In Vitro and In Ovo Evaluation of the Potential Hepatoprotective Effect of Metformin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cellular Viability Assessment
2.4. Cellular Morphology
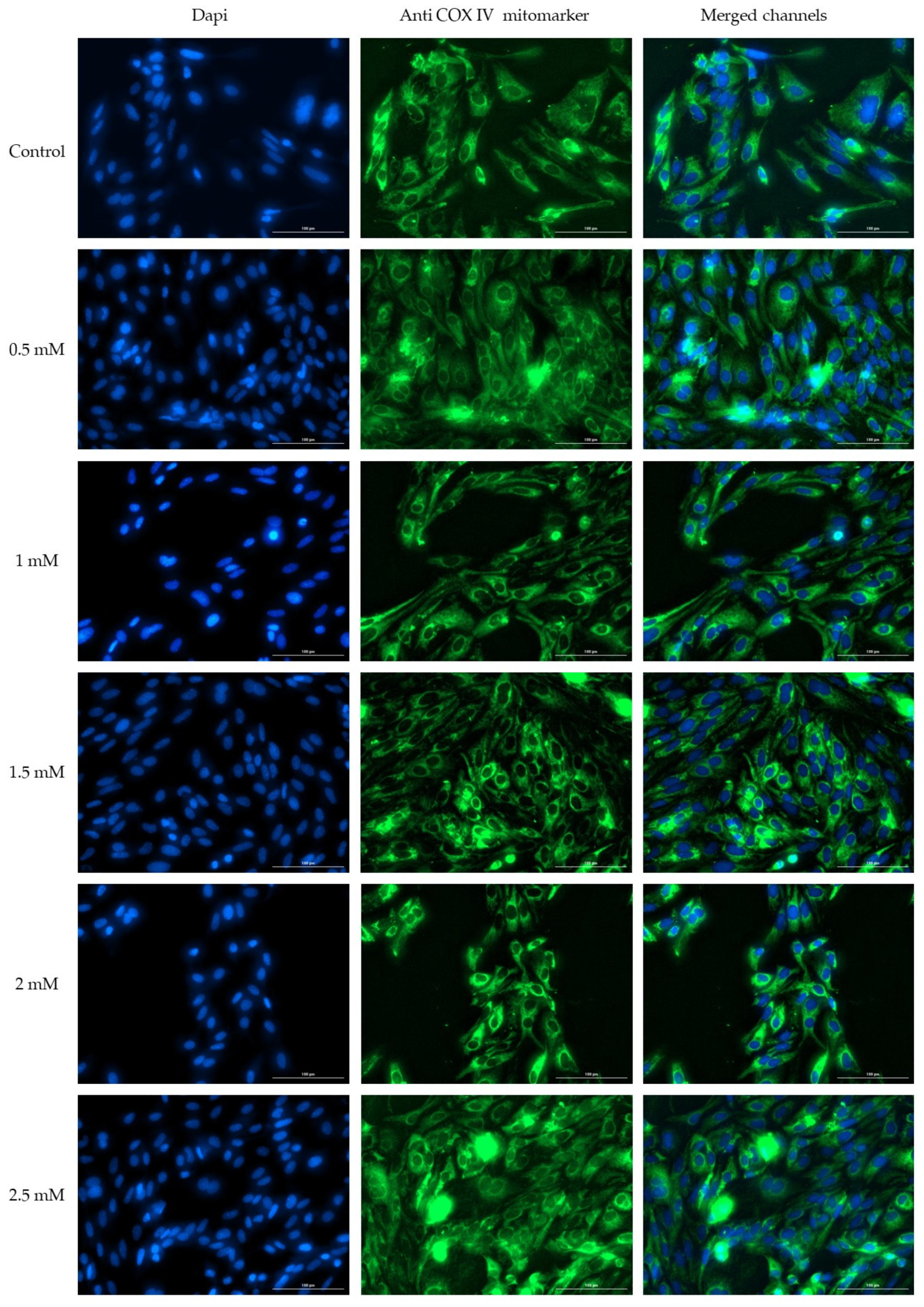
2.5. Immunofluorescence
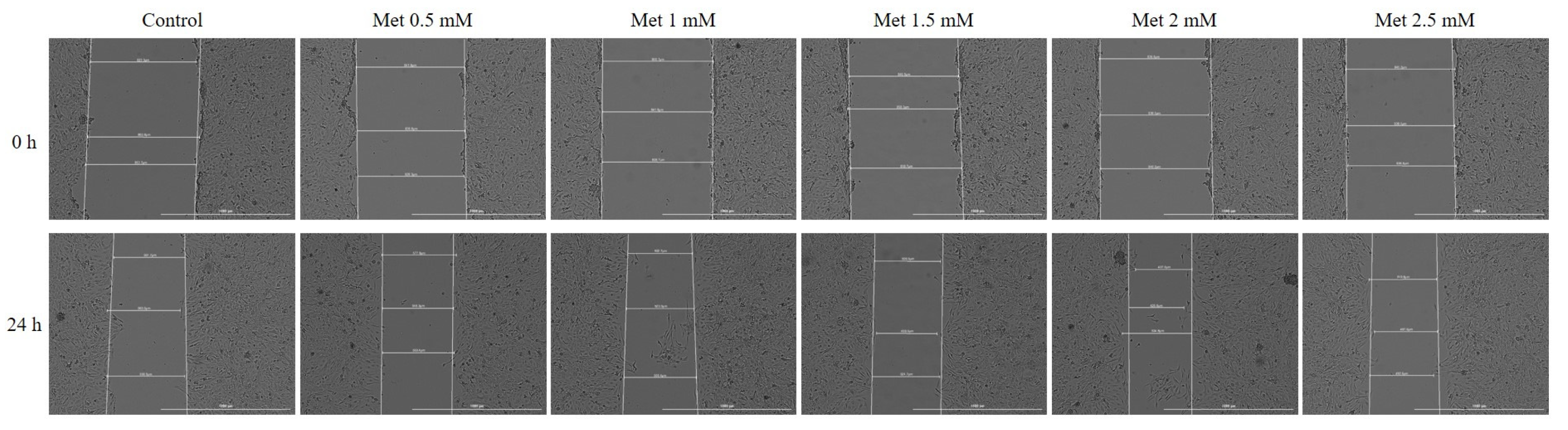
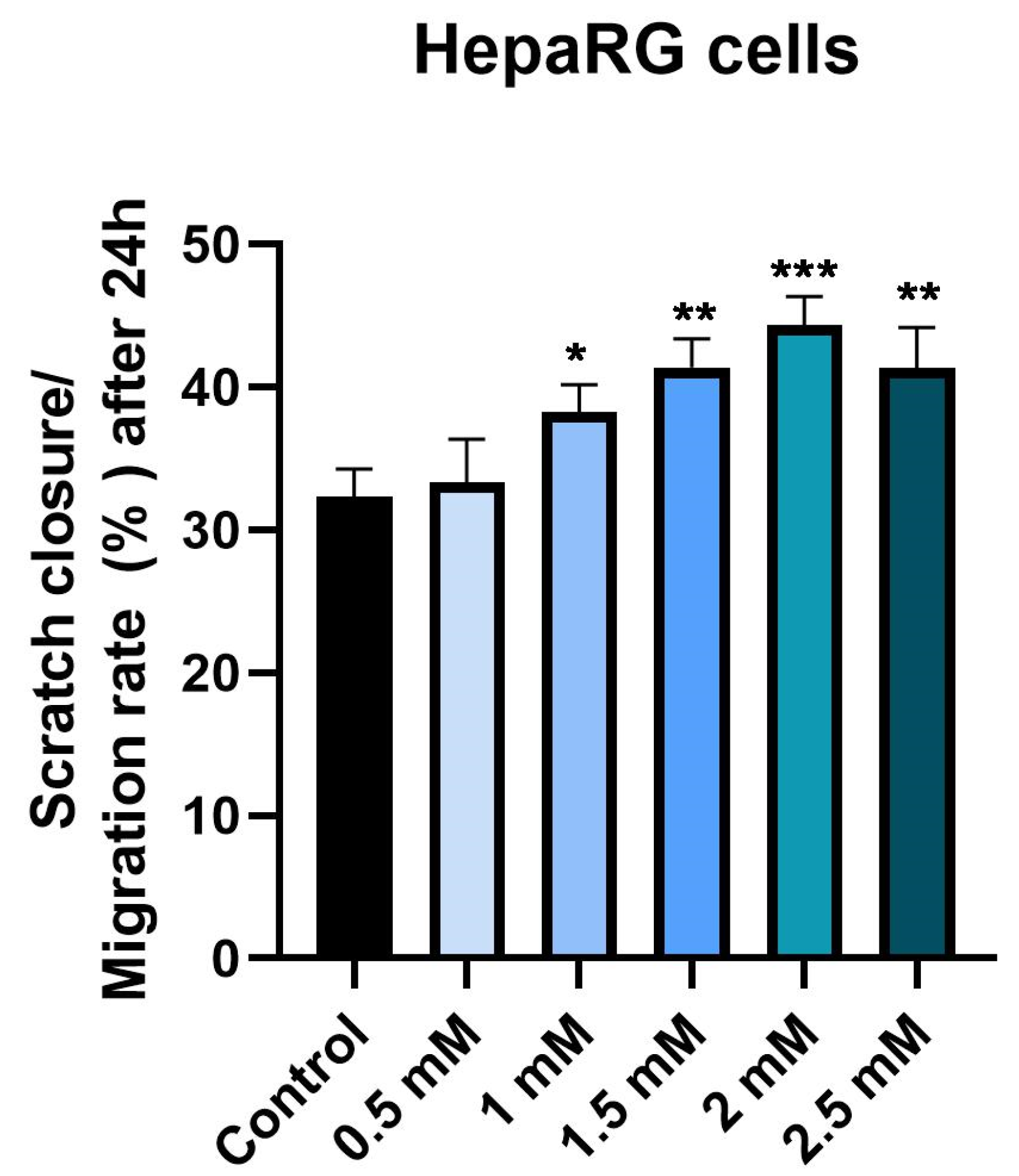
2.6. Wound Healing Assay
2.7. Chorioallantoic Membrane (CAM) Assay
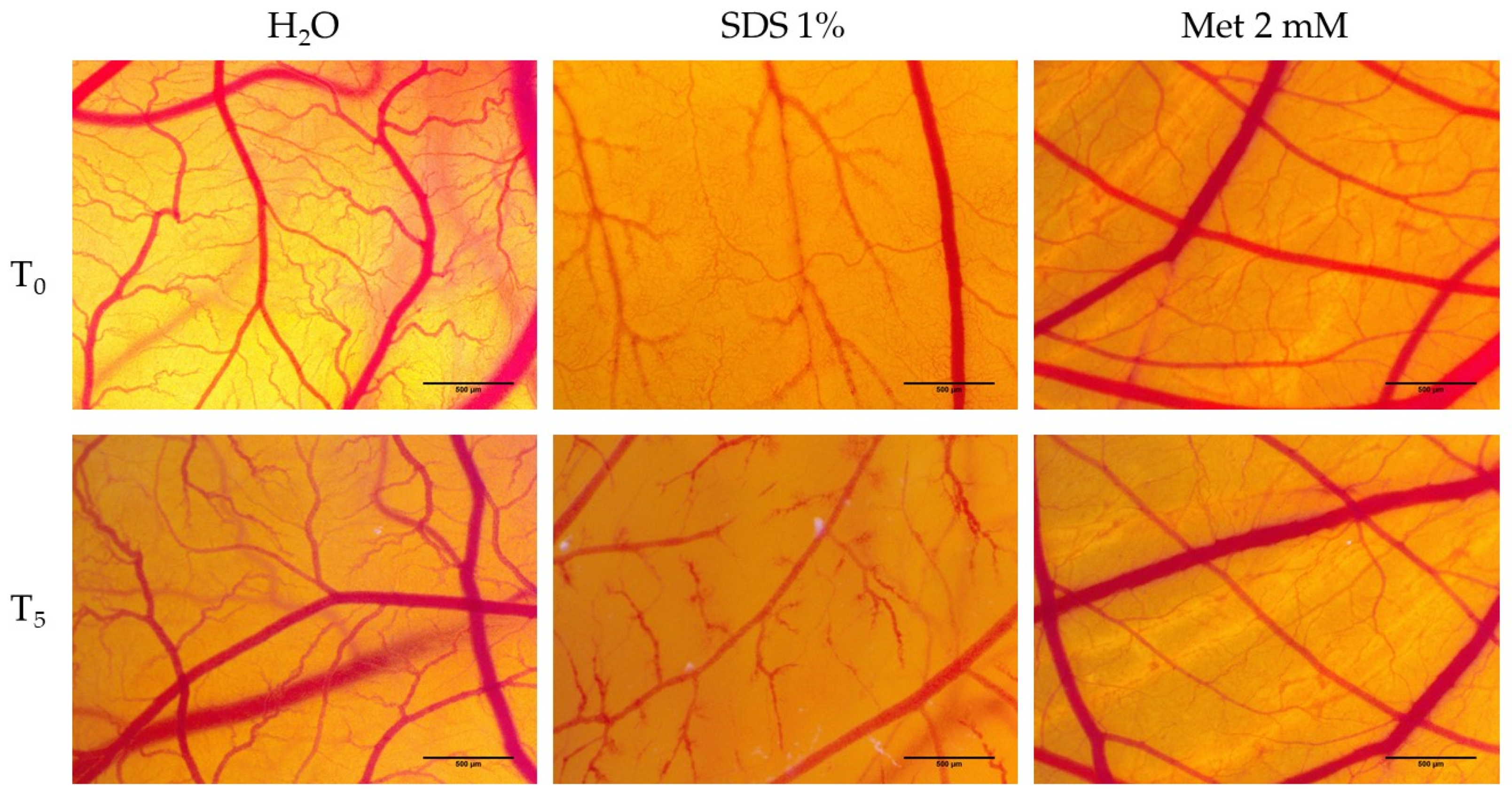
2.8. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Assay
2.9. Determination of Anti-Irritative Potential
2.10. Statistical Analysis
3. Results
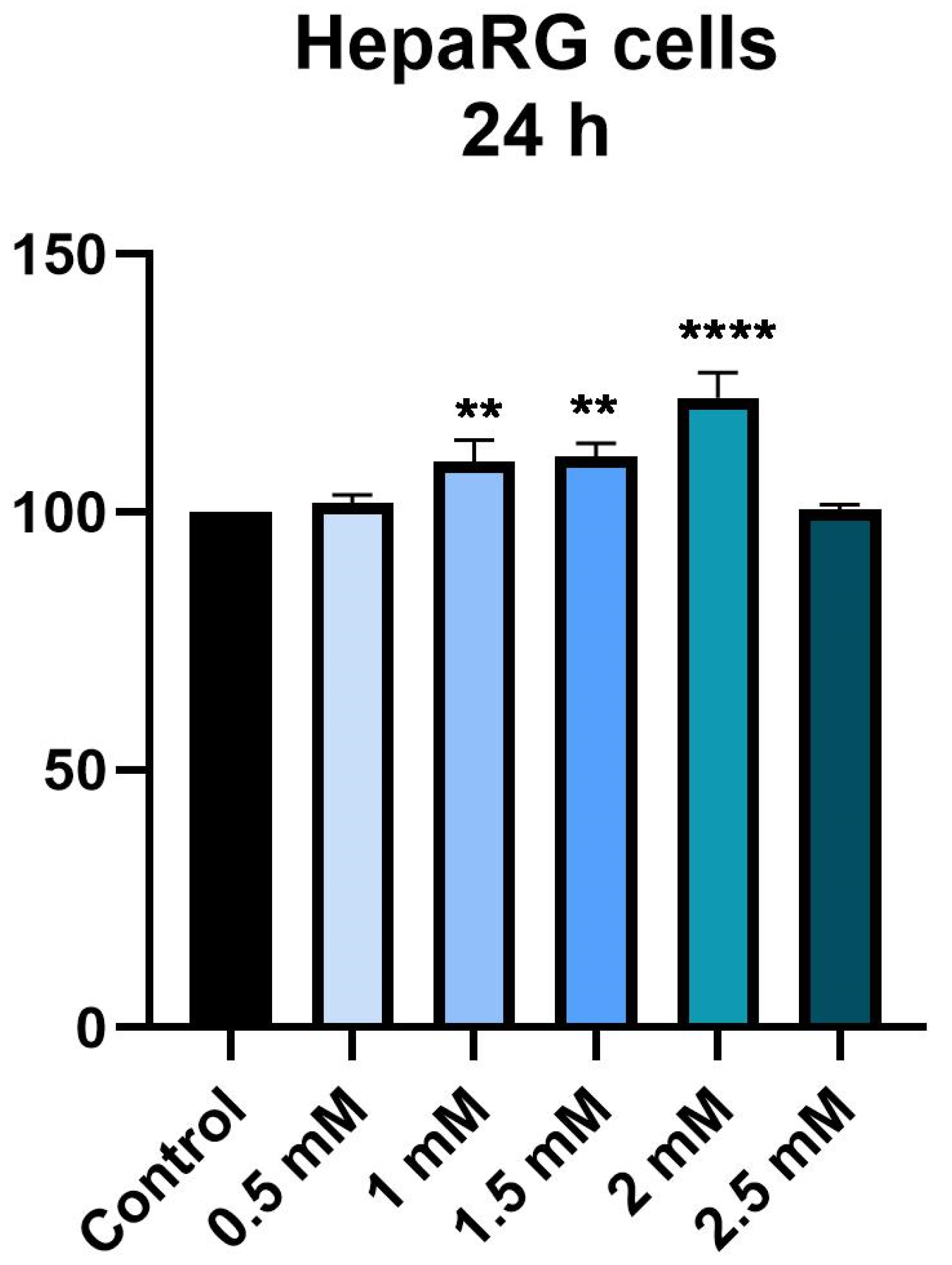
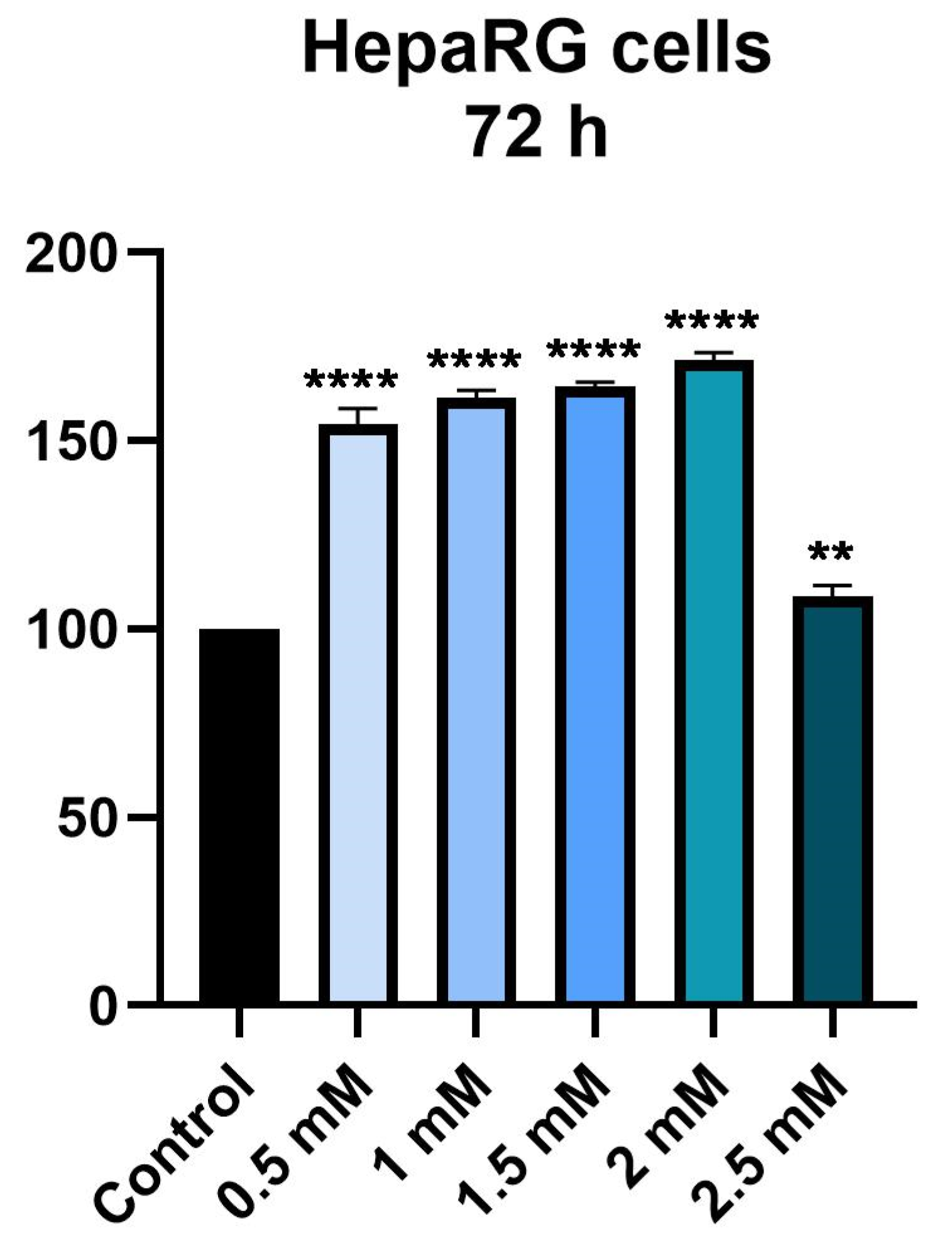
3.1. Cellular Viability Assessment
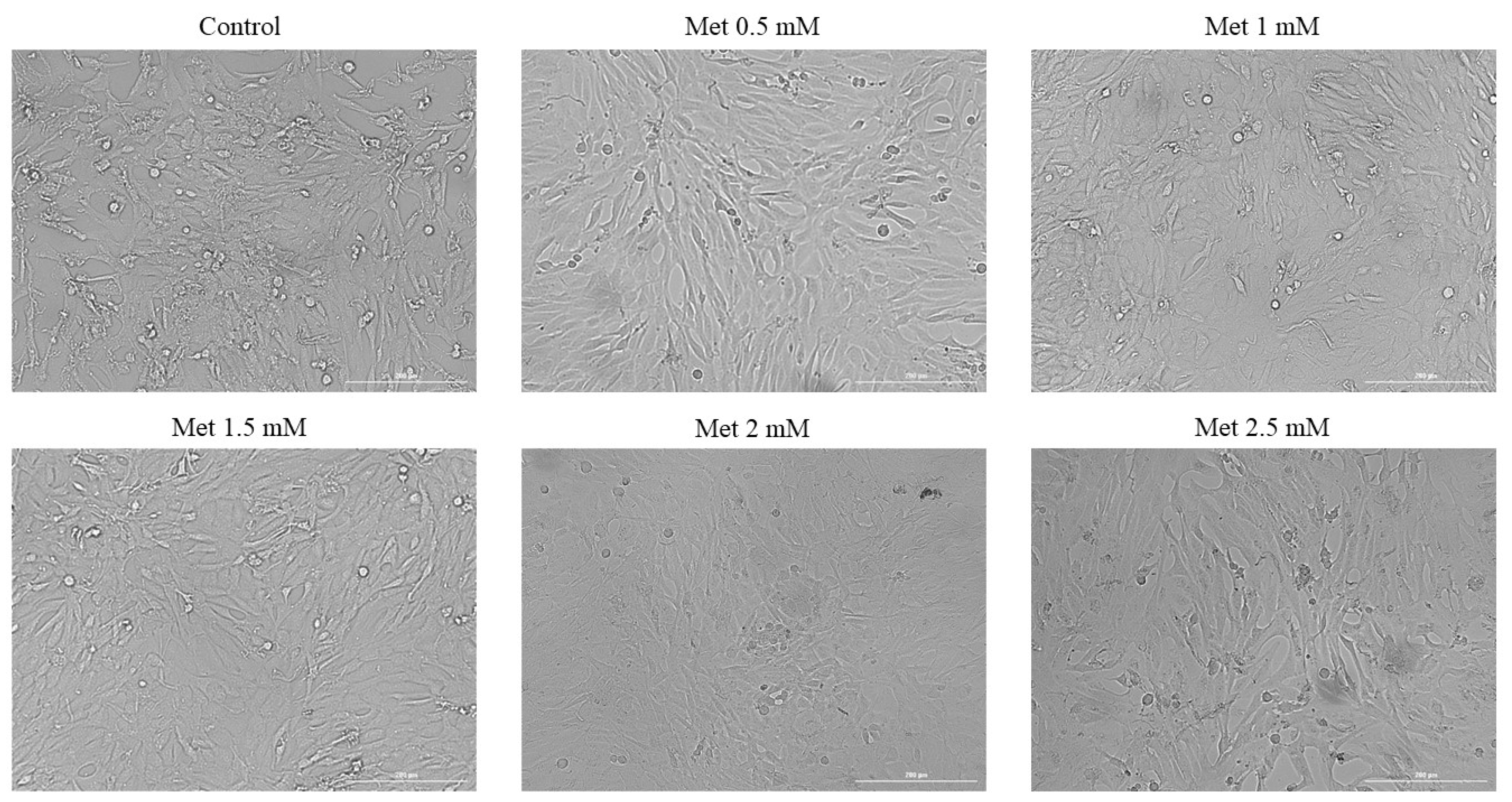
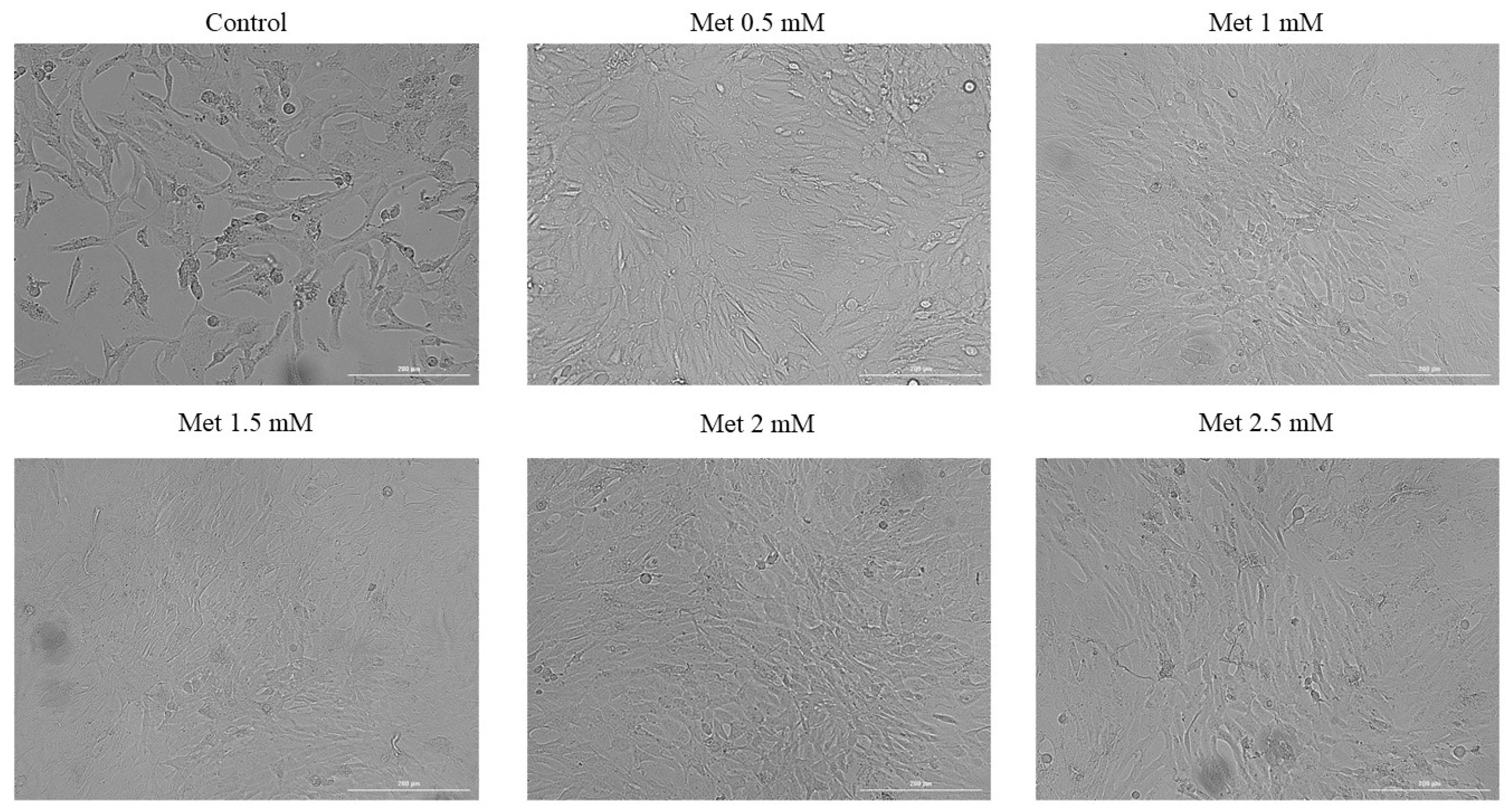
3.2. Cellular Morphology
3.3. Immunofluorescence
3.4. Wound Healing Assay
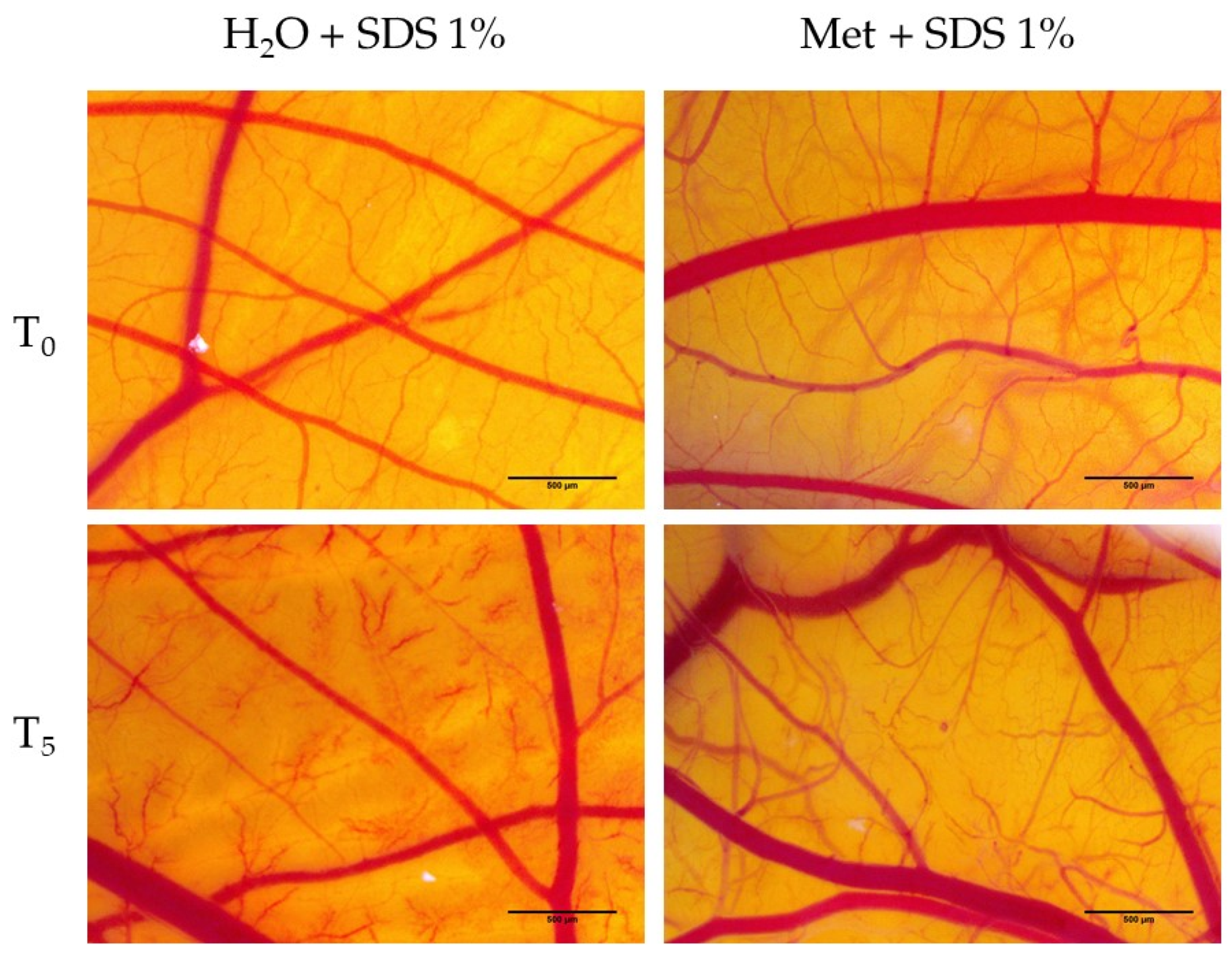
3.5. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Assay
3.6. Determination of Anti-Irritative Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reed, J.; Bain, S.; Kanamarlapudi, V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3567–3602. [Google Scholar] [CrossRef]
- Loria, P.; Lonardo, A.; Anania, F. Liver and Diabetes. A Vicious Circle. Hepatol. Res. 2013, 43, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; He, S.-J.; Feng, X.; Cheng, J.; Luo, Y.-T.; Tian, L.; Huang, Q. Metformin: A Review of Its Potential Indications. Drug Des. Devel. Ther. 2017, 11, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Aljofan, M.; Riethmacher, D. Anticancer Activity of Metformin: A Systematic Review of the Literature. Futur. Sci. OA 2019, 5, FSO410. [Google Scholar] [CrossRef]
- Gandini, S.; Puntoni, M.; Heckman-Stoddard, B.M.; Dunn, B.K.; Ford, L.; DeCensi, A.; Szabo, E. Metformin and Cancer Risk and Mortality: A Systematic Review and Meta-Analysis Taking into Account Biases and Confounders. Cancer Prev. Res. 2014, 7, 867–885. [Google Scholar] [CrossRef]
