Extended Lymphadenectomy for Proximal Transverse Colon Cancer: Is There a Place for Standardization?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
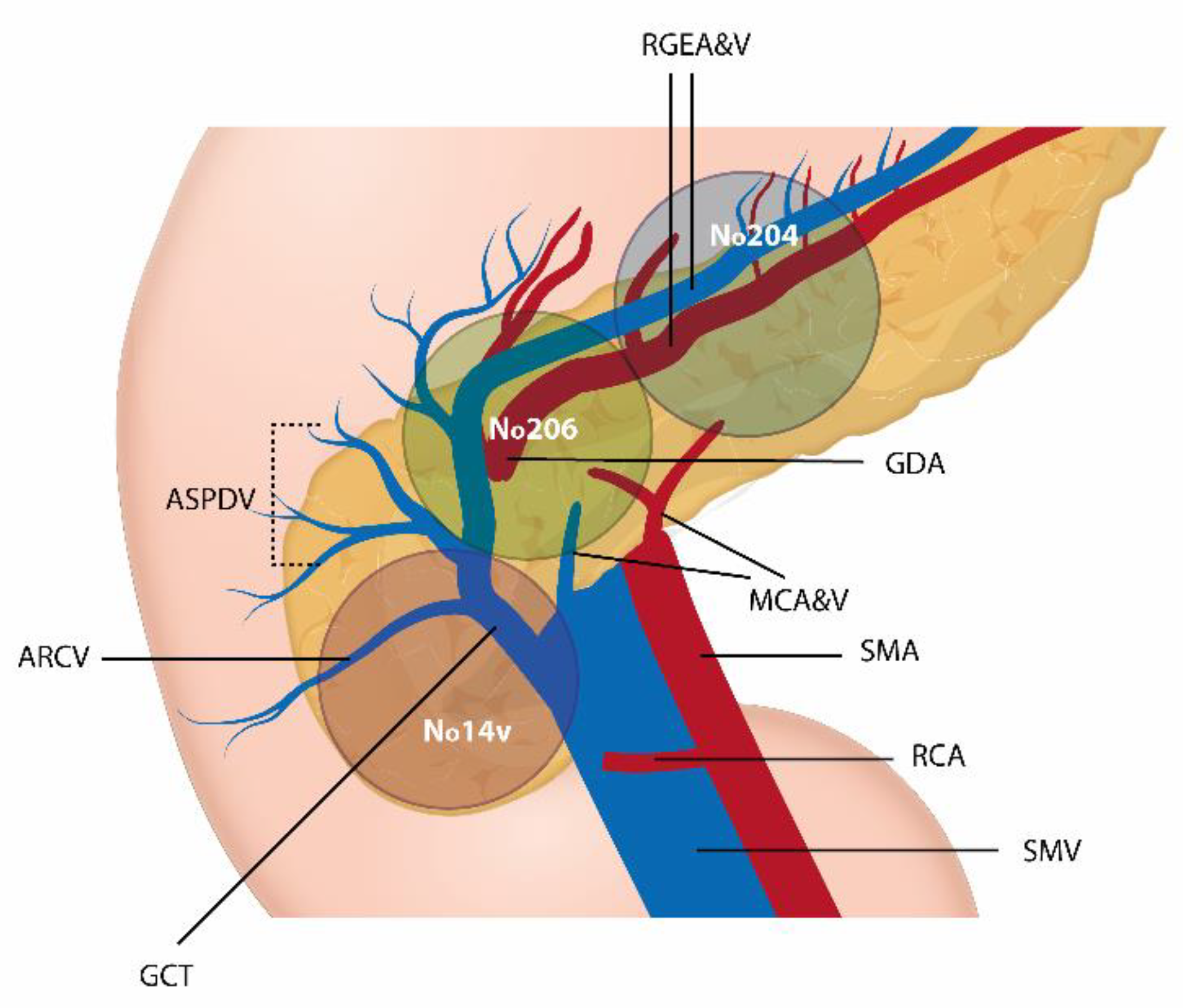
2.2. Surgical Technique
2.3. Study Endpoints
2.4. Pathology
2.5. Follow-Up
2.6. Statistical Analysis
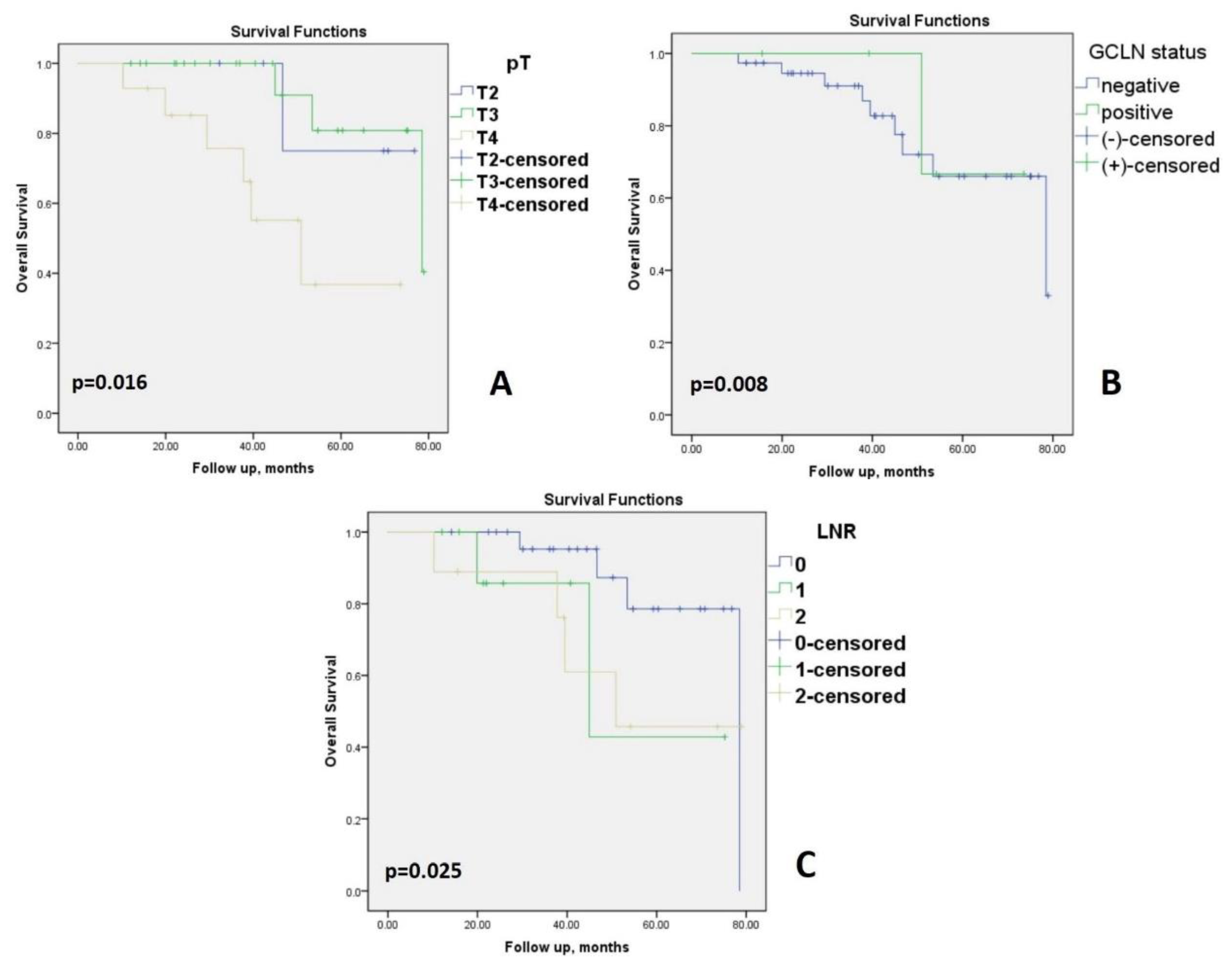
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Kobayashi, H.; Takahashi, K.; Perrakis, A.; Weber, K.; Hohenberger, W.; Sugihara, K.; Quirke, P. Understanding optimal colonic cancer surgery: Comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J. Clin. Oncol. 2012, 30, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.J.; Tveit, K.M.; Gibson, F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin. Colorectal Cancer 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009, 11, 354–364. [Google Scholar] [CrossRef]
- Kim, H.R. Complete Mesocolic Excision With Central Vascular Ligation for the Treatment of Patients With Colon Cancer. Ann. Coloproctol. 2018, 34, 165–166. [Google Scholar] [CrossRef] [Green Version]
- Perrakis, A.; Weber, K.; Merkel, S.; Matzel, K.; Agaimy, A.; Gebbert, C.; Hohenberger, W. Lymph node metastasis of carcinomas of transverse colon including flexures. Consideration of the extramesocolic lymph node stations. Int. J. Colorectal Dis. 2014, 29, 1223–1229. [Google Scholar] [CrossRef]
