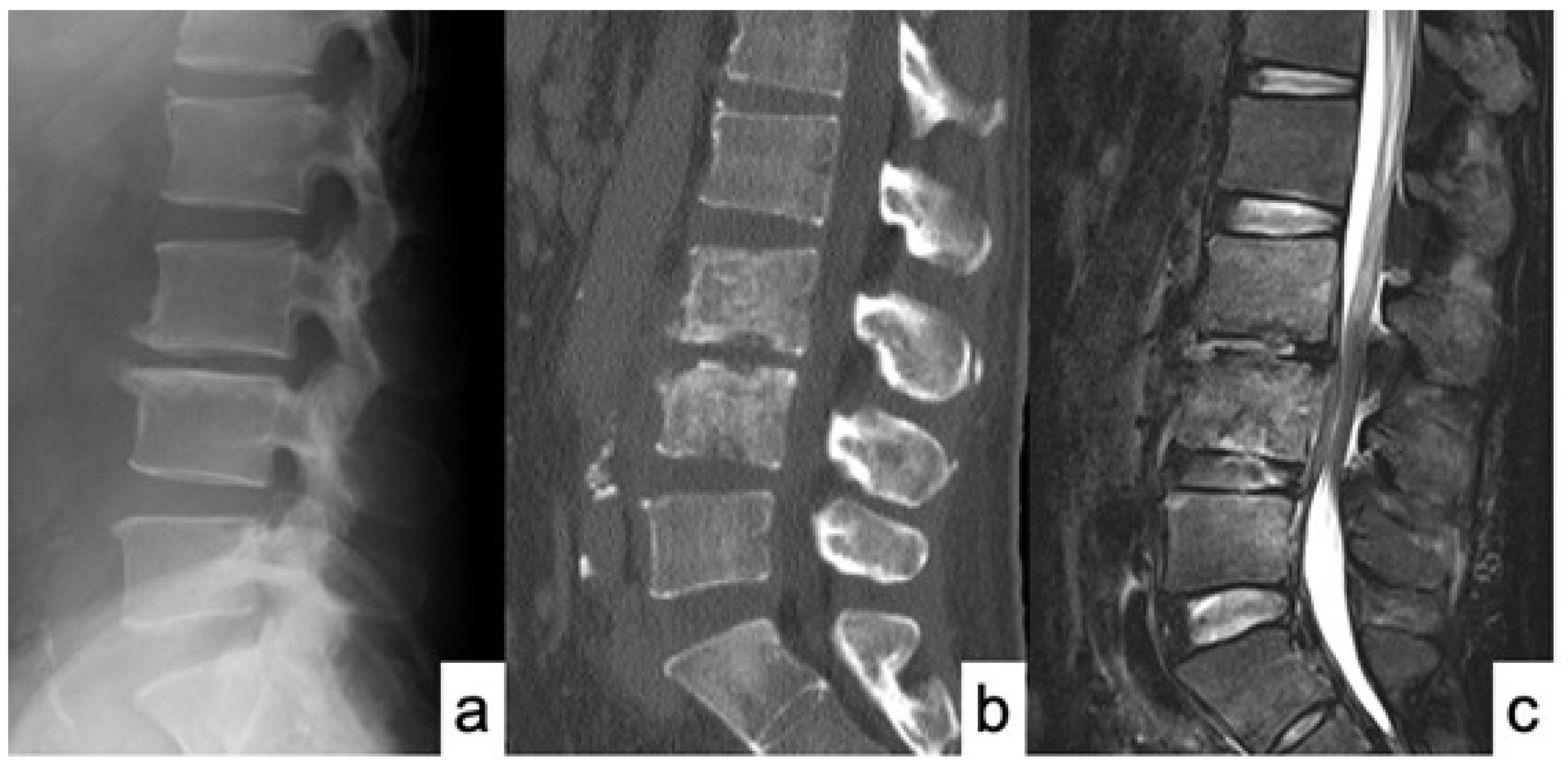
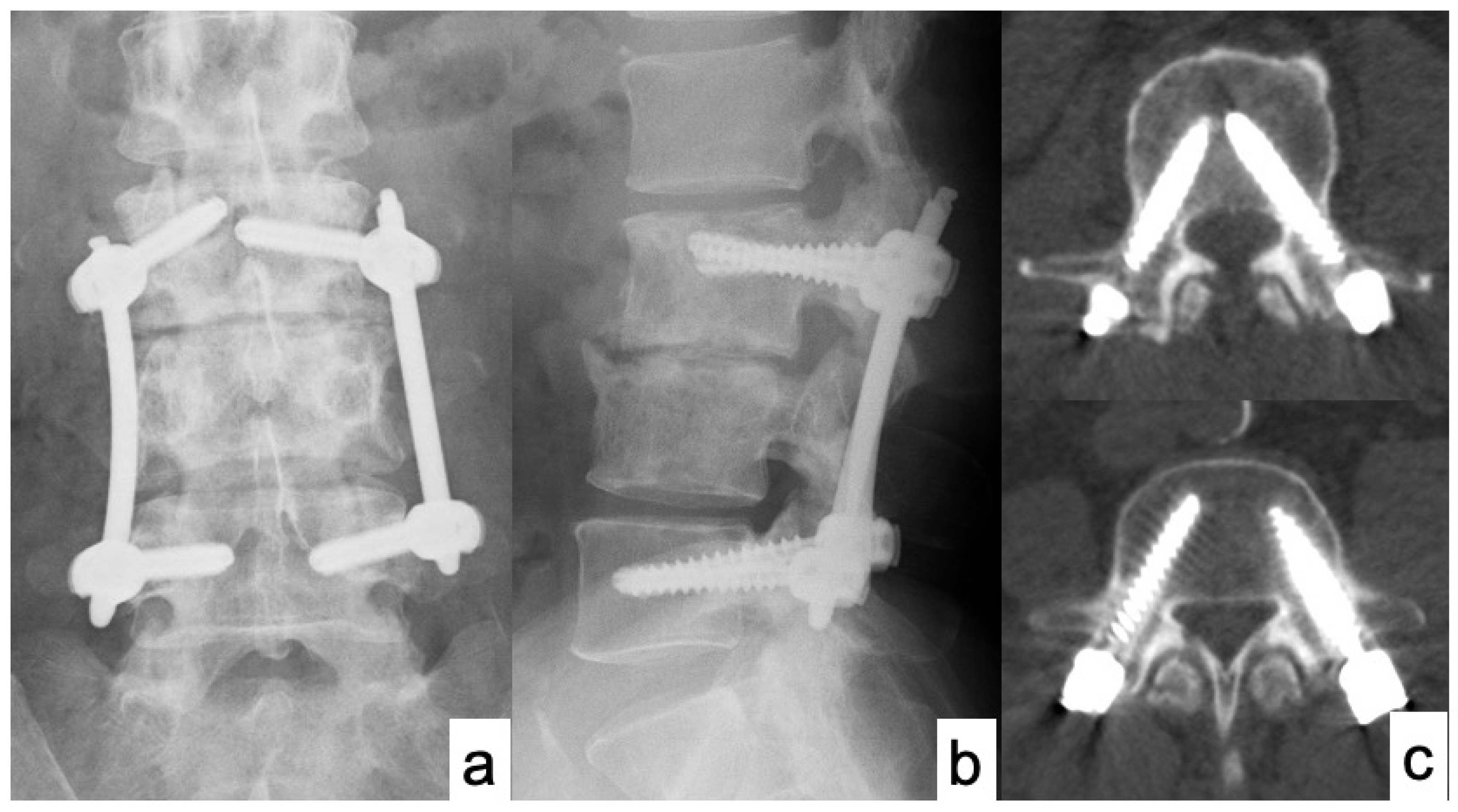
3. Results
Case
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gouliouris, T.; Aliyu, S.H.; Brown, N.M. Spondylodiscitis: Update on diagnosis and management. J. Antimicrob. Chemother. 2010, 65 (Suppl. 3), iii11–iii24. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.Y.; Luk, K.D.K. Pyogenic spondylitis. Int. Orthop. 2012, 36, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Papadopoulos, D.V.; Vrioni, G.; Sioutis, S.; Sapkas, G.; Benzakour, A.; Benzakour, T.; Angelini, A.; Ruggieri, P.; Mavrogenis, A.F.; et al. Spinal Infections: An Update. Microorganisms 2020, 8, 476. [Google Scholar] [CrossRef] [PubMed]
- Kourbeti, I.S.; Tsiodras, S.; Boumpas, D.T. Spinal infections: Evolving concepts. Curr. Opin. Rheumatol. 2008, 20, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Rutges, J.P.H.J.; Kempen, D.H.; van Dijk, M.; Oner, F.C. Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: A systematic literature review. Eur. Spine J. 2016, 25, 983–999. [Google Scholar] [CrossRef] [PubMed]
- Guerado, E.; Cerván, A.M. Surgical treatment of spondylodiscitis. An update. Int. Orthop. 2012, 36, 413–420. [Google Scholar] [CrossRef]
- Cervan, A.M.; Colmenero, J.D.; Del Arco, A.; Villanueva, F.; Guerado, E. Spondylodiscitis in patients under haemodyalisis. Int. Orthop. 2012, 36, 421–426. [Google Scholar] [CrossRef]
- Karchevsky, M.; Schweitzer, M.E.; Morrison, W.B.; Parellada, J.A. MRI findings of septic arthritis and associated osteomyelitis in adults. Am. J. Roentgenol. 2004, 182, 119–122. [Google Scholar] [CrossRef]
- Skaf, G.S.; Domloj, N.T.; Fehlings, M.G.; Bouclaous, C.H.; Sabbagh, A.S.; Kanafani, Z.A.; Kanj, S.S. Pyogenic spondylodiscitis: An overview. J. Infect. Public Health 2010, 3, 5–16. [Google Scholar] [CrossRef]
- Zarghooni, K.; Rollinghoff, M.; Sobottke, R.; Eysel, P. Treatment of spondylodiscitis. Int. Orthop. 2012, 36, 405–411. [Google Scholar] [CrossRef]