- Bannister, C.A.; Holden, S.E.; Jenkins-Jones, S.; Morgan, C.L.; Halcox, J.P.; Schernthaner, G.; Mukherjee, J.; Currie, C.J. Can People with Type 2 Diabetes Live Longer than Those without? A Comparison of Mortality in People Initiated with Metformin or Sulphonylurea Monotherapy and Matched, Non-Diabetic Controls. Diabetes Obes. Metab. 2014, 16, 1165–1173. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, Y.; Lai, S.; Lv, A.; Su, Q.; Dong, Y.; Zhou, Z.; Tang, W.; Zhao, J.; Cui, L.; et al. Effects of Metformin versus Glipizide on Cardiovascular Outcomes in Patients with Type 2 Diabetes and Coronary Artery Disease. Diabetes Care 2013, 36, 1304–1311. [Google Scholar] [CrossRef]
- Mohammed, I.; Hollenberg, M.D.; Ding, H.; Triggle, C.R. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front. Endocrinol. 2021, 12, 718942. [Google Scholar] [CrossRef]
- Nkontchou, G.; Cosson, E.; Aout, M.; Mahmoudi, A.; Bourcier, V.; Charif, I.; Ganne-Carrie, N.; Grando-Lemaire, V.; Vicaut, E.; Trinchet, J.-C.; et al. Impact of Metformin on the Prognosis of Cirrhosis Induced by Viral Hepatitis C in Diabetic Patients. J. Clin. Endocrinol. Metab. 2011, 96, 2601–2608. [Google Scholar] [CrossRef]
- Hassan, M.M.; Curley, S.A.; Li, D.; Kaseb, A.; Davila, M.; Abdalla, E.K.; Javle, M.; Moghazy, D.M.; Lozano, R.D.; Abbruzzese, J.L.; et al. Association of Diabetes Duration and Diabetes Treatment with the Risk of Hepatocellular Carcinoma. Cancer 2010, 116, 1938–1946. [Google Scholar] [CrossRef]
- Cunha, V.; Cotrim, H.P.; Rocha, R.; Carvalho, K.; Lins-Kusterer, L. Metformin in the Prevention of Hepatocellular Carcinoma in Diabetic Patients: A Systematic Review. Ann. Hepatol. 2020, 19, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Schimmack, G.; Defronzo, R.A.; Musi, N. AMP-Activated Protein Kinase: Role in Metabolism and Therapeutic Implications. Diabetes Obes. Metab. 2006, 8, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Zoli, M.; Melchionda, N. Metformin in Non-Alcoholic Steatohepatitis. Lancet 2001, 358, 893–894. [Google Scholar] [CrossRef]