- Kessler, H.; Hohenberger, W. Extended lymphadenectomy in colon cancer is crucial. World J. Surg. 2013, 37, 1789–1798. [Google Scholar] [CrossRef]
- Bertelsen, C.A.; Bols, B.; Ingeholm, P.; Jansen, J.E.; Jepsen, L.V.; Kristensen, B.; Neuenschwander, A.U.; Gögenur, I. Lymph node metastases in the gastrocolic ligament in patients with colon cancer. Dis. Colon. Rectum. 2014, 57, 839–845. [Google Scholar] [CrossRef]
- Yuksel, B.C.; Er, S.; Çetinkaya, E.; Aşlar, A.K. Does transverse colon cancer spread to the extramesocolic lymph node stations? Acta Chir. Belg. 2021, 121, 102–108. [Google Scholar] [CrossRef]
- Strombom, P.; Widmar, M.; Keskin, M.; Gennarelli, R.L.; Lynn, P.; Smith, J.J.; Guillem, J.G.; Paty, P.B.; Nash, G.M.; Weiser, M.R.; et al. Assessment of the Value of Comorbidity Indices for Risk Adjustment in Colorectal Surgery Patients. Ann. Surg. Oncol. 2019, 26, 2797–2804. [Google Scholar] [CrossRef]
- Bolliger, M.; Kroehnert, J.A.; Molineus, F.; Kandioler, D.; Schindl, M.; Riss, P. Experiences with the standardized classification of surgical complications (Clavien-Dindo) in general surgery patients. Eur. Surg. 2018, 50, 256–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, O.; Sekishita, Y.; Shiono, T.; Ono, K.; Fujimori, M.; Kondo, S. Number of lymph node metastases is better predictor of prognosis than level of lymph node metastasis