Managing the Systemic Impact of Periodontitis
Abstract
:1. Introduction
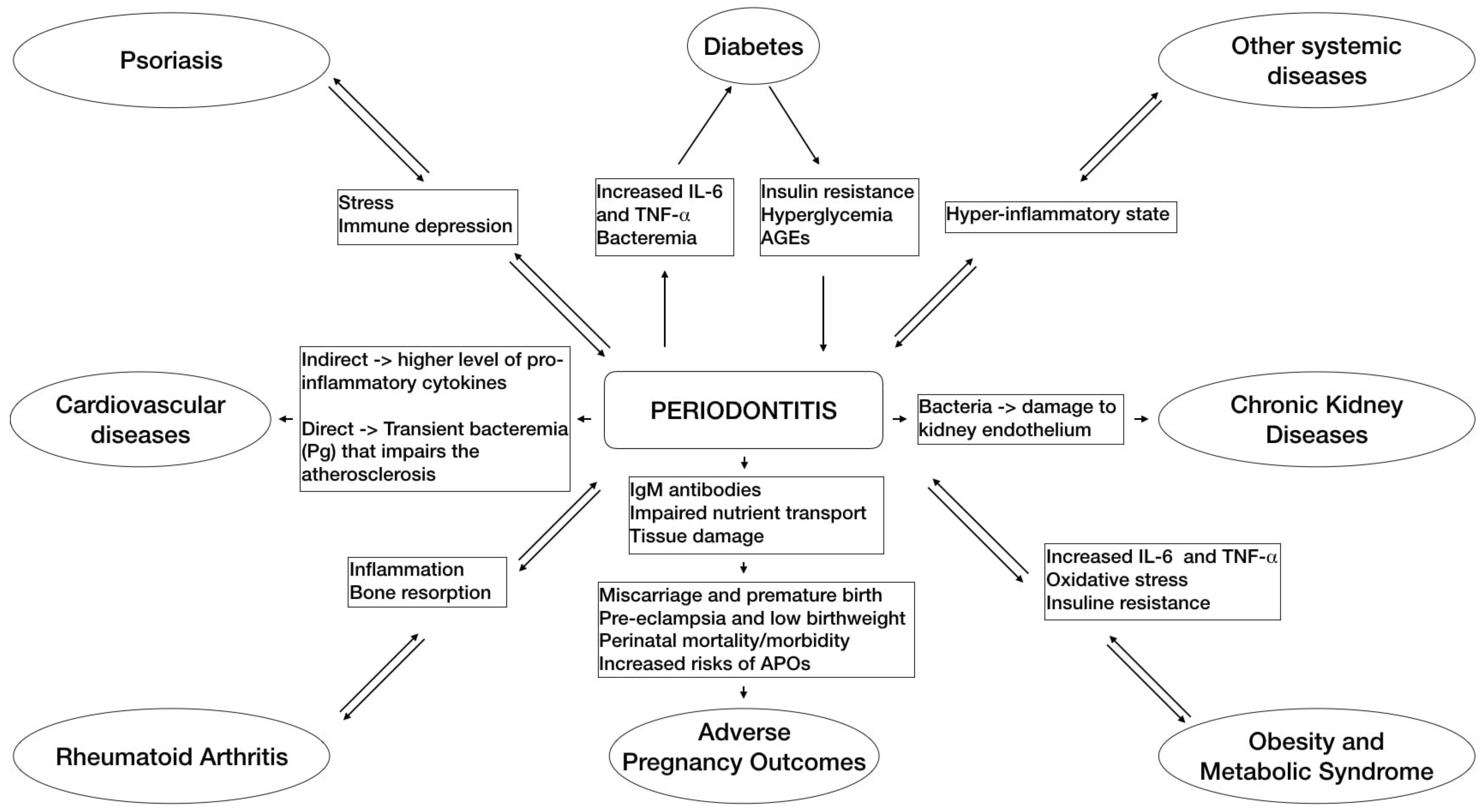
2. Periodontitis and Systemic Diseases
3. Impact of Periodontitis on Quality of Life and Public Health
4. Effects of Periodontal Treatment on Systemic Health in Both the Short- and Long-Term
4.1. Diabetes
4.2. Cardiovascular Diseases
4.3. Adverse Pregnancy Outcomes
4.4. Obesity and Metabolic Syndrome
4.5. Rheumatoid Arthritis
4.6. Chronic Kidney Disease
4.7. Psoriasis
4.8. Other Systemic Diseases
5. How We Can Reduce the Effect of Periodontal Treatment on Systemic Inflammation?
6. Conclusions and Recommendation for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linden, G.J.; Herzberg, M.C. Periodontitis and systemic diseases: A record of discussions of working group 4 of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Borgnakke, W.S.; Ylöstalo, P.V.; Taylor, G.W.; Genco, R.J. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J. Periodontol. 2013, 84, S135–S152. [Google Scholar] [CrossRef] [Green Version]
- Ide, M.; Papapanou, P.N. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes–systematic review. J. Periodontol. 2013, 84, S181–S194. [Google Scholar] [CrossRef]
- Graziani, F.; Cei, S.; Tonetti, M.; Paolantonio, M.; Serio, R.; Sammartino, G.; Gabriele, M.; D’Aiuto, F. Systemic inflammation following non-surgical and surgical periodontal therapy. J. Clin. Periodontol. 2010, 37, 848–854. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Nibali, L.; Mohamed-Ali, V.; Vallance, P.; Tonetti, M.S. Periodontal therapy: A novel non-drug-induced experimental model to study human inflammation. J. Periodontal Res. 2004, 39, 294–299. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; van Winkelhoff, A.J. Infection and inflammatory mechanisms. J. Periodontol. 2013, 84, S1–S7. [Google Scholar] [CrossRef]
- Chapple, I.L.; Genco, R. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40, S106–S112. [Google Scholar] [CrossRef]
- Taylor, J.J.; Preshaw, P.M.; Lalla, E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Clin. Periodontol. 2013, 40, S113–S134. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Orlandi, M.; Gunsolley, J.C. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J. Periodontol. 2013, 84, S85–S105. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Sharma, P.; Walter, C.; Weston, P.; Beck, J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J. Periodontol. 2013, 84, S70–S84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenkein, H.A.; Loos, B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Periodontol. 2013, 84, S51–S69. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Van Dyke, T.E. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40, S24–S29. [Google Scholar] [CrossRef]