- Kamalian, L.; Douglas, O.; Jolly, C.E.; Snoeys, J.; Simic, D.; Monshouwer, M.; Williams, D.P.; Kevin Park, B.; Chadwick, A.E. The Utility of HepaRG Cells for Bioenergetic Investigation and Detection of Drug-Induced Mitochondrial Toxicity. Toxicol. Vitr. 2018, 53, 136–147. [Google Scholar] [CrossRef]
- Iranshahy, M.; Rezaee, R.; Karimi, G. Hepatoprotective Activity of Metformin: A New Mission for an Old Drug? Eur. J. Pharmacol. 2019, 850, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Shan, Q.; Liu, P.; Feng, T.; Zhang, X.; Xiang, P.; Chen, K.; Xie, H.; Song, P.; Zhou, L.; et al. Metformin Ameliorates Arsenic Trioxide Hepatotoxicity via Inhibiting Mitochondrial Complex I. Cell Death Dis. 2017, 8, e3159. [Google Scholar] [CrossRef]
- Al-Hashem, F.; AlHumayed, S.; Ellatif, M.A.; Amin, S.N.; Kamar, S.S.; Al-Ani, B.; Haidara, M.A. Metformin Protects against Thioacetamide Induced Liver Injury in Rats. Int. J. Morphol. 2018, 36, 984–990. [Google Scholar] [CrossRef][Green Version]
- Saeedi Saravi, S.S.; Hasanvand, A.; Shahkarami, K.; Dehpour, A.R. The Protective Potential of Metformin against Acetaminophen-Induced Hepatotoxicity in BALB/C Mice. Pharm. Biol. 2016, 54, 2830–2837. [Google Scholar] [CrossRef]
- Kis, A.M.; Macasoi, I.; Paul, C.; Radulescu, M.; Buzatu, R.; Watz, C.G.; Cheveresan, A.; Berceanu, D.; Pinzaru, I.; Dinu, S.; et al. Methotrexate and Cetuximab—Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina 2022, 58, 167. [Google Scholar] [CrossRef]
- Batista-duharte, A.; Murillo, G.J.; Betancourt, J.E.; Oliver, P.; Damiana, T. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef]
- Budai, P.; Kormos, É.; Buda, I.; Somody, G.; Lehel, J. Comparative Evaluation of HET-CAM and ICE Methods for Objective Assessment of Ocular Irritation Caused by Selected Pesticide Products. Toxicol. Vitr. 2021, 74, 105150. [Google Scholar] [CrossRef]