- Lin, T.Y.; Tsai, T.T.; Lu, M.L.; Niu, C.C.; Hsieh, M.K.; Fu, T.S.; Lai, P.L.; Chen, L.H.; Chen, W.J. Comparison of two-stage open versus percutaneous pedicle screw fixation in treating pyogenic spondylodiscitis. BMC Musculoskelet Disord. 2014, 15, 443. [Google Scholar] [CrossRef] [PubMed]
- Valancius, K.; Hansen, E.S.; Høy, K.; Helmig, P.; Niedermann, B.; Bünger, C. Failure modes in conservative and surgical management of infectious spondylodiscitis. Eur. Spine J. 2013, 22, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Herren, C.; Jung, N.; Pishnamaz, M.; Breuninger, M.; Siewe, J.; Sobottke, R. Spondylodiscitis: Diagnosis and Treatment Options. Dtsch. Arztebl. Int. 2017, 114, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Özmen, D.; Özkan, N.; Guberina, N.; Fliessbach, K.; Suntharalingam, S.; Theysohn, J.; Büchter, M.; Forsting, M.; Buer, J.; Dudda, M.; et al. Computed-tomography-guided biopsy in suspected spondylodiscitis: Single-center experience including 201 biopsy procedures. Orthop. Rev. 2019, 11, 7793. [Google Scholar] [CrossRef]
- Tsai, T.T.; Yang, S.C.; Niu, C.C.; Lai, P.L.; Lee, M.H.; Chen, L.H.; Chen, W.J. Early surgery with antibiotics treatment had better clinical outcomes than antibiotics treatment alone in patients with pyogenic spondylodiscitis: A retrospective cohort study. BMC Musculoskelet Disord. 2017, 18, 175. [Google Scholar] [CrossRef]
- Guo, W.; Wang, M.; Chen, G.; Chen, K.H.; Wan, Y.; Chen, B.; Zou, X.; Peng, X. Early surgery with antibiotic medication was effective and efficient in treating pyogenic spondylodiscitis. BMC Musculoskelet Disord. 2021, 22, 288. [Google Scholar] [CrossRef]
- Butler, J.S.; Shelly, M.J.; Timlin, M.; Powderly, W.G.; O’Byrne, J.M.O. Nontuberculous pyogenic spinal infection in adults: A 12-year experience from a tertiary referral center. Spine 2006, 31, 2695–2700. [Google Scholar] [CrossRef]
- Flury, B.B.; Elzi, L.; Kolbe, M.; Frei, R.; Weisser, M.; Scharen, S.; Widmer, A.F.; Battegay, M. Is switching to an oral antibiotic regimen safe after 2 weeks of intravenous treatment for primary bacterial vertebral osteomyelitis? BMC Infect. Dis. 2014, 14, 226. [Google Scholar]
