ZBTB16-RARα-Positive Atypical Promyelocytic Leukemia: A Case Report
Abstract
:1. Introduction
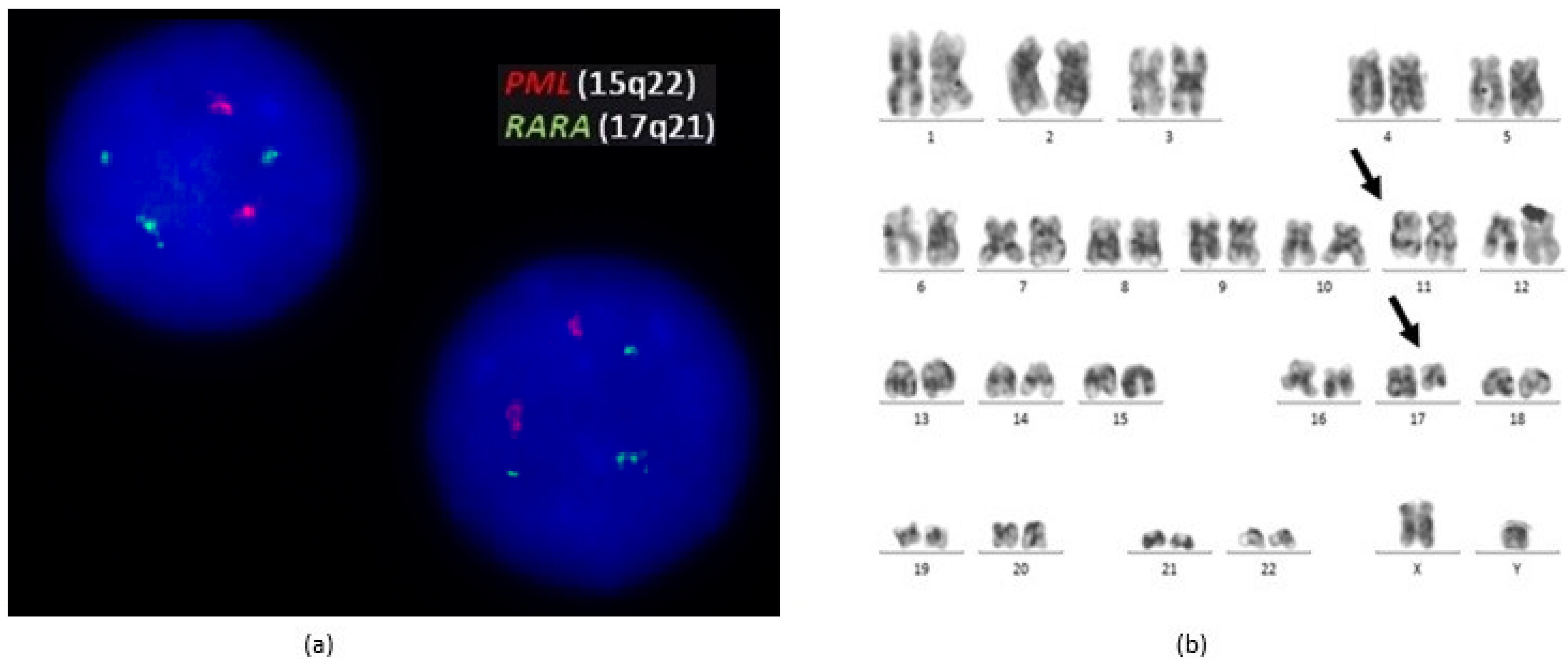
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, W.; Zhang, G. Molecular characteristics and clinical significance of 12 fusion genes in acute promyelocytic leukemia: A systematic review. Acta Haematol. 2016, 136, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Langabeer, S.E.; Preston, L.; Kelly, J.; Goodyer, M.; Elhassadi, E.; Hayat, A. Molecular profiling: A case of ZBTB16-RARA acute promyelocytic leukemia. Case Rep. Hematol. 2017, 2017, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licht, J.; Chomienne, C.; Goy, A.; Chen, A.; Scott, A.; Head, D.; Michaux, J.; Wu, Y.; deBlasio, A.; Miller, W.J. Clinical and molecular characterization of a rare syndrome of acute promyelocytic leukemia associated with translocation (11;17). Blood 1995, 85, 1083–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sainty, D.; Liso, V.; Fenu, S.; Mancini, M.; Duchayne, E.; Mahon, F.-X.; Gutierrez, N.; Birg, F.; Biondi, A.; Grimwade, D.; et al. A new morphologic classification system for acute promyelocytic leukemia distinguishes cases with underlying PLZF/RARA gene rearrangements. Blood J. Am. Soc. Hematol. 2000, 96, 10. [Google Scholar]
- Dowse, R.T.; Ireland, R.M. Variant ZBTB16-RARA translocation: Morphological changes predict cytogenetic variants of APL. Blood 2017, 129, 2038. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, J.V.; Rennie, K.; Culligan, D.; Peniket, A.; Lennard, A.; Harrison, J.; Vyas, P.; Grimwade, D. Development of real-time quantitative polymerase chain reaction assays to track treatment response in retinoid resistant acute promyelocytic leukemia. Front. Oncol. 2011, 1, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobas, M.; Talarn-Forcadell, M.C.; Martínez-Cuadrón, D.; Escoda, L.; García-Pérez, M.J.; Mariz, J.; Mela-Osorio, M.J.; Fernández, I.; Alonso-Domínguez, J.M.; Cornago-Navascués, J.; et al. PLZF-RARα, NPM1-RARα, and other acute promyelocytic leukemia variants: The PETHEMA registry experience and systematic literature review. Cancers 2020, 12, 1313. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, E.; Cicconi, L.; Nardozza, A.M.; Cristiano, A.; Rossi, M.; Ottone, T.; Falconi, G.; Divona, M.; Testi, A.M.; Annibali, O.; et al. Mutational profile of ZBTB16-RARA-positive acute myeloid leukemia. Cancer Med. 2021, 10, 3839–3847. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Tremblay, M.; Humbert, M.; Grondin, B.; Haman, A.; Labrecque, J.; Chen, B.; Chen, Z.; Chen, S.