- Macașoi, I.; Pavel, I.Z.; Moacă, A.E.; Avram, Ș.; David, V.L.; Coricovac, D.; Mioc, A.; Spandidos, D.A.; Tsatsakis, A.; Șoica, C.; et al. Mechanistic Investigations of Antitumor Activity of a Rhodamine B-oleanolic Acid Derivative Bioconjugate. Oncol. Rep. 2020, 44, 1169–1183. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.S.; Singh, S.; Garg, G.; Kumar, R.; Verma, A.K.; Singh, A.K.; Bissoyi, A.; Rizvi, S.I. Metformin Ameliorates Acetaminophen-Induced Sub-Acute Toxicity via Antioxidant Property. Drug Chem. Toxicol. 2022, 45, 52–60. [Google Scholar] [CrossRef]
- Zhang, X.; Harmsen, W.S.; Mettler, T.A.; Kim, W.R.; Roberts, R.O.; Therneau, T.M.; Roberts, L.R.; Chaiteerakij, R. Continuation of Metformin Use after a Diagnosis of Cirrhosis Significantly Improves Survival of Patients with Diabetes. Hepatology 2014, 60, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Jin, M.; Yang, M.; Liu, K.; Li, J. Type 2 Diabetes Mellitus and the Risk of Hepatitis C Virus Infection: A Systematic Review. Sci. Rep. 2013, 3, 2981. [Google Scholar] [CrossRef]
- Coman, L.I.; Coman, O.A.; Bădărău, I.A.; Păunescu, H.; Ciocîrlan, M. Association between Liver Cirrhosis and Diabetes Mellitus: A Review on Hepatic Outcomes. J. Clin. Med. 2021, 10, 262. [Google Scholar] [CrossRef]
- Torbenson, M.; Chen, Y.-Y.; Brunt, E.; Cummings, O.W.; Gottfried, M.; Jakate, S.; Liu, Y.-C.; Yeh, M.M.; Ferrell, L. Glycogenic Hepatopathy: An Underrecognized Hepatic Complication of Diabetes Mellitus. Am. J. Surg. Pathol. 2006, 30, 508–513. [Google Scholar] [CrossRef]
- Hudacko, R.M.; Sciancalepore, J.P.; Fyfe, B.S. Diabetic Microangiopathy in the Liver: An Autopsy Study of Incidence and Association with Other Diabetic Complications. Am. J. Clin. Pathol. 2009, 132, 494–499. [Google Scholar] [CrossRef]
- Association, A.D. Erratum. Classification and Diagnosis of Diabetes. Sec. 2. In Standards of Medical Care in Diabetes-2016. Diabetes Care 2016; 39 (Suppl. 1): S13–S22. Diabetes Care 2016, 39, 1653. [Google Scholar] [CrossRef]
