Inverse Relationship between Mean Corpuscular Volume and T-Score in Chronic Dialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. DXA Assessment
2.3. BMD
2.4. Diagnosis of Osteoporosis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damasiewicz, M.J.; Nickolas, T.L. Rethinking Bone Disease in Kidney Disease. JBMR Plus 2018, 2, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Kimura, T.; Kuragano, T. The Hepcidin-Anemia Axis: Pathogenesis of Anemia in Chronic Kidney Disease. Contrib. Nephrol. 2019, 198, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, S.; Cianciolo, G.; De Pascalis, A.; Guglielmo, C.; Torres, P.A.U.; Bover, J.; Tartaglione, L.; Pasquali, M.; La Manna, G. Bone, inflammation and the bone marrow niche in chronic kidney disease: What do we know? Nephrol. Dial. Transplant. 2018, 33, 2092–2100. [Google Scholar] [CrossRef]
- Balci, Y.I.; Akpinar, F.O.; Polat, A.; Uzun, S.U.; Ergin, A. Evaluation of Reticulocyte Parameters in Iron Deficiency, Vitamin B12 Deficiency and Mixed Anemia. Clin. Lab. 2016, 62, 343–347. [Google Scholar] [CrossRef]
- Kamble, R.T.; Hamadani, M.; Selby, G.B. Increased Mean Corpuscular Volume after Autologous Hematopoietic Stem Cell Transplantation: Incidence and Significance. Biol. Blood Marrow Transplant. 2006, 12, 111–112. [Google Scholar] [CrossRef][Green Version]
- Takahashi, N.; Kameoka, J.; Takahashi, N.; Tamai, Y.; Murai, K.; Honma, R.; Noji, H.; Yokoyama, H.; Tomiya, Y.; Kato, Y.; et al. Causes of macrocytic anemia among 628 patients: Mean corpuscular volumes of 114 and 130 fL as critical markers for categorization. Int. J. Hematol. 2016, 104, 344–357. [Google Scholar] [CrossRef]
- The Writing Group for the ISCD Position Development Conference. Indications and Reporting for Dual-Energy X-Ray Absorptiometry. J. Clin. Densitom. 2004, 7, 37–44. [Google Scholar] [CrossRef]
- Kanis, J.A.; Glüer, C.-C.; Committee of Scientific Advisors; International Osteoporosis Foundation. An Update on the Diagnosis and Assessment of Osteoporosis with Densitometry. Osteoporos. Int. 2000, 11, 192–202. [Google Scholar] [CrossRef]
- Do, H.J.; Shin, J.-S.; Lee, J.; Lee, Y.J.; Kim, M.-R.; Nam, D.; Kim, E.-J.; Park, Y.; Suhr, K.; Ha, I.-H. Association between liver enzymes and bone mineral density in Koreans: A cross-sectional study. BMC Musculoskelet. Disord. 2018, 19, 410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.-Y.; Cong, Q.-W.; Liu, F.; Yao, L.-Y.; Zhu, Y. Nonlinear Relationship Between Macrocytic Anemia and Decompensated Hepatitis B Virus Associated Cirrhosis: A Population-Based Cross-Sectional Study. Front. Pharmacol. 2021, 12, 755625. [Google Scholar] [CrossRef] [PubMed]
- Myojo, M.; Iwata, H.; Kohro, T.; Sato, H.; Kiyosue, A.; Ando, J.; Sawaki, D.; Takahashi, M.; Fujita, H.; Hirata, Y.; et al. Prognostic implication of macrocytosis on adverse outcomes after coronary intervention. Atherosclerosis 2012, 221, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Kawakami, R.; Horii, M.; Sugawara, Y.; Matsumoto, T.; Okada, S.; Nishida, T.; Soeda, T.; Okayama, S.; Somekawa, S.; et al. High Mean Corpuscular Volume Is a New Indicator of Prognosis in Acute Decompensated Heart Failure. Circ. J. 2013, 77, 2766–2771. [Google Scholar] [CrossRef]
- Mizuno, H.; Yuasa, N.; Takeuchi, E.; Miyake, H.; Nagai, H.; Yoshioka, Y.; Miyata, K. Blood cell markers that can predict the long-term outcomes of patients with colorectal cancer. PLoS ONE 2019, 14, e0220579. [Google Scholar] [CrossRef]
- Yoshida, N.; Kosumi, K.; Tokunaga, R.; Baba, Y.; Nagai, Y.; Miyamoto, Y.; Iwagami, S.; Iwatsuki, M.; Hiyoshi, Y.; Ishimoto, T.; et al. Clinical Importance of Mean Corpuscular Volume as a Prognostic Marker After Esophagectomy for Esophageal Cancer: A Retrospective Study. Ann. Surg. 2020, 271, 494–501. [Google Scholar] [CrossRef]
- Yoon, H.-J.; Kim, K.; Nam, Y.-S.; Yun, J.-M.; Park, M. Mean corpuscular volume levels and all-cause and liver cancer mortality. Clin. Chem. Lab. Med. 2016, 54, 1247–1257. [Google Scholar] [CrossRef]
- Tennankore, K.K.; Soroka, S.D.; West, K.A.; Kiberd, B.A. Macrocytosis may be associated with mortality in chronic hemodialysis patients: A prospective study. BMC Nephrol. 2011, 12, 19. [Google Scholar] [CrossRef]
- Dratch, A.; Kleine, C.-E.; Streja, E.; SooHoo, M.; Park, C.; Hsiung, J.-T.; Rhee, C.M.; Obi, Y.; Molnar, M.Z.; Kovesdy, C.P.; et al. Mean Corpuscular Volume and Mortality in Incident Hemodialysis Patients. Nephron Exp. Nephrol. 2019, 141, 188–200. [Google Scholar] [CrossRef]
