The Possible Effects of Zinc Supplementation on Postpartum Depression and Anemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Data
2.2. Statistical Analysis
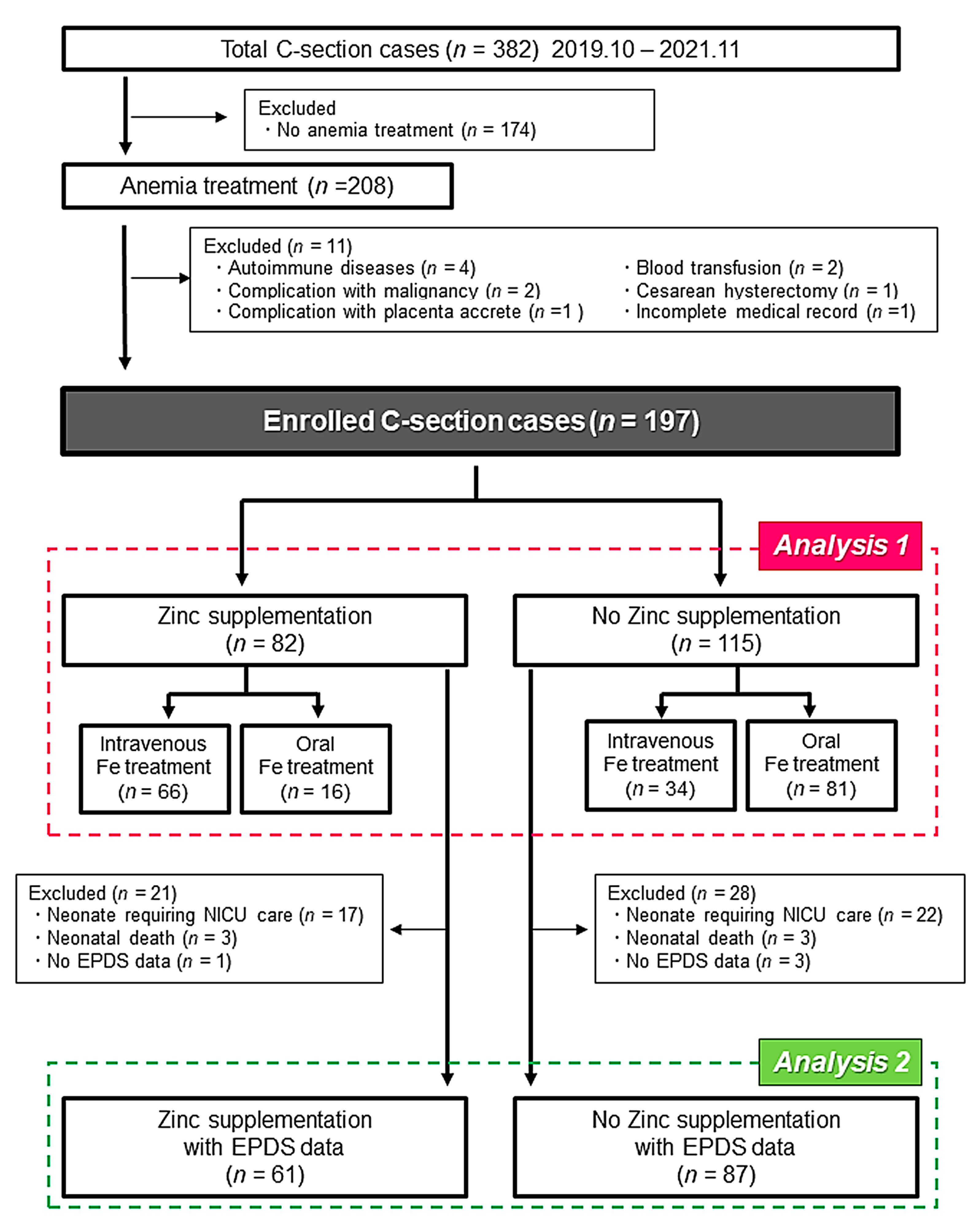
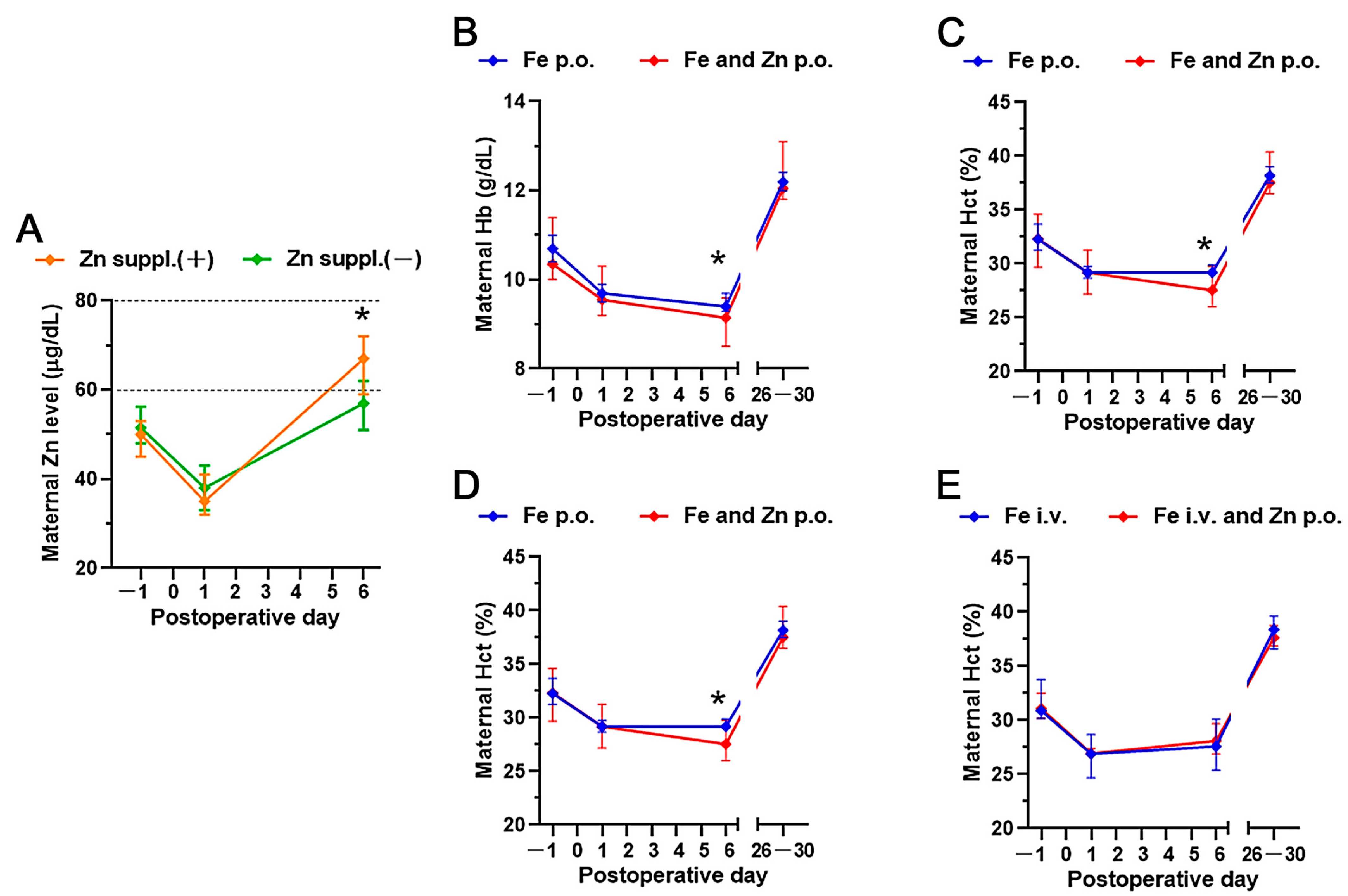
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The Role of Fe, Zn, and Cu in Pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.J.; Gong, B.; Xu, F.Y.; Luo, Y. Four trace elements in pregnant women and their relationships with adverse pregnancy outcomes. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4690–4697. [Google Scholar] [PubMed]
- Wilson, R.L.; Grieger, J.A.; Bianco-Miotto, T.; Roberts, C.T. Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy Complications: A Systematic Review. Nutrients 2016, 8, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlpine, J.M.; McKeating, D.R.; Vincze, L.; Vanderlelie, J.J.; Perkins, A.V. Essential Mineral Intake During Pregnancy and Its Association With Maternal Health and Birth Outcomes in South East Queensland, Australia. Nutr. Metab. Insights 2019, 12, 1178638819879444. [Google Scholar] [CrossRef]
- Caan, B.; Horgen, D.M.; Margen, S.; King, J.C.; Jewell, N.P. Benefits Associated with Wic Supplemental Feeding during the Interpregnancy Interval. Am. J. Clin. Nutr. 1987, 45, 29–41. [Google Scholar] [CrossRef]
- Irwinda, R.; Wibowo, N.; Putri, A.S. The Concentration of Micronutrients and Heavy Metals in Maternal Serum, Placenta, and Cord Blood: A Cross-Sectional Study in Preterm Birth. J. Pregnancy 2019, 2019, 5062365. [Google Scholar] [CrossRef] [Green Version]
- Carducci, B.; Keats, E.C.; Bhutta, Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2021, 3, 1–99. [Google Scholar] [CrossRef]
- Hess, S.Y.; King, J.C. Effects of maternal zinc supplementation on pregnancy and lactation outcomes. Food Nutr. Bull. 2009, 30, S60–S78. [Google Scholar] [CrossRef]
- Nishiyama, S.; Kiwaki, K.; Miyazaki, Y.; Hasuda, T. Zinc and IGF-I concentrations in pregnant women with anemia before and after supplementation with iron and or zinc. J. Am. Coll. Nutr. 1999, 18, 261–267. [Google Scholar] [CrossRef]
- Sparling, T.M.; Henschke, N.; Nesbitt, R.C.; Gabrysch, S. The role of diet and nutritional supplementation in perinatal depression: A systematic review. Matern. Child Nutr. 2017, 13, 1–36. [Google Scholar] [CrossRef]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442S–446S. [Google Scholar] [CrossRef] [PubMed]