- Andersson, T.B.; Kanebratt, K.P.; Kenna, J.G. The HepaRG Cell Line: A Unique in Vitro Tool for Understanding Drug Metabolism and Toxicology in Human. Expert Opin. Drug Metab. Toxicol. 2012, 8, 909–920. [Google Scholar] [CrossRef]
- Hart, S.N.; Li, Y.; Nakamoto, K.; Subileau, E.; Steen, D.; Zhong, X. A Comparison of Whole Genome Gene Expression Profiles of HepaRG Cells and HepG2 Cells to Primary Human Hepatocytes and Human Liver Tissues. Drug Metab. Dispos. 2010, 38, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Choi, A.; Lee, M.; Lee, J.A. Metformin Displays in Vitro and in Vivo Antitumor Effect against Osteosarcoma. Korean J. Pediatrics 2016, 59, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Babaei Khorzoughi, R.; Namvarjah, F.; Teimouri, M.; Hosseini, H.; Meshkani, R. In-Vitro Synergistic Effect of Metformin and Berberine on High Glucose-Induced Lipogenesis. Iran. J. Pharm. Res. IJPR 2019, 18, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Kato, K.; Iwama, H.; Maeda, E.; Sakamoto, T.; Fujita, K.; Toyota, Y.; Tani, J.; Nomura, T.; Mimura, S.; et al. Effect of the Anti-Diabetic Drug Metformin in Hepatocellular Carcinoma in Vitro and in Vivo. Int. J. Oncol. 2014, 45, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yan, H.; Xia, M.; Chang, X.; Xu, X.; Wang, L.; Sun, X.; Lu, Y.; Bian, H.; Li, X.; et al. Metformin Attenuates Triglyceride Accumulation in HepG2 Cells through Decreasing Stearyl-Coenzyme A Desaturase 1 Expression. Lipids Health Dis. 2018, 17, 114. [Google Scholar] [CrossRef]
- Hu, X.; Li, M.; Zhang, C.; Pang, S. Constitutive Androstane Receptor-Mediated Inhibition of Metformin on Phase II Metabolic Enzyme SULT2A1. Int. J. Endocrinol. 2021, 2021, 8867218. [Google Scholar] [CrossRef]
- Levy, A.; Hasson, P. Correlation between In-vitro and In-vivo Studies based on Pharmacokinetic Considerations. AJBSR 2020, 8, 001236. [Google Scholar] [CrossRef]