- Romandini, M.; Laforí, A.; Romandini, P.; Baima, G.; Cordaro, M. Periodontitis and platelet count: A new potential link with cardiovascular and other systemic inflammatory diseases. J. Clin. Periodontol. 2018, 45, 1299–1310. [Google Scholar] [CrossRef]
- Madianos, P.N.; Bobetsis, Y.A.; Offenbacher, S. Adverse pregnancy outcomes (APOs) and periodontal disease: Pathogenic mechanisms. J. Periodontol. 2013, 84, S170–S180. [Google Scholar] [CrossRef]
- Sanz, M.; Kornman, K. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40, S164–S169. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.; Rohde, J.F.; Raymond, K.; Heitmann, B.L. Association between periodontal disease and overweight and obesity: A systematic review. J. Periodontol. 2015, 86, 766–776. [Google Scholar] [CrossRef]
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e708–e715. [Google Scholar] [CrossRef]
- Gobin, R.; Tian, D.; Liu, Q.; Wang, J. Periodontal Diseases and the Risk of Metabolic Syndrome: An Updated Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 336. [Google Scholar] [CrossRef]
- Lamster, I.B.; Pagan, M. Periodontal disease and the metabolic syndrome. Int. Dent. J. 2017, 67, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Sapey, E.; Yonel, Z.; Edgar, R.; Parmar, S.; Hobbins, S.; Newby, P.; Crossley, D.; Usher, A.; Johnson, S.; Walton, G.M.; et al. The clinical and inflammatory relationships between periodontitis and chronic obstructive pulmonary disease. J. Clin. Periodontol. 2020, 47, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhang, B.; Xing, H.; Yang, S.; Xu, J.; Liu, H. Patients with Chronic Obstructive Pulmonary Disease Suffer from Worse Periodontal Health-Evidence from a Meta-Analysis. Front. Physiol. 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Mastrangelo, F.; Russo, L.L.; Muzio, L.L. The Role of Periodontitis and Periodontal Bacteria in the Onset and Progression of Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2020, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Beydoun, M.A.; Beydoun, H.A.; Hossain, S.; El-Hajj, Z.W.; Weiss, J.; Zonderman, A.B. Clinical and Bacterial Markers of Periodontitis and Their Association with Incident All-Cause and Alzheimer’s Disease Dementia in a Large National Survey. J. Alzheimers Dis. 2020, 75, 157–172. [Google Scholar] [CrossRef]
- Araújo, V.M.; Melo, I.M.; Lima, V. Relationship between Periodontitis and Rheumatoid Arthritis: Review of the Literature. Mediat. Inflamm. 2015, 2015, 259074. [Google Scholar] [CrossRef] [Green Version]
- Chambrone, L.; Foz, A.M.; Guglielmetti, M.R.; Pannuti, C.M.; Artese, H.P.; Feres, M.; Romito, G.A. Periodontitis and chronic kidney disease: A systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J. Clin. Periodontol. 2013, 40, 443–456. [Google Scholar] [CrossRef]
- Wahid, A.; Chaudhry, S.; Ehsan, A.; Butt, S.; Ali Khan, A. Bidirectional Relationship between Chronic Kidney Disease & Periodontal Disease. Pak. J. Med. Sci. 2013, 29, 211–215. [Google Scholar] [CrossRef]
