Impact of Venlafaxine on Platelet Count and Activity—Case Report and Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
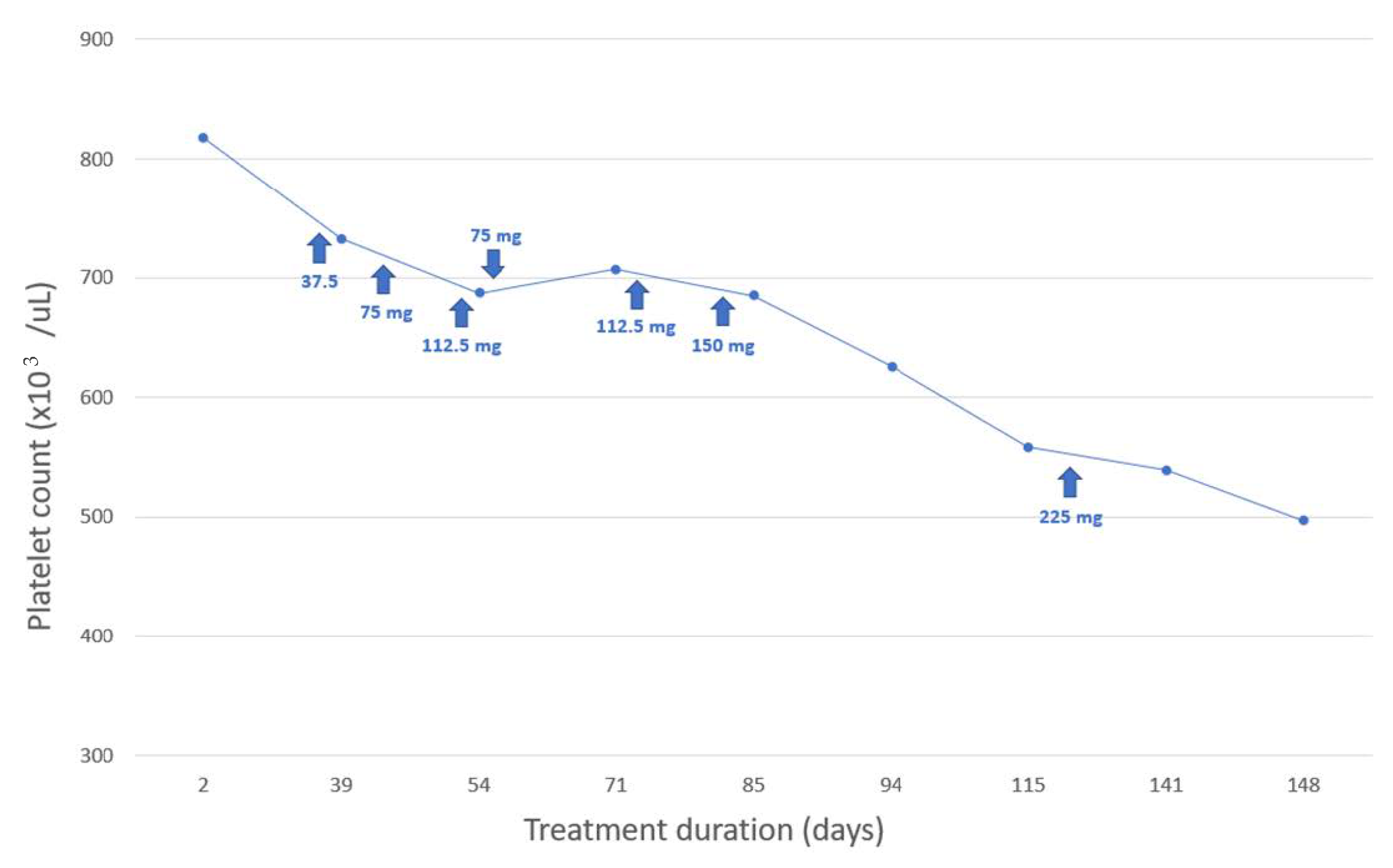
3. Case Study
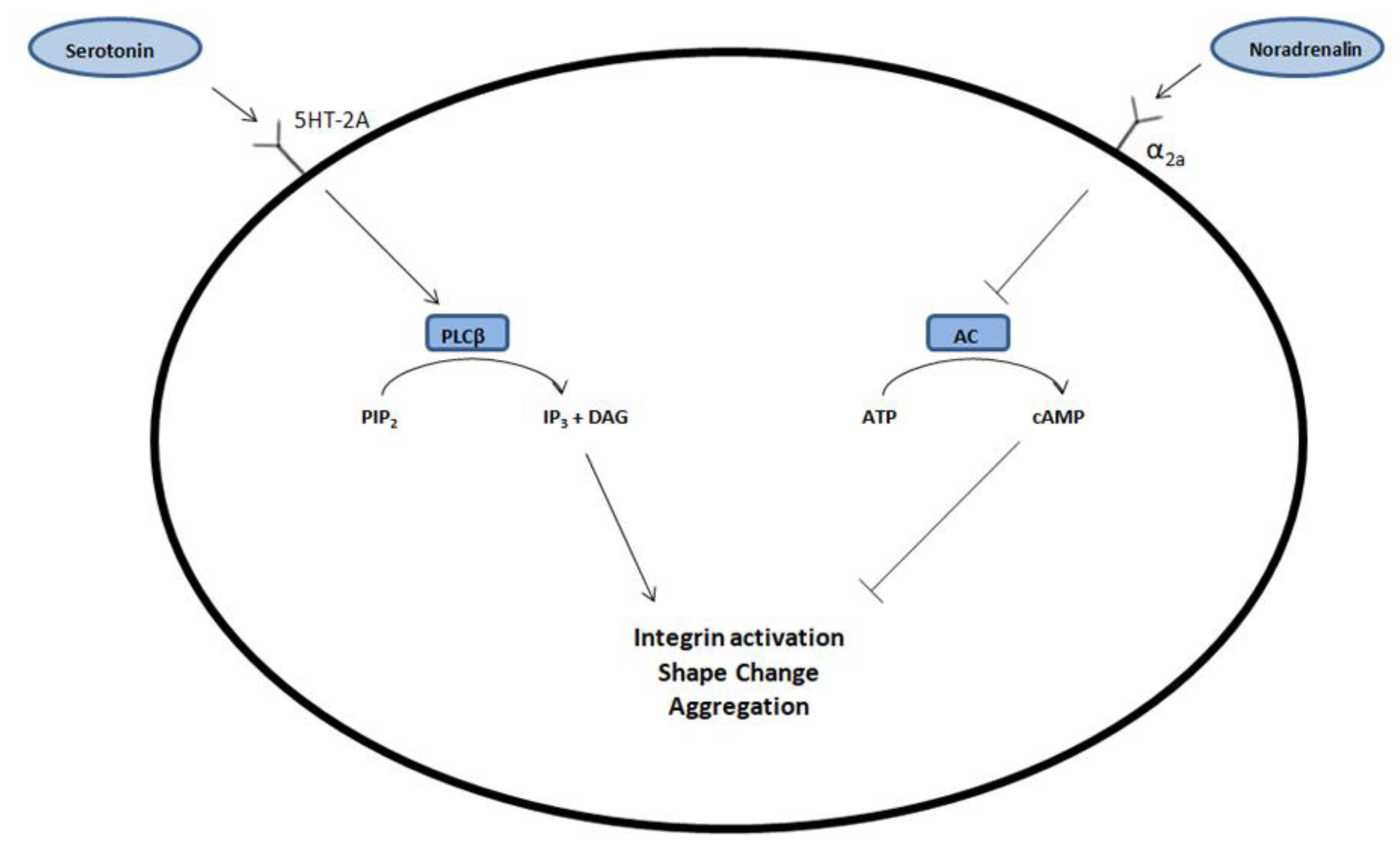
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Moliterno, A.R.; Ginzburg, Y.Z.; Hoffman, R. Clinical insights into the origins of thrombosis in myeloproliferative neoplasms. Blood 2021, 137, 1145–1153. [Google Scholar] [CrossRef]
- Mesa, R.A.; Niblack, J.; Wadleigh, M.; Verstovsek, S.; Camoriano, J.; Barnes, S.; Tan, A.D.; Atherton, P.J.; Sloan, J.A.; Tefferi, A. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): An international Internet-based survey of 1179 MPD patients. Cancer 2007, 109, 68–76. [Google Scholar] [CrossRef]
- Rakel, R.E. Depression. Prim. Care 1999, 26, 211–224. [Google Scholar] [CrossRef]
- Scherber, R.M.; Kosiorek, H.E.; Senyak, Z.; Dueck, A.C.; Clark, M.M.; Boxer, M.A.; Geyer, H.L.; McCallister, A.; Cotter, M.; Van Husen, B.; et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer 2016, 122, 477–485. [Google Scholar] [CrossRef]
- Padrnos, L.; Scherber, R.; Geyer, H.; Langlais, B.T.; Dueck, A.C.; Kosiorek, H.E.; Senyak, Z.; Clark, M.; Boxer, M.; Cotter, M.; et al. Depressive symptoms and myeloproliferative neoplasms: Understanding the confounding factor in a complex condition. Cancer Med. 2020, 9, 8301–8309. [Google Scholar] [CrossRef]
- Singh, D.; Saadabadi, A. Venlafaxine; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Arakawa, R.; Stenkrona, P.; Takano, A.; Svensson, J.; Andersson, M.; Nag, S.; Asami, Y.; Hirano, Y.; Halldin, C.; Lundberg, J. Venlafaxine ER blocks the norepinephrine transporter in the brain of patients with major depressive disorder: A PET study using [18F]FMeNER-D2. Int. J. Neuropsychopharmacol. 2019, 22, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Shelton, R.C. Serotonin and Norepinephrine Reuptake Inhibitors. Handb. Exp. Pharmacol. 2019, 250, 145–180. [Google Scholar]