- Lin, H.Z.; Yang, S.Q.; Chuckaree, C.; Kuhajda, F.; Ronnet, G.; Diehl, A.M. Metformin Reverses Fatty Liver Disease in Obese, Leptin-Deficient Mice. Nat. Med. 2000, 6, 998–1003. [Google Scholar] [CrossRef]
- Krakoff, J.; Clark, J.M.; Crandall, J.P.; Wilson, C.; Molitch, M.E.; Brancati, F.L.; Edelstein, S.L.; Knowler, W.C. Effects of Metformin and Weight Loss on Serum Alanine Aminotransferase Activity in the Diabetes Prevention Program. Obesity 2010, 18, 1762–1767. [Google Scholar] [CrossRef]
- Uygun, A.; Kadayifci, A.; Isik, A.T.; Ozgurtas, T.; Deveci, S.; Tuzun, A.; Yesilova, Z.; Gulsen, M.; Dagalp, K. Metformin in the Treatment of Patients with Non-alcoholic Steatohepatitis. Aliment. Pharmacol. Ther. 2004, 19, 537–544. [Google Scholar] [CrossRef]
- Kadayifçi, A. Nonalcoholic Steatohepatitis: Role of Leptin in Pathogenesis and Benefits of Metformin in Treatment. Am. J. Gastroenterol. 2003, 98, 2330. [Google Scholar] [CrossRef] [PubMed]
- Conde de la Rosa, L.; Vrenken, T.E.; Buist-Homan, M.; Faber, K.N.; Moshage, H. Metformin Protects Primary Rat Hepatocytes against Oxidative Stress-Induced Apoptosis. Pharmacol. Res. Perspect. 2015, 3, e00125. [Google Scholar] [CrossRef]
- Diniz Vilela, D.; Gomes Peixoto, L.; Teixeira, R.R.; Belele Baptista, N.; Carvalho Caixeta, D.; Vieira de Souza, A.; Machado, H.L.; Pereira, M.N.; Sabino-Silva, R.; Espindola, F.S. The Role of Metformin in Controlling Oxidative Stress in Muscle of Diabetic Rats. Oxid. Med. Cell. Longev. 2016, 2016, 6978625. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Birnbaum, M.J. An Energetic Tale of AMPK-Independent Effects of Metformin. J. Clin. Investig. 2010, 120, 2267–2270. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Hwang, J.H.; Kim, K.-S.; Noh, J.-R.; Choi, D.-H.; Kim, D.-K.; Tadi, S.; Yim, Y.-H.; Choi, H.-S.; Lee, C.-H. Metformin Ameliorates Acetaminophen Hepatotoxicity via Gadd45β-Dependent Regulation of JNK Signaling in Mice. J. Hepatol. 2015, 63, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Wu, K.; Lin, L.; Ge, P.; Dai, J.; He, X.; Hu, K.; Zhang, L. Metformin Mitigates Carbon Tetrachloride-Induced TGF-Β1/Smad3 Signaling and Liver Fibrosis in Mice. Biomed. Pharmacother. 2017, 90, 421–426. [Google Scholar] [CrossRef]
