Oral Mucositis Induced by Chemoradiotherapy in Head and Neck Cancer—A Short Review about the Therapeutic Management and the Benefits of Bee Honey
Abstract
1. Introduction
2. Materials and Methods
3. Results
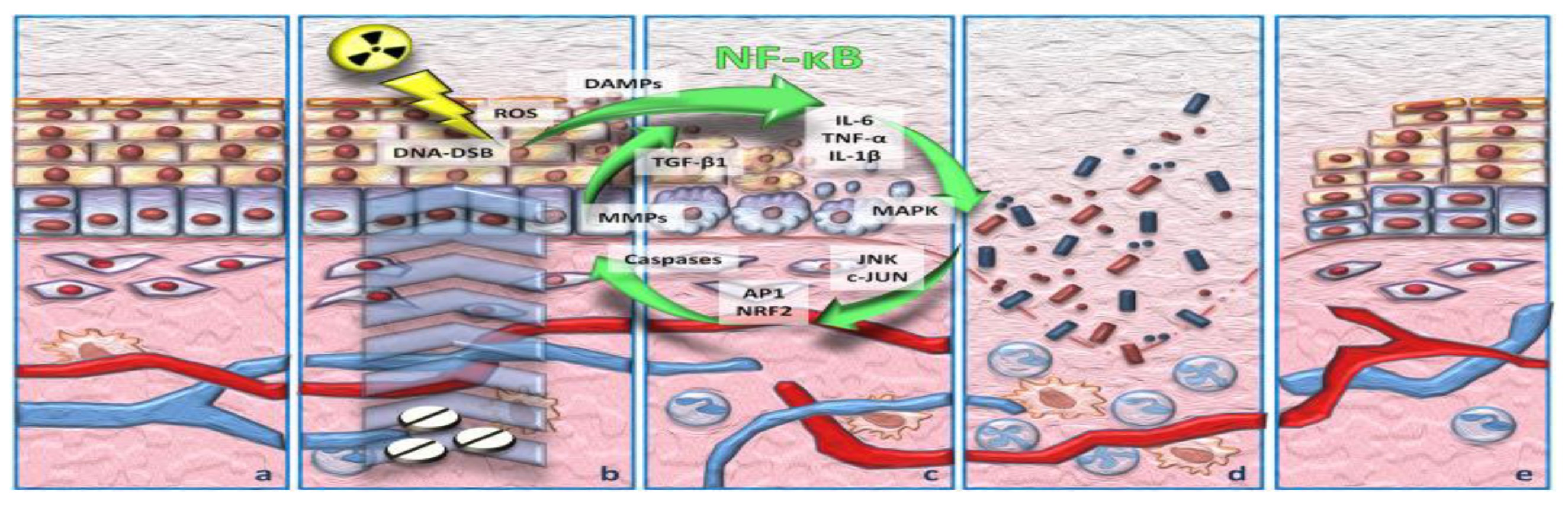
3.1. Incidence and Pathophysiology of Chemoradiotherapy-Induced Oral Mucositis
3.2. Risk Factors and Pathogenesis of Oral Mucositis
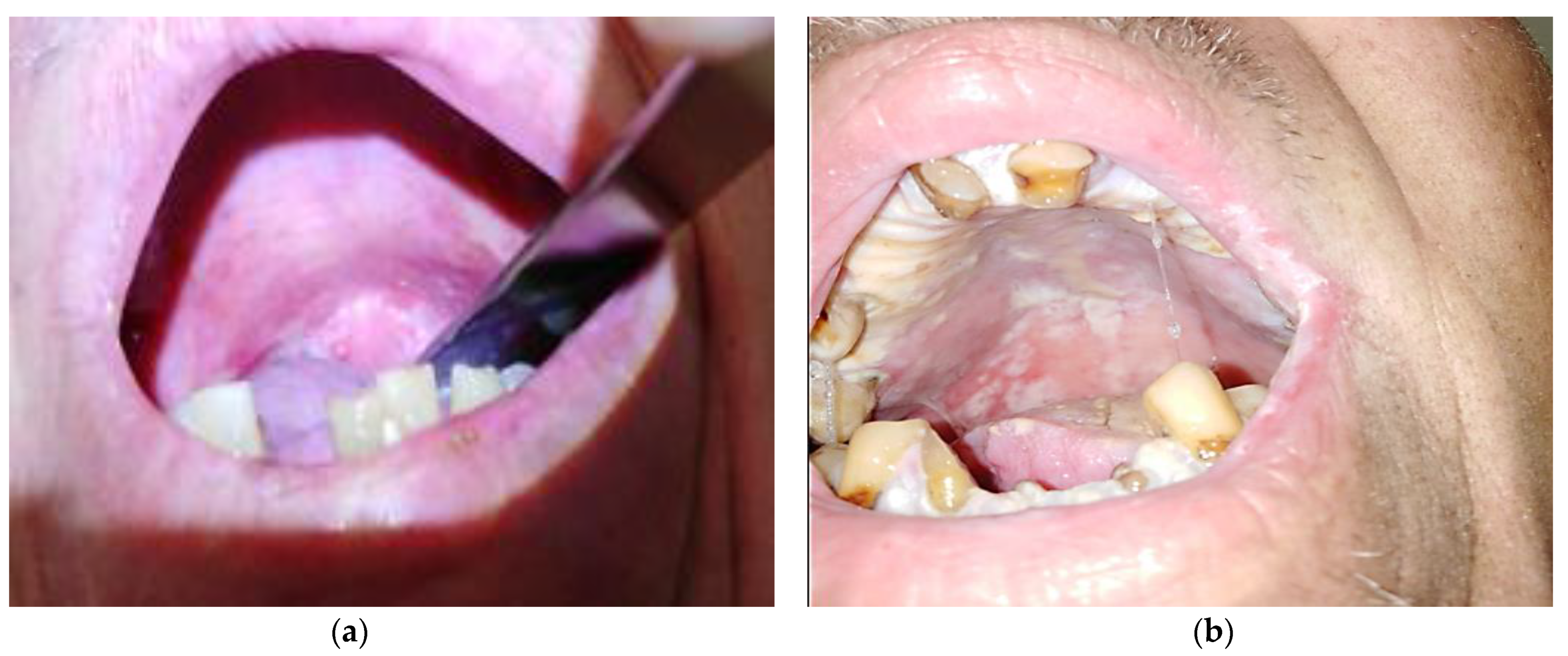
3.3. Tools Used to Assess OM
- The World Health Organization (WHO) scale evaluates OM as having 5 grades, from grade 0—normal mucosa, to grade 4 with deep lesions, when feeding the patient is not possible, making parenteral support necessary [28].
- The “Oral Assessment Guidelines” are used especially in pediatrics, where the degree of stomatitis and the condition of the oral cavity are assessed by inspecting the lips, oral commissures, tongue, the appearance of the oral mucosa membrane, saliva, gums, teeth, voice and swallowing process [29].
- The “Beck Assessment Scale”, in short BOAS, much similar to the previous one, is adapted, oral functionality being assessed with the help of local examination, registering scores from 5 to 20 [30].
- The “Oral Toxicity Scale” is used to evaluate the degree of oral stomatitis, similar to the previous ones. This instrument has been developed by Parulekar and uses symptomatic items for assessing the patients and sorting them by 5 grades [30].