in patients with node-positive colon cancer. J. Am. Coll. Surg. 2006, 202, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.E.; Idrees, K.; Parikh, A.A. Resection of non-regional lymph node metastasis in patients with colorectal cancer. J. Clin. Oncol. 2016, 34 (Suppl. 4), 664. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.; Lu, X.; Huang, Y.; Chi, P. Incidence of and Risk Factors for Gastroepiploic Lymph Node Involvement in Patients with Cancer of the Transverse Colon Including the Hepatic Flexure. World J. Surg. 2021, 45, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, E.M.; Tieranu, C.G.; Maftei, D.; Grivei, A.; Olteanu, A.O.; Arbanas, T.; Calu, V.; Musat, S.; Mihaescu-Pintia, C.; Cucu, I.C. Colorectal Cancer Trends of 2018 in Romania-an Important Geographical Variation Between Northern and Southern Lands and High Mortality Versus European Averages. J. Gastrointest Cancer 2021, 52, 222–228. [Google Scholar] [CrossRef]
- Arslan, N.C.; Sokmen, S.; Canda, A.E.; Terzi, C.; Sarioglu, S. The prognostic impact of the log odds of positive lymph nodes in colon cancer. Colorectal Dis. 2014, 16, O386–O392. [Google Scholar] [CrossRef]
- Kobayashi, H.; Enomoto, M.; Higuchi, T.; Uetake, H.; Iida, S.; Ishikawa, T.; Ishiguro, M.; Kato, S.; Sugihara, K. Clinical significance of lymph node ratio and location of nodal involvement in patients with right colon cancer. Dig. Surg. 2011, 28, 190–197. [Google Scholar] [CrossRef]