- Preus, H.R.; Khanifam, P.; Kolltveit, K.; Mørk, C.; Gjermo, P. Periodontitis in psoriasis patients: A blinded, case-controlled study. Acta Odontol. Scand. 2010, 68, 165–170. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.H.; Lung, C.C.; Su, H.P.; Huang, J.Y.; Ko, P.C.; Jan, S.R.; Sun, Y.H.; Nfor, O.N.; Tu, H.P.; Chang, C.S.; et al. Association between periodontal disease and osteoporosis by gender: A nationwide population-based cohort study. Medicine 2015, 94, e553. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Goberna, B.; Guerrero, J.M.; Segura, J.J.; Perez-Cano, R.; Martinez-Sahuquillo, A. Serum, saliva, and gingival crevicular fluid osteocalcin: Their relation to periodontal status and bone mineral density in postmenopausal women. J. Periodontol. 2005, 76, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontol. 2000 2020, 83, 213–233. [Google Scholar] [CrossRef]
- Myers-Wright, N.; Lamster, I.B. A New Practice Approach for Oral Health Professionals. J. Evid. Based Dent. Pract. 2016, 16, 43–51. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Fourmousis, I.; Suvan, J.; Cortellini, P.; Brägger, U.; Lang, N.P. Healing, post-operative morbidity and patient perception of outcomes following regenerative therapy of deep intrabony defects. J. Clin. Periodontol. 2004, 31, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Richards, P.S.; Inglehart, M.R. Periodontal health, quality of life, and smiling patterns—An exploration. J. Periodontol. 2008, 79, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Needleman, I.; McGrath, C.; Floyd, P.; Biddle, A. Impact of oral health on the life quality of periodontal patients. J. Clin. Periodontol. 2004, 31, 454–457. [Google Scholar] [CrossRef]
- Shanbhag, S.; Dahiya, M.; Croucher, R. The impact of periodontal therapy on oral health-related quality of life in adults: A systematic review. J. Clin. Periodontol. 2012, 39, 725–735. [Google Scholar] [CrossRef]
- Khan, S.; Khalid, T.; Bettiol, S.; Crocombe, L.A. Non-surgical periodontal therapy effectively improves patient-reported outcomes: A systematic review. Int. J. Dent. Hyg. 2021, 19, 18–28. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Jeffcoat, M.K.; Jeffcoat, R.L.; Gladowski, P.A.; Bramson, J.B.; Blum, J.J. Impact of periodontal therapy on general health: Evidence from insurance data for five systemic conditions. Am. J. Prev. Med. 2014, 47, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, S.C.P.; Carter, N.; Cortellini, P.; Kocher, T.; Listl, S.; Loos, B.; Marcenes, W.; Nart, J.; Needleman, I.; Papapanou, P.; et al. Time to Take Gum Disease Seriously. Available online: https://impact.economist.com/perspectives/sites/default/files/eiu-efp-oralb-gum-disease.pdf (accessed on 24 September 2021).