- Lenox-Smith, A.J.; Jiang, Q. Venlafaxine extended release versus citalopram in patients with depression unresponsive to a selective serotonin reuptake inhibitor. Int. Clin. Psychopharmacol. 2008, 23, 113–119. [Google Scholar] [CrossRef]
- Seidel, A.; Arolt, V.; Hunstiger, M.; Rink, L.; Behnisch, A.; Kirchner, H. Major depressive disorder is associated with elevated monocyte counts. Acta Psychiatr. Scand. 1996, 94, 198–204. [Google Scholar] [CrossRef]
- Canan, F.; Dikici, S.; Kutlucan, A.; Celbek, G.; Coskun, H.; Gungor, A.; Aydin, Y.; Kocaman, G. Association of mean platelet volume with DSM-IV major depression in a large community-based population: The MELEN study. J. Psychiatr. Res. 2012, 46, 298–302. [Google Scholar] [CrossRef]
- Lopez-Vilchez, I.; Serra-Millas, M.; Navarro, V.; Rosa Hernandez, M.; Villalta, J.; Diaz-Ricart, M.; Gasto, C.; Escolar, G.; Galan, A.M. Prothrombotic platelet phenotype in major depression: Downregulation by antidepressant treatment. Affect. Disord. 2014, 159, 39–45. [Google Scholar] [CrossRef]
- Hausberg, M.; Hillebrand, U.; Kisters, K. Addressing sympathetic overactivity in major depressive disorder. J. Hypertens. 2007, 25, 2004–2005. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.K. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.E.; Yang, T.Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 1985, 242, 56–92. [Google Scholar] [CrossRef]
- Stegner, D.; Nieswandt, B. Platelet receptor signaling in thrombus formation. J. Mol. Med. 2011, 89, 109–121. [Google Scholar] [CrossRef]
- Halperin, D.; Reber, G. Influence of antidepressants on hemostasis. Dialogues Clin. Neurosci. 2007, 9, 47–59. [Google Scholar] [CrossRef]
- Andersohn, F.; Konzen, C.; Bronder, E.; Klimpel, A.; Garbe, E. Citalopram-induced bleeding due to severe thrombocytopenia. Psychosomatics 2009, 50, 297–298. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Wachowicz, B. Platelet haemostatic function in psychiatric disorders: Effects of antidepressants and antipsychotic drugs. World J. Biol. Psychiatry 2017, 18, 564–574. [Google Scholar] [CrossRef]