- Uematsu, D.; Akiyama, G.; Sugihara, T.; Magishi, A.; Yamaguchi, T.; Sano, T. Laparoscopic radical lymph node dissection for advanced colon cancer close to the hepatic flexure. Asian J. Endosc. Surg. 2017, 10, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wang, X.; Deng, Y.; Jiang, W.; Huang, Y.; Chi, P. Gastrocolic Ligament Lymph Node Dissection for Transverse Colon and Hepatic Flexure Colon Cancer: Risk of Nodal Metastases and Complications in a Large-Volume Center. J. Gastrointest. Surg. 2020, 24, 2658–2660. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) The TNM Classification of Malignant Tumours; Wiley Blackwell: Oxford, UK, 2017. [Google Scholar]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Feng, B.; Sun, J.; Ling, T.L.; Lu, A.G.; Wang, M.L.; Chen, X.Y.; Ma, J.J.; Li, J.W.; Zang, L.; Han, D.P.; et al. Laparoscopic complete mesocolic excision (CME) with medial access for right-hemi colon cancer: Feasibility and technical strategies. Surg. Endosc. 2012, 26, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, H.; Kawamura, J.; Ueda, K.; Yane, Y.; Yoshioka, Y.; Daito, K.; Tokoro, T.; Hida, J.I.; Okuno, K. Visualization of lymphatic flow in laparoscopic colon cancer surgery using indocyanine green fluorescence imaging. Sci. Rep. 2020, 10, 14274. [Google Scholar] [CrossRef]