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Aass, A.M.; Aimetti, M.; et al. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, F.; Parkar, M.; Tonetti, M.S. Acute effects of periodontal therapy on bio-markers of vascular health. J. Clin. Periodontol. 2007, 34, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, V.; Botelho, J.; Escalda, C.; Hussain, S.B.; Luthra, S.; Mascarenhas, P.; Orlandi, M.; Mendes, J.J.; D’Aiuto, F. Serum C-Reactive Protein and Periodontitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 706432. [Google Scholar] [CrossRef] [PubMed]
- Bascones-Martinez, A.; Matesanz-Perez, P.; Escribano-Bermejo, M.; González-Moles, M.; Bascones-Ilundain, J.; Meurman, J.H. Periodontal disease and diabetes-Review of the Literature. Med. Oral Patol. Oral Cir. Bucal. 2011, 16, e722–e729. [Google Scholar] [CrossRef] [Green Version]
- Engebretson, S.; Kocher, T. Evidence that periodontal treatment improves diabetes outcomes: A systematic review and meta-analysis. J. Periodontol. 2013, 84, S153–S169. [Google Scholar] [CrossRef] [Green Version]
- Correa, F.O.; Gonçalves, D.; Figueredo, C.M.; Bastos, A.S.; Gustafsson, A.; Orrico, S.R. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J. Clin. Periodontol. 2010, 37, 53–58. [Google Scholar] [CrossRef]
- Artese, H.P.; Foz, A.M.; Rabelo, M.d.S.; Gomes, G.H.; Orlandi, M.; Suvan, J.; D’Aiuto, F.; Romito, G.A. Periodontal therapy and systemic inflammation in type 2 diabetes mellitus: A meta-analysis. PLoS ONE 2015, 10, e0128344. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.P.E.; Belém, F.V.; Abreu, L.G.; Cunha, F.A.; Cota, L.O.M.; da Costa, J.E.; Costa, F.O. Effect of Periodontal Therapy on Serum Levels of IL-6 in Type 2 Diabetics: A Systematic Review. Int. J. Periodontics Restor. Dent. 2019, 39, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, V.E.; Kornman, K.S.; Beck, J.D.; Genco, R.; Goldfine, A.; Libby, P.; Offenbacher, S.; Ridker, P.M.; Van Dyke, T.E.; Roberts, W.C. The American Journal of Cardiology and Journal of Periodontology editors’ consensus: Periodontitis and atherosclerotic cardiovascular disease. J. Periodontol. 2009, 80, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang; Pickett, F.A. AHA statement on periodontal disease and heart disease. J. Can. Dent. Assoc. 2012, 77, c54. [Google Scholar]
- Mesa, F.; Magan-Fernandez, A.; Castellino, G.; Chianetta, R.; Nibali, L.; Rizzo, M. Periodontitis and mechanisms of cardiometabolic risk: Novel insights and future perspectives. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 476–484. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Osinbowale, O.; Trevisan, M.; Levison, M.E.; Taubert, K.A.; Newburger, J.W.; Gornik, H.L.; Gewitz, M.H.; et al. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association?: A scientific statement from the American Heart Association. Circulation 2012, 125, 2520–2544. [Google Scholar] [CrossRef]
- Reyes, L.; Herrera, D.; Kozarov, E.; Roldá, S.; Progulske-Fox, A. Periodontal bacterial invasion and infection: Contribution to atherosclerotic pathology. J. Periodontol. 2013, 84, S30–S50. [Google Scholar] [CrossRef] [Green Version]
- Teeuw, W.J.; Slot, D.E.; Susanto, H.; Gerdes, V.E.; Abbas, F.; D’Aiuto, F.; Kastelein, J.J.; Loos, B.G. Treatment of periodontitis improves the atherosclerotic profile: A systematic review and meta-analysis. J. Clin. Periodontol. 2014, 41, 70–79. [Google Scholar] [CrossRef]