3.4. Management of OM
3.5. Evidence Regarding the Effectiveness of Bee Honey in Preventing and Treating Chemoradiotherapy-Induced OM
3.6. Other Benefits of Honey
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jicman (Stan), D.; Niculet, E.; Lungu, M.; Onisor, C.; Rebegea, L.; Vesa, D.; Bezman, L.; Bujoreanu, F.C.; Sarbu, M.I.; Mihailov, R.; et al. Nasopharyngeal carcinoma: A new synthesis of literature data (Review). Exp. Ther. Med. 2022, 23, 136. [Google Scholar] [CrossRef] [PubMed]
- Rebegea, L.F.; Firescu, D.; Anghel, R.M.; Gales, L.; Ilie, A.M.; Dumitru, M.E.; Craescu, M.; Niculet, E.; Tatu, A.L.; Cretu, M.S.; et al. Clinical, histological and therapeutical aspects in the management of uterine and extrauterine stromal sarcomas: Case reports. Exp. Ther. Med. 2021, 22, 1456. [Google Scholar] [CrossRef]
- Koyfman, S.A.; Ismaila, N.; Crook, D.; D’Cruz, A.; Rodriguez, C.P.; Sher, D.J.; Silbermins, D.; Sturgis, E.M.; Tsue, T.T.; Weiss, J.; et al. Management of the Neck in Squamous Cell Carcinoma of the Oral Cavity and Oropharynx: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1753–1774. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Macedo, C.; Silva, A.M.; Delerue-Matos, C.; Costa, P.; Rodrigues, F. Natural Products for the Prevention and Treatment of Oral Mucositis—A Review. Int. J. Mol. Sci. 2022, 23, 4385. [Google Scholar] [CrossRef]
- Isozaki, A.B.; Brant, J.M. Clinical Updates in Mucositis-Related Symptom Management. Semin. Oncol. Nurs. 2022, 38, 151252. [Google Scholar] [CrossRef]
- Rebegea, L.; Firescu, D.; Dumitru, M.; Dumitrache, M.; Diaconu, C. Linear-quadratic model applied in reirradiation of brain meastases. Rom. J. Phys. 2015, 60, 1095–1102. [Google Scholar]
- Tatu, A.L.; Nwabudike, L.C. The treatment options of male genital lichen sclerosus et atrophicus: Treatments of genital lichen sclerosus. In Proceedings of the 14th National Congress of Urogynecology and the National Conference of the Romanian Association for the Study of Pain, Eforie, Romania, 26–27 October 2017; pp. 262–264. [Google Scholar]
- Amanat, A.; Ahmed, A.; Kazmi, A.; Aziz, B. The effect of honey on radiation-induced oral mucositis in head and neck cancer patients. Indian J. Palliat. Care 2017, 23, 317–320. [Google Scholar] [CrossRef]
- Hbibi, A.; Sikkou, K.; Khedid, K.; El Hamzaoui, S.; Bouziane, A.; Benazza, D. Antimicrobial activity of honey in periodontal disease: A systematic review. J. Antimicrob. Chemother. 2020, 75, 807–826. [Google Scholar] [CrossRef]
- Jibril, F.I.; Hilmi, A.B.M.; Manivannan, L. Isolation and characterization of polyphenols in natural honey for the treatment of human diseases. Bull. Natl. Res. Cent. 2019, 43, 4. [Google Scholar] [CrossRef]
- Hunter, M.; Kellett, J.; D’Cunha, N.M.; Toohey, K.; McKune, A.; Naumovski, N. The Effect of Honey as a Treatment for Oral Ulcerative Lesions: A Systematic Review. Explor. Res. Hypothesis Med. 2020, 5, 27–37. [Google Scholar] [CrossRef]
- Ciobotaru, O.R.; Lupu, M.-N.; Rebegea, L.; Ciobotaru, O.C.; Duca, O.M.; Tatu, A.L.; Voinescu, C.D.; Stoleriu, G.; Earar, K.; Miulescu, M. Dexamethasone-Chemical Structure and Mechanisms of Action in Prophylaxis of Postoperative Side Effects. Rev. Chim. 2019, 70, 843–847. [Google Scholar] [CrossRef]
- Kudva, A.K.; Rao, S.; Rao, P.; Pais, M.L.; Adnan, M.; Pai, K.S.R.; Baliga, M.S. Evidence for anticancer properties of honey with emphasis on mechanistic overview. Funct. Foods Cancer Prev. Ther. 2020, 7, 121–135. [Google Scholar] [CrossRef]
- Bissonnette, C.; McNamara, K.; Kalmar, J.R.; Grade, W.H.O. Oral Complications in Cancer Patients: A Review of Practical Interventions in the Dental Setting. J. Mich. Dent. Assoc. 2020, 37, 36–43. [Google Scholar]
- Available online: https://www.cancer.gov/about-cancer/treatment/drugs (accessed on 18 May 2021).