- Piozzi, G.N.; Rusli, S.M.; Baek, S.J.; Kwak, J.M.; Kim, J.; Kim, S.H. Infrapyloric and gastroepiploic node dissection for hepatic flexure and transverse colon cancer: A systematic review. Eur. J. Surg. Oncol. 2021, 48, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, S.; Chi, P.; Huang, Y.; Ye, D.; Xu, Y. Prognostic value of gastroepiploic lymph node metastasis in transverse colon cancer. Chin. J. Dig. Surg. 2021, 12, 315–322. [Google Scholar]
- NCCN Guidelines Version 3.2021. Colon Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 10 December 2021).
- Bertelsen, C.A. Complete mesocolic excision an assessment of feasibility and outcome. Dan. Med. J. 2017, 64, B5334. [Google Scholar] [PubMed]
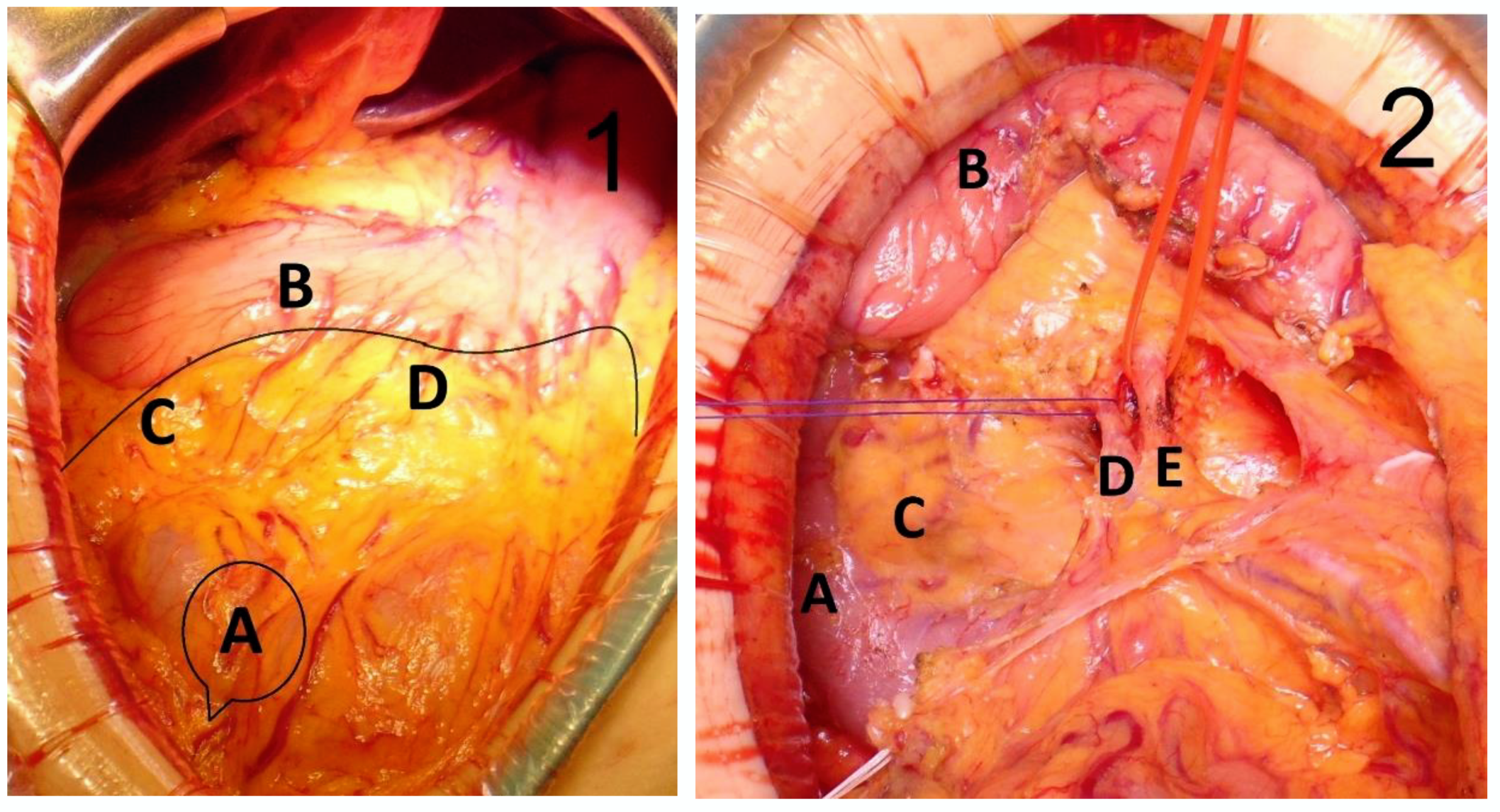
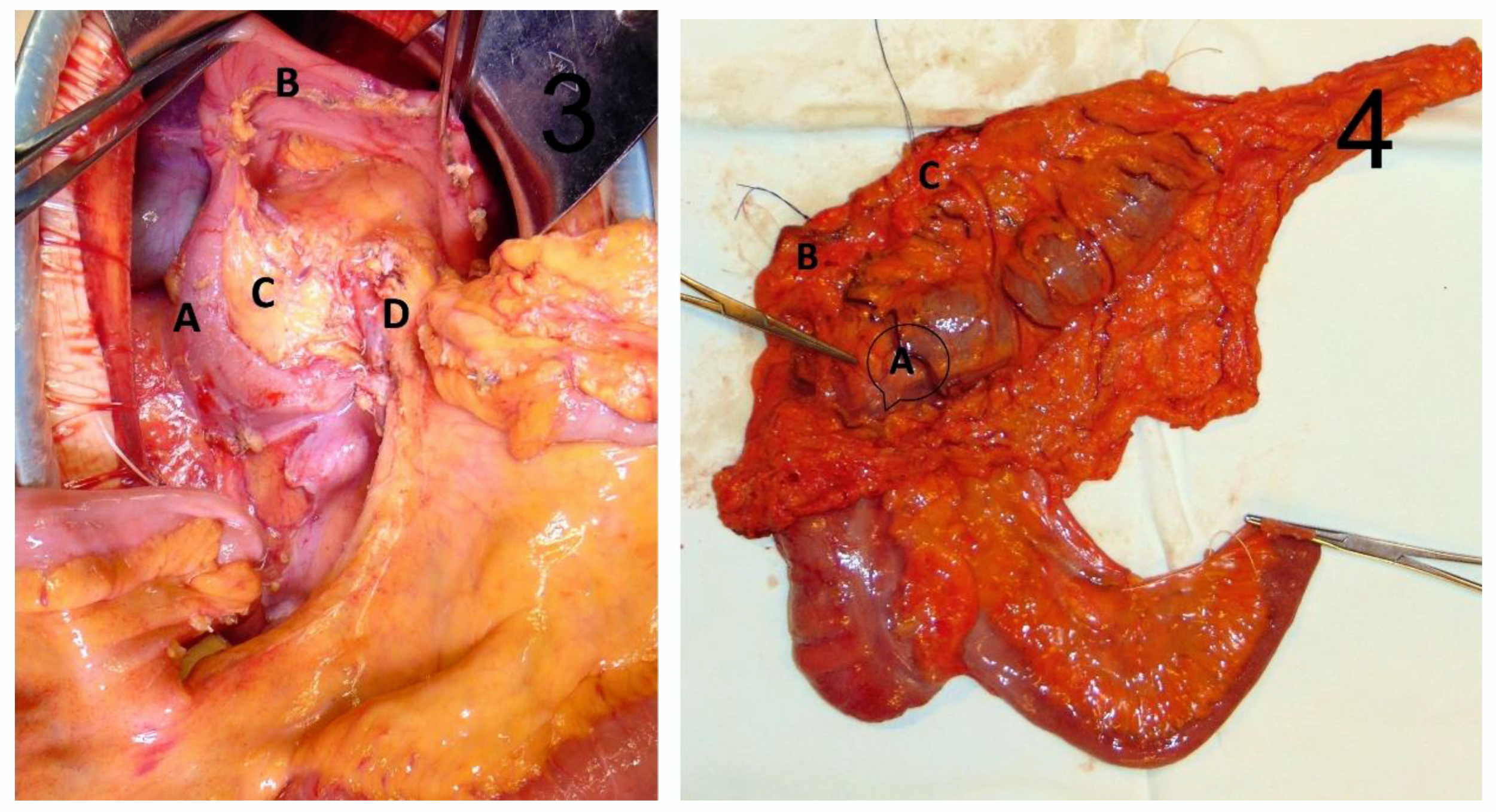
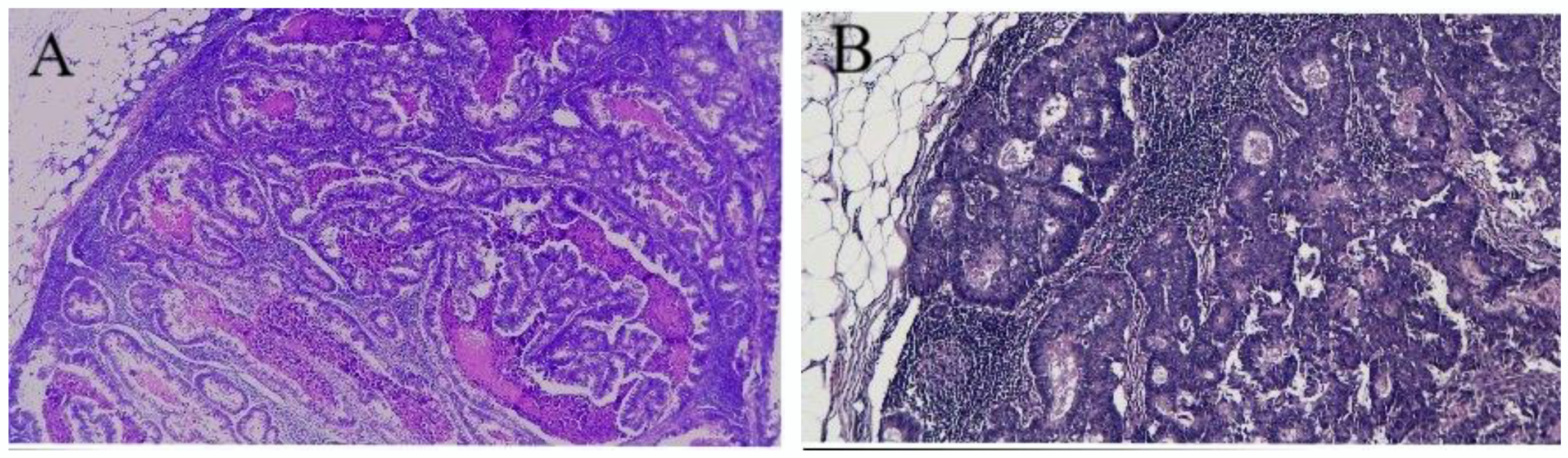
| Variables | Patients (n = 43) | Percentage (%) |
|---|---|---|
| Age ** (y) | 65.09 ± 12.63 (35–86) | |
| Gender Male Female | 18 25 | 41.9 58.1 |
| BMI * Underweight Normal Overweight Obesity I Obesity II Obesity III | 26.3 (17.8–43) 1 15 16 9 1 1 | 2.3 34.9 37.2 20.9 2.3 2.3 |
| ACCI Mean ± SD 0–1 2–3 4–5 ≥6 | 4.95 ± 1.78 0 7 22 14 | 0 16.3 51.2 32.6 |
| Abdominal operation history | 4 | 9.3 |
| Surgical approach Laparoscopic Open | 13 30 | 30.2 69.8 |
| Operation time (min) ** | 193.14 ± 22.15 (150–240) | |