- Roomruangwong, C.; Kanchanatawan, B.; Sirivichayakul, S.; Mahieu, B.; Nowak, G.; Maes, M. Lower Serum Zinc and Higher CRP Strongly Predict Prenatal Depression and Physio-somatic Symptoms, Which All Together Predict Postnatal Depressive Symptoms. Mol. Neurobiol. 2017, 54, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Ogawa, K.; Morisaki, N.; Tachibana, Y.; Horikawa, R.; Sago, H. Association between perinatal anemia and postpartum depression: A prospective cohort study of Japanese women. Int. J. Gynecol. Obstet. 2020, 148, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brummelte, S.; Galea, L.A.M. Postpartum depression: Etiology, treatment and consequences for maternal care. Horm. Behav. 2016, 77, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Evers, S.E.; Avison, W.R.; Campbell, M.K. Higher zinc intake buffers the impact of stress on depressive symptoms in pregnancy. Nutr. Res. 2010, 30, 695–704. [Google Scholar] [CrossRef]
- Ozeki, I.; Arakawa, T.; Suii, H.; Tatsumi, R.; Yamaguchi, M.; Nakajima, T.; Kuwata, Y.; Toyota, J. Zinc deficiency in patients with chronic liver disease in Japan. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2020, 50, 396–401. [Google Scholar] [CrossRef]
- Matsumura, K.; Hamazaki, K.; Tsuchida, A.; Kasamatsu, H.; Inadera, H.; Japan Environment and Children’s Study, G. Causal model of the association of social support during pregnancy with a perinatal and postpartum depressive state: A nationwide birth cohort-the Japan Environment and Children’s Study. J. Affect. Disord. 2022, 300, 540–550. [Google Scholar] [CrossRef]
- Grieger, J.A.; Clifton, V.L. A Review of the Impact of Dietary Intakes in Human Pregnancy on Infant Birthweight. Nutrients 2015, 7, 153–178. [Google Scholar] [CrossRef] [Green Version]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Josefsson, A.; Angelsioo, L.; Berg, G.; Ekstrom, C.M.; Gunnervik, C.; Nordin, C.; Sydsjo, G. Obstetric, somatic, and demographic risk factors for postpartum depressive symptoms. Obstet. Gynecol. 2002, 99, 223–228. [Google Scholar] [CrossRef]
- American Coll, O. Screening for Perinatal Depression. Obstet. Gynecol. 2018, 132, E208–E212. [Google Scholar] [CrossRef]