- Bettini, N.; Girardo, M.; Dema, E.; Cervellati, S. Evaluation of conservative treatment of non spcific spondylodiscitis. Eur. Spine J. 2009, 18 (Suppl. 1), 143–150. [Google Scholar] [CrossRef]
- O’Daly, B.J.; Morris, S.F.; O’Rourke, S.K. Long-term functional outcome in pyogenic spinal infection. Spine 2008, 33, E246–E253. [Google Scholar] [CrossRef]
- Cottle, L.; Riordan, T. Infectious spondylodiscitis. J. Infect. 2008, 56, 401–412. [Google Scholar] [CrossRef]
- Karadimas, E.J.; Bunger, C.; Lindblad, B.E.; Høy, K.; Helmig, P.; Kannerup, A.S.; Niedermann, B. Spondylodiscitis. A retrospective study of 163 patients. Acta Orthop. 2008, 79, 650–659. [Google Scholar] [CrossRef]
- Pee, Y.H.; Park, J.D.; Choi, Y.G.; Lee, S.H. Anterior debridement and fusion followed by posterior pedicle screw fixation in pyogenic spondylodiscitis: Autologous iliac bone strut versus cage. J. Neurosurg. Spine 2008, 8, 405–412. [Google Scholar] [CrossRef]
- Gonzalvo, A.; Abdulla, I.; Riazi, A.; De La Harpe, D. Single- level/single-stage debridement and posterior instrumented fusion in the treatment of spontaneous pyogenic osteomyelitis/discitis: Long-term functional outcome and health-related quality of life. J. Spinal Disord. Tech. 2011, 24, 110–115. [Google Scholar] [CrossRef]
- Duan, K.; Qin, Y.; Ye, J.; Zhang, W.; Hu, X.; Zhou, J.; Gao, L.; Tang, Y. Percutaneous endoscopic debridement with percutaneous pedicle screw fixation for lumbar pyogenic spondylodiscitis: A preliminary study. Int. Orthop. 2020, 44, 495–502. [Google Scholar] [CrossRef]
- Janssen, I.K.; Jörger, A.K.; Barz, M.; Sarkar, C.; Wostrack, M.; Meyer, B. Minimally invasive posterior pedicle screw fixation versus open instrumentation in patients with thoracolumbar spondylodiscitis. Acta Neurochir. 2021, 163, 1553–1560. [Google Scholar] [CrossRef]
- Farshad, M.; Aichmair, A.; Gerber, C.; Bauer, D.E. Classification of perioperative complications in spine surgery. Spine J. 2020, 20, 730–736. [Google Scholar] [CrossRef]
- Bernard, L.; Dinh, A.; Ghout, I.; Simo, D.; Zeller, V.; Issartel, B.; Le Moing, V.; Belmatoug, N.; Lesprit, P.; Bru, J.P.; et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: An open-label, non-inferiority, randomised, controlled trial. Lancet 2015, 385, 875–882. [Google Scholar] [CrossRef]