| Estimate blood loss (mL) ** | 114.19 ± 35.87 (0–210) | |
| Postoperative hospital stay * | 6 (5–11) | |
| Follow-up * (m) | 40.77 (10.27–78.93) |
| Variables | Patients (n) | Percentage (%) |
|---|---|---|
| Complications | 11 | 25.6 |
| Reoperation | 0 | 0 |
| Clavien–Dindo classification | ||
| Grade I | 2 | 4.7 |
| Grade II | 8 | 18.6 |
| Grade IIIA | 3 | 7 |
| Grade IIIB | 0 | 0 |
| Grade IVA | 0 | 0 |
| Grade IVB | 0 | 0 |
| Grade V | 0 | 0 |
| Most severe complication | ||
| Grade I | 1 | 2.3 |
| Grade II | 7 | 16.3 |
| Grade III | 3 | 7 |
| Grade ≥IV | 0 | 0 |
| Variables | Patients (n = 43) | Percentage (%) | |
|---|---|---|---|
| pTstage | T2 | 6 | 14 |
| T3 | 23 | 53.5 | |
| T4 | 14 | 32.6 | |
| pN stage | N0 | 21 | 48.8 |
| N1 | 11 | 25.6 | |
| N2 | 11 | 25.6 | |
| GCLN | Involvement | 5 | 11.6 |
| No. 204 No. 204 + 206 No. 204 + 214v | 3 1 1 | 7 2.3 2.3 | |
| LNR | <0.05 | 25 | 58.2 |
| ≥0.05 to <0.20 ≥0.20 | 9 9 | 20.9 20.9 | |
| pM stage | M0 | 38 | 88.4 |
| M1a | 5 | 11.6 | |
| Microscopical type | Adenocarcinoma Other | 37 6 | 86 14 |
| Tumor grade | Low grade High grade | 34 9 | 79.1 20.9 |
| Invasion | Venous | 22 | 51.2 |
| Lymphatic | 21 | 48.8 | |
| Perineural | 14 | 32.6 | |
| Stage | I | 6 | 14 |
| II | 15 | 34.9 | |
| III | 17 | 39.5 | |
| IV | 5 | 11.6 | |