- Sliwinska, A.; Drzewoski, J. Molecular Action of Metformin in Hepatocytes: An Updated Insight. Curr. Diabetes Rev. 2015, 11, 175–181. [Google Scholar] [CrossRef]
- Degli Esposti, D.; Hamelin, J.; Bosselut, N.; Saffroy, R.; Sebagh, M.; Pommier, A.; Martel, C.; Lemoine, A. Mitochondrial Roles and Cytoprotection in Chronic Liver Injury. Biochem. Res. Int. 2012, 2012, 387626. [Google Scholar] [CrossRef]
- Mihajlovic, M. Mitochondria as the Target of Hepatotoxicity and Drug-Induced Liver Injury: Molecular Mechanisms and Detection Methods. Int. J. Mol. Sci. 2022, 23, 3315. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. Methods Mol. Biol. 2020, 2109, 225–229. [Google Scholar] [CrossRef]
- Tanaka, M.; Miyajima, A. Liver Regeneration and Fibrosis after Inflammation. Inflamm. Regen. 2016, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tao, Y.; Deng, Y.; Yu, J.; Sun, Y.; Jiang, G. Metformin Accelerates Wound Healing in Type 2 Diabetic Db/Db Mice. Mol. Med. Rep. 2017, 16, 8691–8698. [Google Scholar] [CrossRef] [PubMed]
- Desouza, C.V. Does Drug Therapy Reverse Endothelial Progenitor Cell Dysfunction in Diabetes? J. Diabetes Complicat. 2013, 27, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-T.; Chen, H.-M.; Chiu, C.-H.; Liang, Y.-J. AMP-Activated Protein Kinase Activators in Diabetic Ulcers: From Animal Studies to Phase II Drugs under Investigation. Expert Opin. Investig. Drugs 2014, 23, 1253–1265. [Google Scholar] [CrossRef]
- Ochoa-Gonzalez, F.; Cervantes-Villagrana, A.R.; Fernandez-Ruiz, J.C.; Nava-Ramirez, H.S.; Hernandez-Correa, A.C.; Enciso-Moreno, J.A.; Castañeda-Delgado, J.E. Metformin induces Cell Cycle Arrest, Reduced Proliferation, Wound Healing Impairment in Vivo and Is Associated to Clinical Outcomes in Diabetic Foot Ulcer Patients. PLoS ONE 2016, 11, e0150900. [Google Scholar]