- Fuhr, D.C.; Calvert, C.; Ronsmans, C.; Chandra, P.S.; Sikander, S.; De Silva, M.J.; Patel, V. Contribution of suicide and injuries to pregnancy-related mortality in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Psychiatry 2014, 1, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, M.; Hantoushzadeh, S.; Shariat, M.; Farahani, Z.; Ebrahiminasab, O. The efficacy of early iron supplementation on postpartum depression, a randomized double-blind placebo-controlled trial. Eur. J. Nutr. 2017, 56, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Vandoolaeghe, E.; Neels, H.; Demedts, P.; Wauters, A.; Meltzer, H.Y.; Altamura, C.; Desnyder, R. Lower serum zinc in major depression is a sensitive marker of treatment resistance and of the immune/inflammatory response in that illness. Biol. Psychiatry 1997, 42, 349–358. [Google Scholar] [CrossRef]
- Swardfager, W.; Herrmann, N.; Mazereeuw, G.; Goldberger, K.; Harimoto, T.; Lanctot, K.L. Zinc in Depression: A Meta-Analysis. Biol. Psychiatry 2013, 74, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Nossier, S.A.; Naeim, N.E.; El-Sayed, N.A.; Abu Zeid, A.A. The effect of zinc supplementation on pregnancy outcomes: A double-blind, randomised controlled trial, Egypt. Br. J. Nutr. 2015, 114, 274–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merialdi, M.; Caulfield, L.E.; Zavaleta, N.; Figueroa, A.; Dominici, F.; DiPietro, J.A. Randomized controlled trial of prenatal zinc supplementation and the development of fetal heart rate. Am. J. Obstet. Gynecol. 2004, 190, 1106–1112. [Google Scholar] [CrossRef]
- Merialdi, M.; Caulfield, L.E.; Zavaleta, N.; Figueroa, A.; DiPietro, J.A. Adding zinc to prenatal iron and folate tablets improves fetal neurobehavioral development. Am. J. Obstet. Gynecol. 1999, 180, 483–490. [Google Scholar] [CrossRef]
- Fard, F.E.; Mirghafourvand, M.; Charandabi, S.M.A.; Farshbaf-Khalili, A.; Javadzadeh, Y.; Asgharian, H. Effects of zinc and magnesium supplements on postpartum depression and anxiety: A randomized controlled clinical trial. Women Health 2017, 57, 1115–1128. [Google Scholar] [CrossRef]
- Nikseresht, S.; Etebary, S.; Karimian, M.; Nabavizadeh, F.; Zarrindast, M.R.; Sadeghipour, H.R. Acute Administration of Zn, Mg, and Thiamine Improves Postpartum Depression Conditions in Mice. Arch. Iran. Med. 2012, 15, 306–311. [Google Scholar]