| Adjuvant chemotherapy | 22 | 51.2 | |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| GCLN (−) n = 38 | GCLN (+) n = 5 | p Value | OR [95%CI] | p Value | ||
| Gender | Male Female | 17 (44.7) 21 (55.3) | 1 (20) 4 (80) | 0.066 | ||
| Depth of tumor invasion | T3 T4 | 22 (57.9) 10 (26.3) | 1 (20) 4 (80) | 0.019 | 1.360 [1.075–3.067] | 0.016 |
| Tumor grade | Low grade High Grade | 31 (81.6) 7 (18.4) | 3 (60) 2 (40) | 0.072 | 2.030 [0.820–5.051] | 0.092 |
| Lymphatic invasion | Present Absent | 22 (57.9) 16 (42.1) | 0 5 (100) | 0.011 | 0.421 [0.290–0.611] | 0.001 |
| Venous invasion | Present Absent | 21 (55.3) 17 (44.7) | 0 5 (100) | 0.024 | 0.447 [0.314–0.637] | 0.144 |
| Perineural invasion | Present Absent | 29 (76.3) 9 (23.7) | 0 5 (100) | 0.008 | 0.237 [0.134–0.419] | 0.250 |
| Variables | Open Surgery (n = 16) | Laparoscopic Surgery (n = 7) | p-Value |
|---|---|---|---|
| Age * (y) | 68.81 ± 10.66 (35–78) | 53.29 ± 10.84 (36–67) | 0.193 |
| Gender Male Female | 3 (18.8) 13 (81.3) | 5 (71.4) 2 (28.6) | 0.015 |
| BMI * | 26.03 ± 5.79 (17.8–38.1) | 27.01 ± 4.06 (21.6–32.9) | 0.481 |
| ACCI 0–1 2–3 4–5 ≥6 | 0 1 (6.3) 9 (56.2) 6 (37.5) | 0 3 (42.9) 4 (57.1) 0 | 0.163 |
| Abdominal operation history | 4 (25) | 0 | 0.146 |
| Operation time (min) * | 181.56 ± 11.51 (160–210) | 220 ± 20 (200–240) | 0.007 |
| Clavien–Dindo classification Grade I Grade II Grade IIIA ≥Grade IIIB | 1 (6.3) 6 (37.5) 2 (12.5) 0 | 0 0 0 0 | |