- Loibl, M.; Stoyanov, L.; Doenitz, C.; Brawanski, A.; Wiggermann, P.; Krutsch, W.; Nerlich, M.; Oszwald, M.; Neumann, C.; Salzberger, B.; et al. Outcome-related co-factors in 105 cases of vertebral osteomyelitis in a tertiary care hospital. Infection 2014, 42, 503–510. [Google Scholar] [CrossRef]
- Roblot, F.; Besnier, J.M.; Juhel, L.; Vidal, C.; Ragot, S.; Bastides, F.; Le Moal, G.; Godet, C.; Mulleman, D.; Azais, I.; et al. Optimal duration of antibiotic therapy in vertebral osteomyelitis. Semin. Arthritis Rheum. 2007, 36, 269–277. [Google Scholar] [CrossRef]
- Park, K.H.; Chong, Y.P.; Kim, S.H.; Lee, S.O.; Choi, S.H.; Lee, M.S.; Jeong, J.Y.; Woo, J.H.; Kim, Y.S. Clinical characteristics and therapeutic outcomes of hematogenous vertebral osteomyelitis caused by methicillin-resistant Staphylococcus aureus. J. Infect. 2013, 67, 556–564. [Google Scholar] [CrossRef]
- Funao, H.; Kebaish, K.M.; Isogai, N.; Koyanagi, T.; Matsumoto, M.; Ishii, K. Utilization of a technique of percutaneous S2 alariliacfixation in immunocompromised patients with spondylodiscitis. World Neurosurg. 2017, 97, e11–e18. [Google Scholar] [CrossRef]
- Shinohara, A.; Ueno, Y.; Marumo, K. Weekly teriparatide therapy rapidly accelerates bone healing in pyogenic spondylitis with severe osteoporosis. Asian Spine J. 2014, 8, 498–501. [Google Scholar] [CrossRef]
- Morita, M.; Iwasaki, R.; Sato, Y.; Kobayashi, T.; Watanabe, R.; Oike, T.; Nakamura, S.; Keneko, Y.; Miyamoto, K.; Ishihara, K.; et al. Elevation of pro-inflammatory cytokine levels following anti-resorptive drug treatment is required for osteonecrosis development in infectious osteomyelitis. Sci. Rep. 2017, 7, 46322. [Google Scholar] [CrossRef]

| Characteristic | N = 23 (%) |
|---|---|
| Gender | |
| Male | 18 (78.3%) |
| Female | 5 (21.7%) |
| Comorbidities (including duplications) | |
| Solid cancer | 8 (34.8%) |
| Diabetes mellitus | 5 (21.7%) |
| Renal failure | 3 (13.0%) |
| Cerebrovascular disease | 3 (13.0%) |