- Nowak, G.; Kubera, M.; Maes, M. Neuroimmunological aspects of the alterations in zinc homeostasis in the pathophysiology and treatment of depression. Acta Neuropsychiatr. 2000, 12, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J. Neurobiology of Zinc and Zinc-Containing Neurons. Int. Rev. Neurobiol. 1989, 31, 145–238. [Google Scholar] [PubMed]
- Bodiga, S.; Krishnapillai, M.N. Concurrent repletion of iron and zinc reduces intestinal oxidative damage in iron- and zinc-deficient rats. World J. Gastroenterol. 2007, 13, 5707–5717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrick, M.D.; Singleton, S.T.; Vargas, F.; Kuo, H.C.; Zhao, L.; Knopfel, M.; Davidson, T.; Costa, M.; Paradkar, P.; Roth, J.A.; et al. DMT1: Which metals does it transport? Biol. Res. 2006, 39, 79–85. [Google Scholar] [CrossRef] [Green Version]
| Zinc Supplementation | No Zinc Supplementation | p | |
| (n = 82) | (n = 115) | ||
| Maternal characteristics | |||
| Age (years) | 34.0 (30.0–39.0) | 34.0 (30.0–37.0) | 0.549 |
| Primiparity | 43 (52.4) | 55 (47.8) | 0.523 |
| Infertility treatment | 33(40.2) | 43 (37.4) | 0.685 |
| Body Mass Index | 25.0 (22.9–26.9) | 25.2 (22.6–27.4) | 0.764 |
| Smoking | 2 (2.4) | 3 (2.6) | 0.656 |
| Gestational age at delivery (weeks) | 38.0 (36.3–38.0) | 37.0 (37.0–38.0) | 0.943 |
| Bleeding (g) | 954.5 (733.3–1285.0) | 850.0 (463.0–1078.0) | 0.014 |
| Intravenous Fe treatment | 66 (80.5) | 34 (29.6) | <0.001 |
| Oral Fe treatment | 16 (19.5) | 81 (70.4) | <0.001 |
| Dosage of Fe treatment (mg) | 410 (240–500) | 400 (400–500) | 0.280 |
| Gestational Diabetes Mellitus | 2 (2.4) | 5 (0.43) | 0.724 |
| Thyroid dysfunction | 4 (4.9) | 5(4.3) | 1.000 |
| Zinc Supplementation | No Zinc Supplementation | p | |
| (n = 93) | (n = 125) | ||
| Neonatal characteristics | |||
| Male | 43 (46.2) | 69 (55.2) | 0.366 |
| Birth weight (g) | 2766 (2245–3064) | 2758 (2374–3040) | 0.652 |
| 5-min Apgar score | 9.0 (9.0–9.0) | 9.0 (8.0–9.0) | 0.917 |
| Cord blood pH | 7.34 (7.31–7.35) | 7.32 (7.31–7.34) | 0.047 |
| Cord blood BE | −1.3 (−2.5–−0.3) | −1.2 (−2.5–−0.3) | 0.875 |
| Zinc Supplementation | No Zinc Supplementation | p | |
| (n = 61) | (n = 87) | ||
| Maternal characteristics | |||
| Age (years) | 34.0(31.0–39.3) | 35.0 (30.0–37.0) | 0.355 |
| Primiparity | 31 (50.8) | 43 (49.4) | 0.867 |
| Infertility treatment | 27 (44.3) | 36 (40.9) | 0.727 |
| Body Mass Index | 25.0 (23.0–26.4) | 25.8 (23.8–27.4) | 0.114 |
| Smoking | 2 (3.3) | 3 (3.4) | 0.955 |
| Gestational age at delivery (weeks) | 38.0 (37.0–38.0) | 38.0 (37.0–38.0) | 0.889 |