- Kate, N.; Grover, S.; Aggarwal, M.; Malhotra, P.; Sachdeva, M.S. Clozapine associated thrombocytopenia. J. Pharmacol. Pharmacother. 2013, 4, 149–151. [Google Scholar]
- Sahoo, S.; Singla, H.; Spoorty, M.; Malhotra, P.; Grover, S. Thrombocytopenia associated with olanzapine: A case report and review of literature. Indian J. Psychiatry 2016, 58, 339–341. [Google Scholar]
- De Berardis, D.; Campanella, D.; Matera, V.; Gambi, F.; La Rovere, R.; Sepede, G.; Grimaldi, M.R.; Pacilli, A.M.; Salerno, R.M.; Ferro, F.M. Thrombocytopenia during valproic acid treatment in young patients with new-onset bipolar disorder. J. Clin. Psychopharmacol. 2003, 23, 451–458. [Google Scholar] [CrossRef]
- Focosi, D.; Azzarà, A.; Kast, R.E.; Carulli, G.; Petrini, M. Lithium and hematology: Established and proposeduses. J. Leukoc. Biol. 2009, 85, 20–28. [Google Scholar] [CrossRef]
- Nixon, D.D. Thrombocytopenia following doxepin treatment. JAMA 1972, 220, 4. [Google Scholar] [CrossRef]
- Mirsal, H.; Kalyoncu, A.; Pektaş, O. Ecchymosis associated with the use of fluoxetine: Case report. Turk Psikiyatri Derg. 2002, 13, 320–324. [Google Scholar]
- Nguyen, L.; Reese, J.; George, J.N. Drug-induced thrombocytopenia: An updated systematic review, 2010. Drug Saf. 2011, 34, 437–438. [Google Scholar] [CrossRef]
- Greinacher, A.; Eichler, P.; Lubenow, N.; Kiefel, V. Drug-induced and drug-dependent immune thrombocytopenias. Rev. Clin. Exp. Hematol. 2001, 5, 166–200. [Google Scholar] [CrossRef]
- Song, H.R.; Jung, Y.E.; Wang, H.R.; Woo, Y.S.; Jun, T.Y.; Bahk, W.M. Platelet count alterations associated with escitalopram, venlafaxine and bupropion in depressive patients. Psychiatry Clin. Neurosci. 2012, 66, 457–459. [Google Scholar] [CrossRef]
- Oakes, T.M.; Katona, C.; Liu, P.; Robinson, M.; Raskin, J.; Greist, J.H. Safety and tolerability of duloxetine in elderly patients with major depressive disorder: A pooled analysis of two placebo-controlled studies. Int. Clin. Psychopharmacol. 2013, 28, 1–11. [Google Scholar] [CrossRef]