- Ahirwar, M.K.; Yogi, V.; Singh, O.P.; Ghori, H.U.; Tiwari, V.; Francis, B. Role of induction chemotherapy in downstaging of locally advanced head-and-neck squamous cell cancer. Clin. Cancer Investig. J. 2018, 7, 221–226. [Google Scholar] [CrossRef]
- Pulito, C.; Cristaudo, A.; La Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Scarpace, S.L.; Brodzik, F.A.; Mehdi, S.; Belgam, R. Treatment of Head and Neck Cancers: Issues for Clinical Pharmacists. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 578–592. [Google Scholar] [CrossRef]
- Münstedt, K.; Momm, F.; Hübner, J. Honey in the management of side effects of radiotherapy- or radio/chemotherapy-induced oral mucositis. A systematic review. Complement. Ther. Clin. Pract. 2018, 34, 145–152. [Google Scholar] [CrossRef]
- Al-Serwi, R.H.; Darwish, S.; Mahran, Y.F. Growth hormone modulates the inflammatory and apoptotic pathways incorporated in fluorouracil-induced oral mucositis in rats. Egypt. Dent. J. 2020, 66, 327–336. [Google Scholar] [CrossRef][Green Version]
- Riley, P. The Prevention of Oral Side Effects of Cancer Treatment. 2018. Available online: https://www.research.manchester.ac.uk (accessed on 18 May 2021).
- Kusiak, A.; Jereczek-Fossa, B.A.; Cichońska, D.; Alterio, D. Oncological-Therapy Related Oral Mucositis as an Interdisciplinary Problem—Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 2464. [Google Scholar] [CrossRef]
- Available online: https://oralcancerfoundation.org/ (accessed on 18 May 2021).
- Kawashita, Y.; Soutome, S.; Umeda, M.; Saito, T. Oral management strategies for radiotherapy of head and neck cancer. Jpn. Dent. Sci. Rev. 2020, 56, 62–67. [Google Scholar] [CrossRef]
- Hajisalem, T.; Ghaffary, S.; Nejati, B.; Mashayekhi, S.O.; Fathiazad, F.; Shokri, J.; Bateni, A. Effect of Achillea millefolium Mouthwash on Oral Mucositis Induced by Chemotherapy in AML Patients. Jundishapur J. Nat. Pharm. Prod. 2019, 14, e14077. [Google Scholar] [CrossRef]
- Shankar, A.; Roy, S.; Bhandari, M.; Rath, G.; Biswas, A.; Kanodia, R.; Adhikari, N.; Sachan, R. Current Trends in Management of Oral Mucositis in Cancer Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Razmara, F.; Khayamzadeh, M. An Investigation into the Prevalence and Treatment of Oral Mucositis After Cancer Treatment. Int. J. Cancer Manag. 2019, 12, e88405. [Google Scholar] [CrossRef]
- López-González, Á.; García-Quintanilla, M.; Guerrero-Agenjo, C.; Tendero, J.; Guisado-Requena, I.; Rabanales-Sotos, J. Eficacy of Cryotherapy in the Prevention of Oral Mucosistis in Adult Patients with Chemotherapy. Int. J. Environ. Res. Public Health 2021, 18, 994. [Google Scholar] [CrossRef] [PubMed]
- Sianturi, E.; Irawati, D. The The Effectiveness Of Oral Cryotherapy To Reduce Oral Mucositis Among Cancer Patients Undergoing Chemotherapy: A Literature Review. Int. J. Nurs. Health Serv. 2019, 2, 102–109. [Google Scholar] [CrossRef][Green Version]
- Mohammad, I.R.; Ahmed, N.M.; Magbool, F.R. Effect of Honey mouth wash on the health outcome of patient with stomatitis. Int. J. Nov. Res. Healthc. Nurs. 2020, 7, 210–219. [Google Scholar]
- Kim, S.; Nguyen, N.N.; Haider, A. Symptom control and palliative care in hematopoietic stem cell transplantation, in Hematopoietic Cell Transplantation for Malignant Conditions. Hematop. Cell Transplant. Malig. Cond. 2019, 27, 379–393. [Google Scholar] [CrossRef]