- Freitas, C.O.; Gomes-Filho, I.S.; Naves, R.C.; Filho, G.D.R.N.; Cruz, S.S.; Santos, C.A.; Dunningham, L.; Miranda, L.F.; Barbosa, M.D. Influence of periodontal therapy on C-reactive protein level: A systematic review and meta-analysis. J. Appl. Oral Sci. 2012, 20, 1–8. [Google Scholar] [CrossRef]
- Vidal, F.; Figueredo, C.M.; Cordovil, I.; Fischer, R.G. Periodontal therapy reduces plasma levels of interleukin-6, C-reactive protein, and fibrinogen in patients with severe periodontitis and refractory arterial hypertension. J. Periodontol. 2009, 80, 786–791. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.H.; Kang, S.H.; Yoon, C.H.; Lee, H.J.; Yun, P.Y.; Youn, T.J.; Chae, I.H. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: A population-based study from Korea. Eur. Heart J. 2019, 40, 1138–1145. [Google Scholar] [CrossRef]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Duan, X. Association between periodontitis and hyperlipidaemia: A systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Cappello, F.; Marfil, R.; Nibali, L.; Marino Gammazza, A.; Rappa, F.; Bonaventura, G.; Galindo-Moreno, P.; O’Valle, F.; Zummo, G.; et al. Heat-shock protein 60 kDa and atherogenic dyslipidemia in patients with untreated mild periodontitis: A pilot study. Cell Stress Chaperones 2012, 17, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, M.; Rini, G.B.; Berneis, K. The clinical relevance of LDL size and subclasses modulation in patients with type-2 diabetes. Exp. Clin. Endocrinol. Diabetes 2007, 115, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, M.S.; Pawar, B.R.; Marawar, P.P.; Khairnar, D.M. Estimation of changes in C-reactive protein level and pregnancy outcome after nonsurgical supportive periodontal therapy in women affected with periodontitis in a rural set up of India. Contemp. Clin. Dent. 2015, 6, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Penova-Veselinovic, B.; Keelan, J.A.; Wang, C.A.; Newnham, J.P.; Pennell, C.E. Changes in inflammatory mediators in gingival crevicular fluid following periodontal disease treatment in pregnancy: Relationship to adverse pregnancy outcome. J. Reprod. Immunol. 2015, 112, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Aiuto, F.; Sabbah, W.; Netuveli, G.; Donos, N.; Hingorani, A.D.; Deanfield, J.; Tsakos, G. Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. J. Clin. Endocrinol. Metab. 2008, 93, 3989–3994. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Nam, H.S.; Seo, H.S.; Hwang, S.J. Change of periodontal inflammatory indicators through a 4-week weight control intervention including caloric restriction and exercise training in young Koreans: A pilot study. BMC Oral Health 2015, 15, 109. [Google Scholar] [CrossRef] [Green Version]
- Torumtay, G.; Kırzıoğlu, F.Y.; Tonguç, M.Ö.; Kale, B.; Calapoğlu, M.; Orhan, H. Effects of periodontal treatment on inflammation and oxidative stress markers in patients with metabolic syndrome. J. Periodontal Res. 2016, 51, 489–498. [Google Scholar] [CrossRef]