| Liver cirrhosis | 2 (8.7%) |
| Angina pectoris | 1 (4.3%) |
| Pancreatitis | 1 (4.3%) |
| Depression | 1 (4.3%) |
| Location (including 1 duplication) | |
| Thoracic | 4 (17.4%) |
| Thoracolumbar | 4 (17.4%) |
| Lumbar | 11 (47.8%) |
| Lumbosacral | 5 (21.7%) |
| Bacterial Strain | N = 23 (%) |
|---|---|
| Staphylococcus aureus | 6 (26.1%) |
| MRSA 1 | 3 (13.0%) |
| Streptococcus dysgalactiae | 2 (8.7%) |
| Streptococcus intermedius | 1 (4.3%) |
| Streptococcus mutans | 1 (4.3%) |
| Escherichia coli | 1 (4.3%) |
| Enterobacter aerogens | 1 (4.3%) |
| Corynebacterium | 1 (4.3%) |
| Unknown | 7 (30.4%) |
| Patient No. | Age | Sex | Involved Level | Fixed Vertebrae | Organism | Comorbidities | CRP Become Negative (Days) | Operative Time (min) | EBL (mL) | ADF | F-U (Month) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | M | T7-8 | T5-10 | Unknown | 27 | 140 | 50 | No | 6 | |
| 2 | 66 | M | T8-9 | T6-11 | MRSA | Infectious endocarditis | 16 | 122 | 5 | No | 6 |
| 3 | 85 | F | T9-10 | T6-L1 | S. aureus | Prostate cancer | 28 | 258 | 70 | No | 24 |
| 4 | 78 | M | T9-10 | T7-12 | MRSA | Pancreatitis | 14 | 130 | 300 | No | 7 |
| 5 | 75 | F | T9-11 | T6-L2 | Unknown | 12 | 278 | 130 | No | 6 | |
| 6 | 84 | M | T10-11 | T7-L2 | S. aureus | DM | 28 | 265 | 384 | No | 26 |
| 7 | 69 | M | T10-11 | T7-L2 | Unknown | RF and CD | 25 | 282 | 67 | No | 18 |
| 8 | 64 | M | L1-2 | L1-3 | S. aureus | Liver Cancer | 39 | 65 | 20 | Yes | 6 |
| 9 | 60 | M | L2-3 | T12-L5 | S. aureus | 11 | 212 | 52 | No | 6 | |
| 10 | 62 | M | L2-3 L5-S1 | T12-S1 (Iliac) | S. dysgalactiae | RF | 56 | 364 | 260 | No | 6 |
| 11 | 55 | M | L3-4 | L1-S1 | E. aerogenes | Colon cancer | 47 | 183 | 158 | Yes | 43 |
| 12 | 57 | M | L3-4 | L1-5 | S. mutans | Liver cancer | 10 | 245 | 100 | No | 16 |
| 13 | 75 | M | L3-4 | L1-5 | S. dysgalactiae | DM | 51 | 154 | 280 | No | 15 |
| 14 | 50 | M | L3-5 | L3-5 | S. intermedius | DM and Liver cancer | 56 | 85 | 90 | No | 12 |
| 15 | 39 | F | L4-5 | L4-5 | S. aureus | Depression | 19 | 55 | 20 | No | 18 |
| 16 | 77 | M | L4-5 | L2-S1 (S2AI *) | S. aureus | Lung cancer | 20 | 193 | 26 | No | 6 |