-J.; Hoang, T. RAR-PLZF oncogene inhibits C/EBP function in myeloid cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13522–13527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ablain, J.; de The, H. Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood 2011, 117, 5795–5802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.H.; de Ridder, M.C.; Geertsma, W.M.C.; Erpelinck, C.A.J.; van Lom, K.; Smit, E.M.E.; Slater, R.; Reijden, B.A.; de Greef, G.E.; Sonneveld, P.; et al. Complete remission of t(11;17) positive acute promyelocytic leukemia induced by all-trans retinoic acid and granulocyte colony-stimulating factor. Blood 1999, 94, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimwade, D.; Biondi, A.; Dastugue, N.; Jansen, J.; Radford-Weiss, I.; Coco, F.L.; Lessard, M.; Hernandez, J.-M.; Delabesse, E.; Head, D.; et al. Characterization of acute promyelocytic leukemia cases lacking the classic t(15;17): Results of the European working party. Blood J. Am. Soc. Hematol. 2000, 96, 13. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo Gambarte, L.; Franganillo Suárez, A.; Cornago Navascués, J.; Soto de Ozaeta, C.; Blas López, C.; Atance Pasarisas, M.; Salgado Sánchez, R.N.; Serrano del Castillo, C.; Mata Serna, R.; Velasco-Rodríguez, D.; et al. ZBTB16-RARα-Positive Atypical Promyelocytic Leukemia: A Case Report. Medicina 2022, 58, 520. https://doi.org/10.3390/medicina58040520
Pardo Gambarte L, Franganillo Suárez A, Cornago Navascués J, Soto de Ozaeta C, Blas López C, Atance Pasarisas M, Salgado Sánchez RN, Serrano del Castillo C, Mata Serna R, Velasco-Rodríguez D, et al. ZBTB16-RARα-Positive Atypical Promyelocytic Leukemia: A Case Report. Medicina. 2022; 58(4):520. https://doi.org/10.3390/medicina58040520
Chicago/Turabian StylePardo Gambarte, Laura, Aída Franganillo Suárez, Javier Cornago Navascués, Carlos Soto de Ozaeta, Carlos Blas López, Mireia Atance Pasarisas, Rocío Nieves Salgado Sánchez, Cristina Serrano del Castillo, Raquel Mata Serna, Diego Velasco-Rodríguez, and et al. 2022. "ZBTB16-RARα-Positive Atypical Promyelocytic Leukemia: A Case Report" Medicina 58, no. 4: 520. https://doi.org/10.3390/medicina58040520
APA StylePardo Gambarte, L., Franganillo Suárez, A., Cornago Navascués, J., Soto de Ozaeta, C., Blas López, C., Atance Pasarisas, M., Salgado Sánchez, R. N., Serrano del Castillo, C., Mata Serna, R., Velasco-Rodríguez, D., López-Lorenzo, J. L., Llamas-Sillero, P., & Solán Blanco, L. (2022). ZBTB16-RARα-Positive Atypical Promyelocytic Leukemia: A Case Report. Medicina, 58(4), 520. https://doi.org/10.3390/medicina58040520