- Ndefo, U.A. Oral Mucositis: Update on Prevention and Management Strategies. U.S. Pharm. 2009, 34, 10–14. [Google Scholar]
- Peterson, D.E. Oral and Gastrointestinal Mucosal Adverse Effects. In Supportive Oncology; WB Saunders: Philadelphia, PA, USA, 2011; pp. 102–114. [Google Scholar]
- Peterson, D.E.; Boers-Doets, C.B.; Bensadoun, R.J.; Herrstedt, J.; ESMO Guidelines Committee. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. 2015, 26, v139–v151. [Google Scholar] [CrossRef]
- Pricop, R.; Cristea, V.C.; Gheorghe, I.; Tatu, A.L.; Mihaescu, G.; Chifiriuc, M.C. Matrix-assisted laser desorption/ionization time-of-flight mas spectrometry (MALDI-TOF MS) reveals the anaerobic Slakia exigua as unique etiology of a dental abscess. Biointerface Res. Appl. Chem. 2017, 7, 1995–1997. [Google Scholar]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Dmd, D.E.P.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Rn, K.K.F.C.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Dds, D.P.S.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Sener, D.K.; Aydin, M.; Cangur, S.; Guven, E. The Effect of Oral Care with Chlorhexidine, Vitamin E and Honey on Mucositis in Pediatric Intensive Care Patients: A Randomized Controlled Trial. J. Pediatr. Nurs. 2019, 45, e95–e101. [Google Scholar] [CrossRef]
- Howlader, D.; Singh, V.; Mohammad, S.; Gupta, S.; Pal, U.S.; Pal, M. Effect of Topical Application of Pure Honey in Chemo-radiation-Induced Mucositis and Its Clinical Benefits in Improving Quality of Life in Patients of Oral Squamous Cell Carcinoma. J. Maxillofac. Oral Surg. 2018, 18, 73–79. [Google Scholar] [CrossRef]
- Gupta, M.; Mamgain, R.K.; Mamgain, P.; Verma, S.K.; Pruthi, D.S.; Kandwal, A.; Saini, S. The efficacy of an ayurvedic preparation of yashtimadhu (Glycyrrhiza glabra) on radiation-induced mucositis in head-and-neck cancer patients: A pilot study. J. Cancer Res. Ther. 2020, 16, 458–462. [Google Scholar] [CrossRef]
- Khanal, L.; Yadav, P.; Baral, P.; Shah, R.; Rauniar, G.P. Effect of local bee honey on dihydrofolate reductase enzyme inhibitor-induced mucositis: A histological study on albino wistar rats. Indian J. Dent. Res. 2019, 30, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Ottaviani, G.; et al. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Systematic review of natural and miscellaneous agents, for the management of oral mucositis in cancer patients and clinical practice guidelines-part 2: Honey, herbal compounds, saliva stimulants, probiotics, and miscellaneous agents. Support. Care Cancer 2020, 28, 2457–2472. [Google Scholar] [CrossRef]
- Tharakan, T.; Bent, J.; Tavaluc, R. Honey as a Treatment in Otorhinolaryngology: A Review by Subspecialty. Ann. Otol. Rhinol. Laryngol. 2019, 128, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gong, G.; Jin, E.; Han, X.; Zhuo, Y.; Yang, S.; Song, B.; Zhang, Y.; Piao, C. Topical application of honey in the management of chemo/radiotherapy-induced oral mucositis: A systematic review and network meta-analysis. Int. J. Nurs. Stud. 2018, 89, 80–87. [Google Scholar] [CrossRef]
- Liu, T.-M.; Luo, Y.-W.; Tam, K.-W.; Lin, C.-C.; Huang, T.-W. Prophylactic and therapeutic effects of honey on radiochemotherapy-induced mucositis: A meta-analysis of randomized controlled trials. Support. Care Cancer 2019, 27, 2361–2370. [Google Scholar] [CrossRef]