| 17 | 72 | M | L4-5 | L2-S1 (S2AI *) | Unknown | Gastric cancer | 30 | 181 | 10 | Yes | 24 |
| 18 | 57 | F | L4-5 | L2-S1 (S2AI *) | Unknown | 30 | 215 | 43 | No | 18 | |
| 19 | 77 | M | L4-5 | L2-S1 (S2AI *) | Unknown | Lung cancer and angina | 30 | 188 | 26 | No | 6 |
| 20 | 77 | M | L5-S1 | L3-S1 (Illiac) | Unknown | DM | 14 | 265 | 340 | No | 13 |
| 21 | 59 | M | L5-S1 | L3-S1 (Illiac) | MRSA | CD | 30 | 399 | 550 | No | 24 |
| 22 | 69 | F | L5-S1 | L3-S1 (Illiac) | Coryne bacterium | Uterine cancer | 30 | 261 | 172 | No | 36 |
| 23 | 71 | M | L5-S1 | L3-S1 (S2AI *) | E. coli | DM, RF, and CD | 30 | 196 | 171 | Yes | 24 |
| Numbers of fixed vertebrae | 4.1 vertebrae (2–6) |
| Operative time | 205.1 min (55–399) |
| Estimated blood loss | 145.0 mL (5–550) |
| Anterior debridement and bone graft placement | 4 cases (17.4%) |
| CRP becomes negative after surgery | 28.4 days (10–56 days) |
| Major perioperative complication | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, S.; Funao, H.; Isogai, N.; Ishihara, M.; Saito, T.; Ishii, K. Minimally Invasive Spine Stabilization for Pyogenic Spondylodiscitis: A 23-Case Series and Review of Literature. Medicina 2022, 58, 754. https://doi.org/10.3390/medicina58060754
Ishihara S, Funao H, Isogai N, Ishihara M, Saito T, Ishii K. Minimally Invasive Spine Stabilization for Pyogenic Spondylodiscitis: A 23-Case Series and Review of Literature. Medicina. 2022; 58(6):754. https://doi.org/10.3390/medicina58060754
Chicago/Turabian StyleIshihara, Shinichi, Haruki Funao, Norihiro Isogai, Masayuki Ishihara, Takanori Saito, and Ken Ishii. 2022. "Minimally Invasive Spine Stabilization for Pyogenic Spondylodiscitis: A 23-Case Series and Review of Literature" Medicina 58, no. 6: 754. https://doi.org/10.3390/medicina58060754
APA StyleIshihara, S., Funao, H., Isogai, N., Ishihara, M., Saito, T., & Ishii, K. (2022). Minimally Invasive Spine Stabilization for Pyogenic Spondylodiscitis: A 23-Case Series and Review of Literature. Medicina, 58(6), 754. https://doi.org/10.3390/medicina58060754