| Postoperative hospital stay * | 6.44 ± 1.15 (5–10) | 5.29 ± 0.49 (5–6) | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, R.C.; Botea, F.; Dumitru, E.; Mazilu, L.; Micu, L.G.; Tocia, C.; Dumitru, A.; Croitoru, A.; Leopa, N. Extended Lymphadenectomy for Proximal Transverse Colon Cancer: Is There a Place for Standardization? Medicina 2022, 58, 596. https://doi.org/10.3390/medicina58050596
Popescu RC, Botea F, Dumitru E, Mazilu L, Micu LG, Tocia C, Dumitru A, Croitoru A, Leopa N. Extended Lymphadenectomy for Proximal Transverse Colon Cancer: Is There a Place for Standardization? Medicina. 2022; 58(5):596. https://doi.org/10.3390/medicina58050596
Chicago/Turabian StylePopescu, Răzvan Cătălin, Florin Botea, Eugen Dumitru, Laura Mazilu, Luminița Gențiana Micu, Cristina Tocia, Andrei Dumitru, Adina Croitoru, and Nicoleta Leopa. 2022. "Extended Lymphadenectomy for Proximal Transverse Colon Cancer: Is There a Place for Standardization?" Medicina 58, no. 5: 596. https://doi.org/10.3390/medicina58050596
APA StylePopescu, R. C., Botea, F., Dumitru, E., Mazilu, L., Micu, L. G., Tocia, C., Dumitru, A., Croitoru, A., & Leopa, N. (2022). Extended Lymphadenectomy for Proximal Transverse Colon Cancer: Is There a Place for Standardization? Medicina, 58(5), 596. https://doi.org/10.3390/medicina58050596