- Nibali, L.; Tatarakis, N.; Needleman, I.; Tu, Y.K.; D’Aiuto, F.; Rizzo, M.; Donos, N. Clinical review: Association between metabolic syndrome and periodontitis: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; White, S.; Bartold, P.M. Periodontal disease and rheumatoid arthritis: A systematic review. J. Dent. Res. 2013, 92, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.; Wegner, N.; Yucel-Lindberg, T.; Venables, P.J. Periodontitis in RA—The citrullinated enolase connection. Nat. Rev. Rheumatol. 2010, 6, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bright, R.; Proudman, S.M.; Bartold, P.M. Does periodontal treatment influence clinical and biochemical measures for rheumatoid arthritis? A systematic review and meta-analysis. Semin. Arthritis Rheum. 2014, 44, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Xu, X.; Liu, Q.; Li, X.; Xiao, Y.; Hu, B. Effects of non-surgical periodontal therapy on systemic inflammation and metabolic markers in patients undergoing haemodialysis and/or peritoneal dialysis: A systematic review and meta-analysis. BMC Oral Health 2020, 20, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilela, E.M.; Bastos, J.A.; Fernandes, N.; Ferreira, A.P.; Chaoubah, A.; Bastos, M.G. Treatment of chronic periodontitis decreases serum prohepcidin levels in patients with chronic kidney disease. Clinics 2011, 66, 657–662. [Google Scholar] [CrossRef] [Green Version]
- Nakib, S.; Han, J.; Li, T.; Joshipura, K.; Qureshi, A.A. Periodontal disease and risk of psoriasis among nurses in the United States. Acta Odontol. Scand. 2013, 71, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Raman, A.; Pradeep, A.R. Association of chronic periodontitis and psoriasis: Periodontal status with severity of psoriasis. Oral Dis. 2015, 21, 314–319. [Google Scholar] [CrossRef]
- Yarkac, F.U.; Ogrum, A.; Gokturk, O. Effects of non-surgical periodontal therapy on inflammatory markers of psoriasis: A randomized controlled trial. J. Clin. Periodontol. 2020, 47, 193–201. [Google Scholar] [CrossRef]
- Orlandi, M.; Aguilera, E.M.; Marletta, D.; Petrie, A.; Suvan, J.; D’Aiuto, F. Impact of the treatment of periodontitis on systemic health and quality of life: A systematic review. J. Clin. Periodontol. 2021. [Google Scholar] [CrossRef]
- Graziani, F.; Cei, S.; Orlandi, M.; Gennai, S.; Gabriele, M.; Filice, N.; Nisi, M.; D’Aiuto, F. Acute-phase response following full-mouth versus quadrant non-surgical periodontal treatment: A randomized clinical trial. J. Clin. Periodontol. 2015, 42, 843–852. [Google Scholar] [CrossRef]
- Minassian, C.; D’Aiuto, F.; Hingorani, A.D.; Smeeth, L. Invasive dental treatment and risk for vascular events: A self-controlled case series. Ann. Intern. Med. 2010, 153, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Hatz, C.R.; Cremona, M.; Liu, C.C.; Schmidlin, P.R.; Conen, A. Antibiotic prophylaxis with amoxicillin to prevent infective endocarditis in periodontitis patients reconsidered: A narrative review. Swiss Med. Wkly. 2021, 151, w30078. [Google Scholar] [PubMed]
- Srivastava, A.; Brewer, A.; Mauser-Bunschoten, E.; Key, N.; Kitchen, S.; Llinas, A.; Ludlam, C.; Mahlangu, J.; Mulder, K.; Poon, M.; et al. Guidelines for the management of hemophilia. Haemophilia 2013, 19, e1–e47. [Google Scholar] [CrossRef] [PubMed]
- Research, Science and Therapy Committee, American Academy of Periodontology. Periodontal management of patients with cardiovascular diseases. J. Periodontol. 2002, 73, 954–968. [Google Scholar] [CrossRef]