- Yu, Y.T.; Deng, J.L.; Jin, X.R.; Zhang, Z.Z.; Zhang, X.H.; Zhou, X. Effects of 9 oral care solutions on the prevention of oral mucositis:A network meta-analysis of randomized controlled trials. Medicine 2020, 99, 19661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, D.; Qin, N.; Liu, M.; Zhang, J.; Li, X. Comparative prevention potential of 10 mouthwashes on intolerable oral mucositis in cancer patients: A Bayesian network analysis. Oral Oncol. 2020, 107, 104751. [Google Scholar] [CrossRef] [PubMed]
- Nwabudike, L.C.; Tatu, A.L. Magistral Prescription With Silver Nitrate and Peru Balsam in Difficult-to-Heal Diabetic Foot Ulcers. Am. J. Ther. 2018, 25, 679–680. [Google Scholar] [CrossRef]
- Al Jaouni, S.K.; Al Muhayawi, M.S.; Hussein, A.; Elfiki, I.; Al-Raddadi, R.; Al Muhayawi, S.M.; Almasaudi, S.; Kamal, M.A.; Harakeh, S. Effects of Honey on Oral Mucositis among Pediatric Cancer Patients Undergoing Chemo/Radiotherapy Treatment at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi Arabia. Evid.-Based Complement. Altern. Med. 2017, 2017, 5861024. [Google Scholar] [CrossRef] [PubMed]
- Bulut, H.K.; Tüfekci, F.G. Honey prevents oral mocositis in children undergoing chemotherapy: A quasi-experimental study with a control group. Complement. Ther. Med. 2016, 29, 132–140. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, S.U.; Saleh, T.A.; Fahad, S. Mad honey: Uses, intoxicating/poisoning effects, diagnosis, and treatment. RSC Adv. 2018, 8, 18635–18646. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef]
- Maiti, P.K.; Ray, A.; Mitra, T.N.; Jana, U.; Bhattacharya, J.; Ganguly, S. The effect of honey on mucositis induced by chemoradiation in head and neck cancer. J. Indian Med. Assoc. 2012, 110, 453–456. [Google Scholar]
- Motallebnejad, M.; Akram, S.; Moghadamnia, A.A.; Moulana, Z.; Omidi, S. The Effect of Topical Application of Pure Honey on Radiation-induced Mucositis: A Randomized Clinical Trial. J. Contemp. Dent. Pract. 2008, 9, 40–47. [Google Scholar] [CrossRef]
- Parsons, E.; Begley, A.; Herst, P. Manuka honey mouthwash does not affect oral mucositis in head and neck cancer patients in New Zealand. J. Radiother. Pract. 2011, 11, 249–256. [Google Scholar] [CrossRef]
- Bardy, J.; Molassiotis, A.; Ryder, W.D.; Mais, K.; Sykes, A.; Yap, B.; Lee, L.; Kaczmarski, E.; Slevin, N. A double-blind, placebo-controlled, randomised trial of active manuka honey and standard oral care for radiation-induced oral mucositis. Br. J. Oral Maxillofac. Surg. 2012, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Fakhlaei, R.; Selamat, J.; Khatib, A.; Razis, A.F.A.; Sukor, R.; Ahmad, S.; Babadi, A.A. The Toxic Impact of Honey Adulteration: A Review. Foods 2020, 9, 1538. [Google Scholar] [CrossRef] [PubMed]
- Nwabudike, L.; Tatu, A. Reply to Gambichler T et al.: Altered epigenetic pathways and cell cycle dysregulation in healthy appearing skin of patients with koebnerized squamous cell carcinomas following skin surgery. J. Eur. Acad. Dermatol. Venereol. 2018, 33, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Jain, D.; Mehani, R.; Lal Hingorani, H.; Thawani, V. Novel poly herbal muco-adhesive formulation for treatment of oral aphthous ulcer. Int. J. Basic Clin. Pharmacol. 2021, 10, 906–910. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Natural Compounds for Preventing Ear, Nose, and Throat-Related Oral Infections. Plants 2021, 10, 1847. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Flores, M.A.; Quintero-Cabello, K.P.; Palafox-Rivera, P.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Ortega-Ramirez, L.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Plant-Derived Substances with Antibacterial, Antioxidant, and Flavoring Potential to Formulate Oral Health Care Products. Biomedicines 2021, 9, 1669. [Google Scholar] [CrossRef] [PubMed]