- Gronau, W.; Paslakis, G.; Lederbogen, F.; Weber-Hamann, B.; Gilles, M.; Schilling, C.; Deuschle, M. Increased platelet count after treatment with venlafaxine or mirtazapine in depressed patients. Pharmacopsychiatry 2015, 48, 37–39. [Google Scholar] [CrossRef]
- Hallbäck, I.; Hägg, S.; Eriksson, A.C.; Whiss, P.A. In vitro effects of serotonin and noradrenaline reuptake inhibitors on human platelet adhesion and coagulation. Pharmacol. Rep. 2012, 64, 979–983. [Google Scholar] [CrossRef]
- Sarma, A.; Horne, M.K., 3rd. Venlafaxine-induced ecchymoses and impaired platelet aggregation. Eur. J. Haematol. 2006, 77, 533–537. [Google Scholar] [CrossRef]
- Shaligram, D.; Alqassem, T.; Koby, E. Desvenlafaxine as a possible cause of acquired hemophilia. Gen. Hosp. Psychiatry 2010, 32, 646.e13–646.e15. [Google Scholar] [CrossRef]
- Tully, P.J.; Cardinal, T.; Bennetts, J.S.; Baker, R.A. Selective serotonin reuptake inhibitors, venlafaxine and duloxetine are associated with in hospital morbidity but not bleeding or late mortality after coronary artery bypass graft surgery. Heart Lung Circ. 2012, 21, 206–214. [Google Scholar] [CrossRef]
- Smith, M.M.; Smith, B.B.; Lahr, B.D.; Nuttall, G.A.; Mauermann, W.J.; Weister, T.J.; Dearani, J.A.; Barbara, D.W. Selective Serotonin Reuptake Inhibitors and Serotonin-Norepinephrine Reuptake Inhibitors Are Not Associated with Bleeding or Transfusion in Cardiac Surgical Patients. Anesth. Analg. 2018, 126, 1859–1866. [Google Scholar] [CrossRef]
- Yadav, D.; Vargo, J.; Lopez, R.; Chahal, P. Does serotonin reuptake inhibitor therapy increase the risk of post-sphincterotomy bleeding in patients undergoing endoscopic retrograde cholangio-pancreatography? World J. Gastrointest. Endosc. 2017, 9, 171–176. [Google Scholar] [CrossRef]
- Schultz, J.; Bream-Rouwenhorst, H.; Hobbs, R.; McDanel, D.; Goerbig-Campbell, J. Serotonergic agents increase the incidence of gastrointestinal bleeds in patients with continuous-flow left ventricular assist devices. J. Heart Lung Transplant. 2016, 35, 823–824. [Google Scholar] [CrossRef]