| Gestational Diabetes Mellitus | 1 (1.6) | 3 (3.4) | 0.643 |
| Thyroid dysfunction | 2 (3.3) | 3 (3.4) | 1.000 |
| EPDS score | 3 (1–5) | 2 (1–6) | 0.629 |
| EPDS ≥ 9 | 3 (4.9) | 14 (16.1) | 0.023 |
| Zinc Supplementation | No Zinc Supplementation | p | |
| (n = 70) | (n = 93) | ||
| Neonatal characteristics | |||
| Male | 35 (50.0) | 55 (59.1) | 0.245 |
| Birth weight (g) | 2956 (2584–3164) | 2876 (2590–3095) | 0.640 |
| 5-min Apgar score | 9.0 (9.0–9.0) | 9.0 (9.0–9.0) | 0.919 |
| Cord blood pH | 7.34 (7.31–7.35) | 7.33 (7.31–7.34) | 0.038 |
| Cord blood BE | −1.4 (−2.5–−0.4) | −1.2 (−2.5–−0.3) | 0.674 |
| Crude OR | p | Adjusted OR | p | |
|---|---|---|---|---|
| Age (years) | 0.911 (0.828–1.003) | 0.057 | 0.876 (0.780–0.984) | 0.025 |
| Primiparity | 1.979 (0.691–5.667) | 0.204 | 1.794 (0.582–5.534) | 0.309 |
| Infertility treatment | 1.604 (0.582–4.422) | 0.361 | 2.480 (0.728–8.449) | 0.146 |
| Body Mass Index | 0.997 (0.863–1.151) | 0.964 | 0.946 (0.811–1.104) | 0.481 |
| Gestational age at delivery (weeks) | 0.965 (0.747–1.248) | 0.788 | 0.920 (0.701–1.208) | 0.550 |
| Zinc supplementation | 0.270 (0.074–0.983) | 0.047 | 0.249 (0.062–0.988) | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, C.; Imai, K.; Owaki, T.; Kobayashi-Nakano, T.; Ushida, T.; Iitani, Y.; Nakamura, N.; Kajiyama, H.; Kotani, T. The Possible Effects of Zinc Supplementation on Postpartum Depression and Anemia. Medicina 2022, 58, 731. https://doi.org/10.3390/medicina58060731
Aoki C, Imai K, Owaki T, Kobayashi-Nakano T, Ushida T, Iitani Y, Nakamura N, Kajiyama H, Kotani T. The Possible Effects of Zinc Supplementation on Postpartum Depression and Anemia. Medicina. 2022; 58(6):731. https://doi.org/10.3390/medicina58060731
Chicago/Turabian StyleAoki, Chieko, Kenji Imai, Taro Owaki, Tomoko Kobayashi-Nakano, Takafumi Ushida, Yukako Iitani, Noriyuki Nakamura, Hiroaki Kajiyama, and Tomomi Kotani. 2022. "The Possible Effects of Zinc Supplementation on Postpartum Depression and Anemia" Medicina 58, no. 6: 731. https://doi.org/10.3390/medicina58060731
APA StyleAoki, C., Imai, K., Owaki, T., Kobayashi-Nakano, T., Ushida, T., Iitani, Y., Nakamura, N., Kajiyama, H., & Kotani, T. (2022). The Possible Effects of Zinc Supplementation on Postpartum Depression and Anemia. Medicina, 58(6), 731. https://doi.org/10.3390/medicina58060731