- Mărgăoan, R.; Topal, E.; Balkanska, R.; Yücel, B.; Oravecz, T.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral Honeys as a Potential Source of Natural Antioxidants, Minerals and Medicine. Antioxidants 2021, 10, 1023. [Google Scholar] [CrossRef]
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| WHO | erythema and soreness | ulcers, able to eat solids | ulcers, requires a liquid diet (due to mucositis) | ulcers, alimentation not possible (due to mucositis) | |
| RTOG | (mild) irritation, mild pain, does not necessarily require analgesics | (moderate) patchy mucositis with inflammation and serosanguinal secretions, moderate pain may be present requiring analgesics | (severe) confluent or fibrinous stage of mucositis, with severe pain requiring narcotics | (life threatening) deep ulceration, bleeding, or necrosis | |
| OMAS Erythema Ulceration | Normal | <1 cm2 Not severe | 1–3 cm2 Severe | >3 cm2 | |
| NCI-CTCAE | erythema, painless ulcers or mild pain in the absence of lesions | edema, painful erythema, and ulcers, but patients may eat or swallow | severe ulcers present, the patient requires enteral/parenteral nutrition or prophylactic intubation | death caused by this toxicity |
| Article Type; Authors | Number of Patients or Studies Used | Oncological Treatment | Objective | Type of Honey and How to Use It in the Study Group | Substances Used in the Control Group | Results |
|---|---|---|---|---|---|---|
| 1. Prospective single-blind randomized control study; Howlader, D. et al. [39] | 40 patients divided into 2 arms | radiochemotherapy together with cisplatin-based chemotherapy 4 weeks after completion of induction chemotherapy | to assess clinical benefits and improve quality of life in patients with head and neck cancers after honey administration |
|
|
|
| 2. Prospective randomized control study Mamgain, R. K. et al. [40] | 150 patients initially enrolled randomly assigned to 3 arms | local EBRT at 6 MV LINAC by conventional fractionation, average dose = 60 Gy × 5 days/week 6 weeks concurrent with cisplatin | to evaluate the efficacy of Ayurvedic preparation in oral mucositis in head and neck cancer patients receiving concurrent chemoradiotherapy |
|
|
|
| 3. Murine model study Khanal, L. et al. [41] | 24 albino rats randomly assigned to 4 working arms | intraperitoneal methotrexate at a dose of 60 mg/kg | to demonstrate the efficacy of bee honey on chemotherapy-induced oral mucositis |
|
|
|
| 4. Systematic review Yarom, N. et al. [42] | 78 papers: 49 were included in this review +9 publications reported in the previous update of the guidelines describing 26 different interventions falling within the honey field | radiotherapy with or without chemotherapy in patients with head cancer pediatric patients with hematological or solid cancers treated with chemotherapy | to update the clinical practice guidelines for OM management that have been developed by MASCC/ISOO. This part focuses on honey, herbal compounds, saliva stimulants, probiotics, and miscellaneous agents |
|
|
|