- Sanz, M.; del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Keceli, H.G.; Ercan, N.; Hendek, M.K.; Kisa, U.; Mesut, B.; Olgun, E. The effect of the systemic folic acid intake as an adjunct to scaling and root planing on clinical parameters and homocysteine and C-reactive protein levels in gingival crevicular fluid of periodontitis patients: A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2020, 47, 602–613. [Google Scholar] [CrossRef]
- Žekonis, G.; Žekonis, J.; Gleiznys, A.; Noreikienė, V.; Balnytė, I.; Šadzevičienė, R.; Narbutaitė, J. Effect of Supragingival Irrigation with Aerosolized 0.5% Hydrogen Peroxide on Clinical Periodontal Parameters, Markers of Systemic Inflammation, and Morphology of Gingival Tissues in Patients with Periodontitis. Med. Sci. Monit. 2016, 22, 3713–3721. [Google Scholar] [CrossRef]
- Lee, M.K.; Ide, M.; Coward, P.Y.; Wilson, R.F. Effect of ultrasonic debridement using a chlorhexidine irrigant on circulating levels of lipopolysaccharides and interleukin-6. J. Clin. Periodontol. 2008, 35, 415–419. [Google Scholar] [CrossRef]
- Rasperini, G.; Pellegrini, G.; Sugai, J.; Mauro, C.; Fiocchi, S.; Mora, P.C.; Dellavia, C. Effects of food supplements on periodontal status and local and systemic inflammation after nonoperative periodontal treatment. J. Oral Sci. 2019, 61, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Graziani, F.; Gennai, S.; Petrini, M.; Bettini, L.; Tonetti, M. Enamel matrix derivative stabilizes blood clot and improves clinical healing in deep pockets after flapless periodontal therapy: A Randomized Clinical Trial. J. Clin. Periodontol. 2019, 46, 231–240. [Google Scholar] [CrossRef]
- Luo, P.P.; Xu, H.S.; Chen, Y.W.; Wu, S.P. Periodontal disease severity is associated with micronutrient intake. Aust. Dent. J. 2018, 63, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Neiva, R.F.; Steigenga, J.; Al-Shammari, K.F.; Wang, H.L. Effects of specific nutrients on periodontal disease onset, progression and treatment. J. Clin. Periodontol. 2003, 30, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.G.; Flemmig, T.F.; Nachnani, S.; Rodrigues, A.; Calsina, G.; Lee, Y.S.; de Camargo, P.; Doherty, F.M.; Bakdash, M.B. Irrigation with 0.06% chlorhexidine in naturally occurring gingivitis. II. 6 months microbiological observations. J. Periodontol. 1990, 61, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, M.; Westphal, C.; Froum, S.; Palat, M.; Schoor, R. Comprehensive Periodontics for the Dental Hygienist, 4th ed.; Higher, P., Ed.; Pearson: New York, NY, USA, 2014. [Google Scholar]
- Behle, J.H.; Sedaghatfar, M.H.; Demmer, R.T.; Wolf, D.L.; Celenti, R.; Kebschull, M.; Belusko, P.B.; Herrera-Abreu, M.; Lalla, E.; Papapanou, P.N. Heterogeneity of systemic inflammatory responses to periodontal therapy. J. Clin. Periodontol. 2009, 36, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, K.; Almas, K. The Role of Nutrition in Periodontal Health: An Update. Nutrients 2016, 8, 530. [Google Scholar] [CrossRef]
| Authors | Study | Periodontitis Definition | Follow-up | Treatment(s) | Results |
|---|---|---|---|---|---|