- Mawardi, G.; Markman, T.M.; Muslem, R.; Sobhanian, M.; Converse, M.; Meadows, H.B.; Uber, W.E.; Russell, S.D.; Rouf, R.; Ramu, B.; et al. SSRI/SNRI Therapy is Associated with a Higher Risk of Gastrointestinal Bleeding in LVAD Patients. Heart Lung Circ. 2020, 29, 1241–1246. [Google Scholar] [CrossRef]
- Modica, M.; Ferratini, M.; Torri, A.; Oliva, F.; Martinelli, L.; De Maria, R.; Frigerio, M. Quality of life and emotional distress early after left ventricular assist device implant: A mixed-method study. Artif. Organs 2015, 39, 220–227. [Google Scholar] [CrossRef]
- Brouwers, C.; Denollet, J.; de Jonge, N.; Caliskan, K.; Kealy, J.; Pedersen, S.S. Patient-reported outcomes in left ventricular assist device therapy: A systematic review and recommendations for clinical research and practice. Circ. Heart Fail. 2011, 4, 714–723. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolarczyk-Kosowska, J.; Kosowski, M.; Kunert, Ł.; Filipczyk, K.; Wojciechowski, M.; Piegza, M.; Gorczyca, P.; Okopień, B.; Pudlo, R. Impact of Venlafaxine on Platelet Count and Activity—Case Report and Narrative Review. Medicina 2022, 58, 626. https://doi.org/10.3390/medicina58050626
Smolarczyk-Kosowska J, Kosowski M, Kunert Ł, Filipczyk K, Wojciechowski M, Piegza M, Gorczyca P, Okopień B, Pudlo R. Impact of Venlafaxine on Platelet Count and Activity—Case Report and Narrative Review. Medicina. 2022; 58(5):626. https://doi.org/10.3390/medicina58050626
Chicago/Turabian StyleSmolarczyk-Kosowska, Joanna, Michał Kosowski, Łukasz Kunert, Karolina Filipczyk, Marcin Wojciechowski, Magdalena Piegza, Piotr Gorczyca, Bogusław Okopień, and Robert Pudlo. 2022. "Impact of Venlafaxine on Platelet Count and Activity—Case Report and Narrative Review" Medicina 58, no. 5: 626. https://doi.org/10.3390/medicina58050626
APA StyleSmolarczyk-Kosowska, J., Kosowski, M., Kunert, Ł., Filipczyk, K., Wojciechowski, M., Piegza, M., Gorczyca, P., Okopień, B., & Pudlo, R. (2022). Impact of Venlafaxine on Platelet Count and Activity—Case Report and Narrative Review. Medicina, 58(5), 626. https://doi.org/10.3390/medicina58050626