| 5. Systematic review Münstedt, K. et al. [19] | 17 randomized trials | radiotherapy or radiotherapy with combined chemotherapy | to evaluate the efficacy of conventional bee honey or Manuka honey on radiochemotherapy-induced OM |
|
|
|
| 6. Systematic review Hunter, M. et al. [11] | 13 randomized controlled trials with 634 patients | chemotherapy or radiotherapy | to demonstrate the efficacy of bee honey on oral mucositis induced by chemotherapy or radiotherapy |
|
|
|
| 7. A subspecialty review Tharakan, T. et al. [43] | 13 randomized controlled trials with 634 patients | chemotherapy or radiotherapy | to demonstrate the efficacy of bee honey on oral mucositis induced by chemotherapy or radiotherapy |
|
|
|
| 8. A systematic review and network meta-analysis Yang, C. et al. [44] | 17 studies involving 1265 patients grouped into 13 arms | chemotherapy or radiotherapy | to demonstrate the efficacy of bee honey on oral mucositis induced by chemotherapy or radiotherapy |
|
|
|
| 9. A meta-analysis of randomized controlled trials Liu, Tzu-Ming et al. [45] | 19 randomized controlled trials with 1276 patients | radiochemotherapy | reduction of OM |
|
|
|
| 10. A network meta-analysis of randomized controlled trials Ya-Ying Yu et al. [46] | 28 randomized controlled trials with 1861 patients | radiochemotherapy | prevention and treatment of OM, evaluate the effect of different oral care solutions |
|
|
|
| 11. New systematic review and update the clinical guidelines Sharon Elad et al. [37] | 1197 randomized controlled trials with 1861 patients | radiochemotherapy | prevention and treatment of OM |
|
|
|
| 12. A Bayesiannetwork analysis Xu Zhang et al. [12] | 36 randomized controlled trials with 2594 patients | radiochemotherapy, total radiation dose =50 Gy | to compare the preventive effect of ten mouthwashes in intolerable OM |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jicman, D.; Sârbu, M.I.; Fotea, S.; Nechifor, A.; Bălan, G.; Anghele, M.; Vasile, C.I.; Niculeț, E.; Sârbu, N.; Rebegea, L.-F.; et al. Oral Mucositis Induced by Chemoradiotherapy in Head and Neck Cancer—A Short Review about the Therapeutic Management and the Benefits of Bee Honey. Medicina 2022, 58, 751. https://doi.org/10.3390/medicina58060751
Jicman D, Sârbu MI, Fotea S, Nechifor A, Bălan G, Anghele M, Vasile CI, Niculeț E, Sârbu N, Rebegea L-F, et al. Oral Mucositis Induced by Chemoradiotherapy in Head and Neck Cancer—A Short Review about the Therapeutic Management and the Benefits of Bee Honey. Medicina. 2022; 58(6):751. https://doi.org/10.3390/medicina58060751
Chicago/Turabian StyleJicman (Stan), Daniela, Mihaela Ionela Sârbu, Silvia Fotea, Alexandru Nechifor, Gabriela Bălan, Mihaela Anghele, Claudiu Ionuț Vasile, Elena Niculeț, Nicolae Sârbu, Laura-Florentina Rebegea, and et al. 2022. "Oral Mucositis Induced by Chemoradiotherapy in Head and Neck Cancer—A Short Review about the Therapeutic Management and the Benefits of Bee Honey" Medicina 58, no. 6: 751. https://doi.org/10.3390/medicina58060751
APA StyleJicman, D., Sârbu, M. I., Fotea, S., Nechifor, A., Bălan, G., Anghele, M., Vasile, C. I., Niculeț, E., Sârbu, N., Rebegea, L.-F., & Tatu, A. L. (2022). Oral Mucositis Induced by Chemoradiotherapy in Head and Neck Cancer—A Short Review about the Therapeutic Management and the Benefits of Bee Honey. Medicina, 58(6), 751. https://doi.org/10.3390/medicina58060751