- Wang, J.-L.; Lan, Y.-W.; Tsai, Y.-T.; Chen, Y.-C.; Staniczek, T.; Tsou, Y.-A.; Yen, C.-C.; Chen, C.-M. Additive Antiproliferative and Antiangiogenic Effects of Metformin and Pemetrexed in a Non-Small-Cell Lung Cancer Xenograft Model. Front. Cell Dev. Biol. 2021, 9, 688062. [Google Scholar] [CrossRef]
| H2O | SDS 1% | Met 2 mM | |
|---|---|---|---|
| IS | 0.13 | 19.68 | 0.7 |
| tH | 300 | 22 s | 300 s |
| tL | 300 | 19 s | 300 s |
| tC | 298 | 17 s | 279 s |
| SDS 1% | H2O + SDS 1% | Met 2 mM + SDS 1% | |
|---|---|---|---|
| IS | 19.68 | 17.58 | 7.29 |
| tH | 22 s | 78 s | 270 s |
| tL | 19 s | 55 s | 197 s |
| tC | 17 s | 30 s | 156 s |
| HAI | 3.54 | 12.27 | |
| LAI | 2.89 | 10.36 | |
| CAI | 1.76 | 9.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozma, G.V.; Apostu, A.; Macasoi, I.; Dehelean, C.A.; Cretu, O.M.; Dinu, S.; Gaiță, D.; Manea, A. In Vitro and In Ovo Evaluation of the Potential Hepatoprotective Effect of Metformin. Medicina 2022, 58, 705. https://doi.org/10.3390/medicina58060705
Cozma GV, Apostu A, Macasoi I, Dehelean CA, Cretu OM, Dinu S, Gaiță D, Manea A. In Vitro and In Ovo Evaluation of the Potential Hepatoprotective Effect of Metformin. Medicina. 2022; 58(6):705. https://doi.org/10.3390/medicina58060705
Chicago/Turabian StyleCozma, Gabriel Veniamin, Alexandru Apostu, Ioana Macasoi, Cristina Adriana Dehelean, Octavian Marius Cretu, Stefania Dinu, Dan Gaiță, and Aniko Manea. 2022. "In Vitro and In Ovo Evaluation of the Potential Hepatoprotective Effect of Metformin" Medicina 58, no. 6: 705. https://doi.org/10.3390/medicina58060705
APA StyleCozma, G. V., Apostu, A., Macasoi, I., Dehelean, C. A., Cretu, O. M., Dinu, S., Gaiță, D., & Manea, A. (2022). In Vitro and In Ovo Evaluation of the Potential Hepatoprotective Effect of Metformin. Medicina, 58(6), 705. https://doi.org/10.3390/medicina58060705