| Graziani et al., 2015 [81] | Randomized clinical trial | Proximal attachment loss of ≥3 mm in ≥2 non-adjacent teeth (Tonetti & Claffey 2005), bleeding on probing on at least 25% of their total sites, and documented radiographic bone loss | 3 months | Full-mouth vs. quadrant scaling | Full-mouth PMPR resulted in significant increase of CRP, IL-6 or TNF-α compared to quadrant (conventional) PMPR |
| Keceli et al., 2020 [87] | Randomized placebo-controlled clinical trial | Periodontitis stage II–III (2017 World Workshop) | 6 months | PMPR + Folic acid (FA) + vs. PMPR alone | FA group resulted in no significant change in homocysteine (Hcy) and CRP levels in GCF compared to placebo group |
| Zekonis et al., 2016 [88] | Prospective cohort study | PPD ≥ 6 mm on at least 2 teeth, and radiographic evidence of horizontal and vertical bone loss. | 1-, 2- and 3-years | PMPR + supragingival irrigations with 0.5% hydrogen peroxide | Plasma levels of high sensitivity CRP (hs-CRP) and white blood cell (WBC) count decreased significantly after 1 and 2 years, whereas at 3 years a less evident decrease was found |
| Lee et al., 2008 [89] | Prospective single-masked, split-mouth, crossover interventional study | At least five sites per quadrant with PPD ≥ 5 mm and radiographic evidence of alveolar bone loss. | Day 0 | PMPR (ultrasonic instrumentation) + 0.02% chlorhexidine (CHX) irrigations with sterile water | CHX irrigations did not have any influence on increasing levels of lipopolysaccharide (LPS) and IL-6 |
| Rasperini et al., 2019 [90] | Randomized clinical trial | at least two sites with PPD > 7 mm, bleeding on probing > 25% | Day 0, 1 month and 3 months | PMPR + macronutrient complex vs. PMPR + olive oil-filled capsules | No statistically significant differences were observed between groups in terms of salivary and serum MMP-8/-9 levels at any time point. CRP serum levels were reduced after 3 months in both groups, but not significantly |
| Graziani et al., 2019 [91] | Randomized clinical trial | AL of ≥3 mm in ≥2 non-adjacent teeth (Tonetti, Claffey, & European Workshop in Periodontology Group C, 2005), bleeding on probing on at least 25% of total sites and documented radiographic bone loss | 3 months | PMPR + EMD vs. PMPR | At 24 h significant increase of CRP, D-dimer and cystatin C in control group and, only increase in CRP and fibrinogen in EMD group. At 3 months, biomarkers levels significantly decreased and returned to baseline values in both groups, except for glucose that was significant lower in the EMD group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mainas, G.; Ide, M.; Rizzo, M.; Magan-Fernandez, A.; Mesa, F.; Nibali, L. Managing the Systemic Impact of Periodontitis. Medicina 2022, 58, 621. https://doi.org/10.3390/medicina58050621
Mainas G, Ide M, Rizzo M, Magan-Fernandez A, Mesa F, Nibali L. Managing the Systemic Impact of Periodontitis. Medicina. 2022; 58(5):621. https://doi.org/10.3390/medicina58050621
Chicago/Turabian StyleMainas, Giuseppe, Mark Ide, Manfredi Rizzo, Antonio Magan-Fernandez, Francisco Mesa, and Luigi Nibali. 2022. "Managing the Systemic Impact of Periodontitis" Medicina 58, no. 5: 621. https://doi.org/10.3390/medicina58050621
APA StyleMainas, G., Ide, M., Rizzo, M., Magan-Fernandez, A., Mesa, F., & Nibali, L. (2022). Managing the Systemic Impact of Periodontitis. Medicina, 58(5), 621. https://doi.org/10.3390/medicina58050621