- Taichman, R.S.; Emerson, S.G. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J. Exp. Med. 1994, 179, 1677–1682. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, C.; Ye, L.; Huang, H.; He, X.; Tong, W.-G.; Ross, J.; Haug, J.; Johnson, T.; Feng, J.Q.; et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003, 425, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Aleksinskaya, M.A.; Monge, M.; Siebelt, M.; Slot, E.M.; Koekkoek, K.M.; De Bruin, R.G.; Massy, Z.A.; Weinans, H.; Rabelink, T.J.; Fibbe, W.E.; et al. Chronic kidney failure mineral bone disorder leads to a permanent loss of hematopoietic stem cells through dysfunction of the stem cell niche. Sci. Rep. 2018, 8, 15385. [Google Scholar] [CrossRef]
- Ott, S.M. Cortical or Trabecular Bone: What’s the Difference? Am. J. Nephrol. 2018, 47, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Woodard, H.Q.; Holodny, E. A Summary of the Data of Mechanik on the Distribution of Human Bone Marrow. Phys. Med. Biol. 1960, 5, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.E. The Distribution of Active Bone Marrow in the Adult. Phys. Med. Biol. 1961, 5, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Hayman, J.A.; Callahan, J.W.; Herschtal, A.; Everitt, S.; Binns, D.S.; Hicks, R.J.; Mac Manus, M. Distribution of Proliferating Bone Marrow in Adult Cancer Patients Determined Using FLT-PET Imaging. Int. J. Radiat. Oncol. 2011, 79, 847–852. [Google Scholar] [CrossRef]
- Campbell, B.A.; Callahan, J.; Bressel, M.; Simoens, N.; Everitt, S.; Hofman, M.; Hicks, R.; Burbury, K.; MacManus, M. Distribution Atlas of Proliferating Bone Marrow in Non-Small Cell Lung Cancer Patients Measured by FLT-PET/CT Imaging, With Potential Applicability in Radiation Therapy Planning. Int. J. Radiat. Oncol. 2015, 92, 1035–1043. [Google Scholar] [CrossRef]
- Laor, T.; Jaramillo, D. MR Imaging Insights into Skeletal Maturation: What Is Normal? Radiology 2009, 250, 28–38. [Google Scholar] [CrossRef]
- Cannata-Andía, J.B.; Martín-Carro, B.; Martín-Vírgala, J.; Rodríguez-Carrio, J.; Bande-Fernández, J.J.; Alonso-Montes, C.; Carrillo-López, N. Chronic Kidney Disease—Mineral and Bone Disorders: Pathogenesis and Management. Calcif. Tissue Res. 2020, 108, 410–422. [Google Scholar] [CrossRef]
- Evenepoel, P.; Cunningham, J.; Ferrari, S.; Haarhaus, M.; Javaid, M.K.; Lafage-Proust, M.-H.; Prieto-Alhambra, D.; Torres, P.U.; Cannata-Andia, J.; Vervloet, M.; et al. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4–G5D. Nephrol. Dial. Transplant. 2020, 36, 42–59. [Google Scholar] [CrossRef]
- Kato, T.; Mizobuchi, M.; Sasa, K.; Yamada, A.; Ogata, H.; Honda, H.; Sakashita, A.; Kamijo, R. Osteoblastic differentiation of bone marrow mesenchymal stem cells in uremic rats. Biochem. Biophys. Res. Commun. 2020, 532, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Morrone, L.; Di Iorio, B.; Andreucci, M.; De Gregorio, M.G.; Errichiello, C.; Russo, L.; Locatelli, F. Parathyroid hormone may be an early predictor of low serum hemoglobin concentration in patients with not advanced stages of chronic kidney disease. J. Nephrol. 2014, 28, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Capuano, A.; Serio, V.; Pota, A.; Memoli, B.; Andreucci, V.E. Beneficial effects of better control of secondary hyperparathyroidism with paricalcitol in chronic dialysis patients. J. Nephrol. 2009, 22, 59–68. [Google Scholar] [PubMed]
- Tanaka, M.; Komaba, H.; Fukagawa, M. Emerging Association between Parathyroid Hormone and Anemia in Hemodialysis Patients. Ther. Apher. Dial. 2018, 22, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.S.; Shih, M.-S.; Mohini, R. Effect of Serum Parathyroid Hormone and Bone Marrow Fibrosis on the Response to Erythropoietin in Uremia. N. Engl. J. Med. 1993, 328, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Iseri, K.; Qureshi, A.R.; Ripsweden, J.; Heimbürger, O.; Barany, P.; Bergström, I.B.; Stenvinkel, P.; Brismar, T.B.; Lindholm, B. Sparing effect of peritoneal dialysis vs hemodialysis on BMD changes and its impact on mortality. J. Bone Miner. Metab. 2020, 39, 260–269. [Google Scholar] [CrossRef]
- Iseri, K.; Carrero, J.J.; Evans, M.; Felländer-Tsai, L.; Berg, H.; Runesson, B.; Stenvinkel, P.; Lindholm, B.; Qureshi, A.R. Major fractures after initiation of dialysis: Incidence, predictors and association with mortality. Bone 2020, 133, 115242. [Google Scholar] [CrossRef]
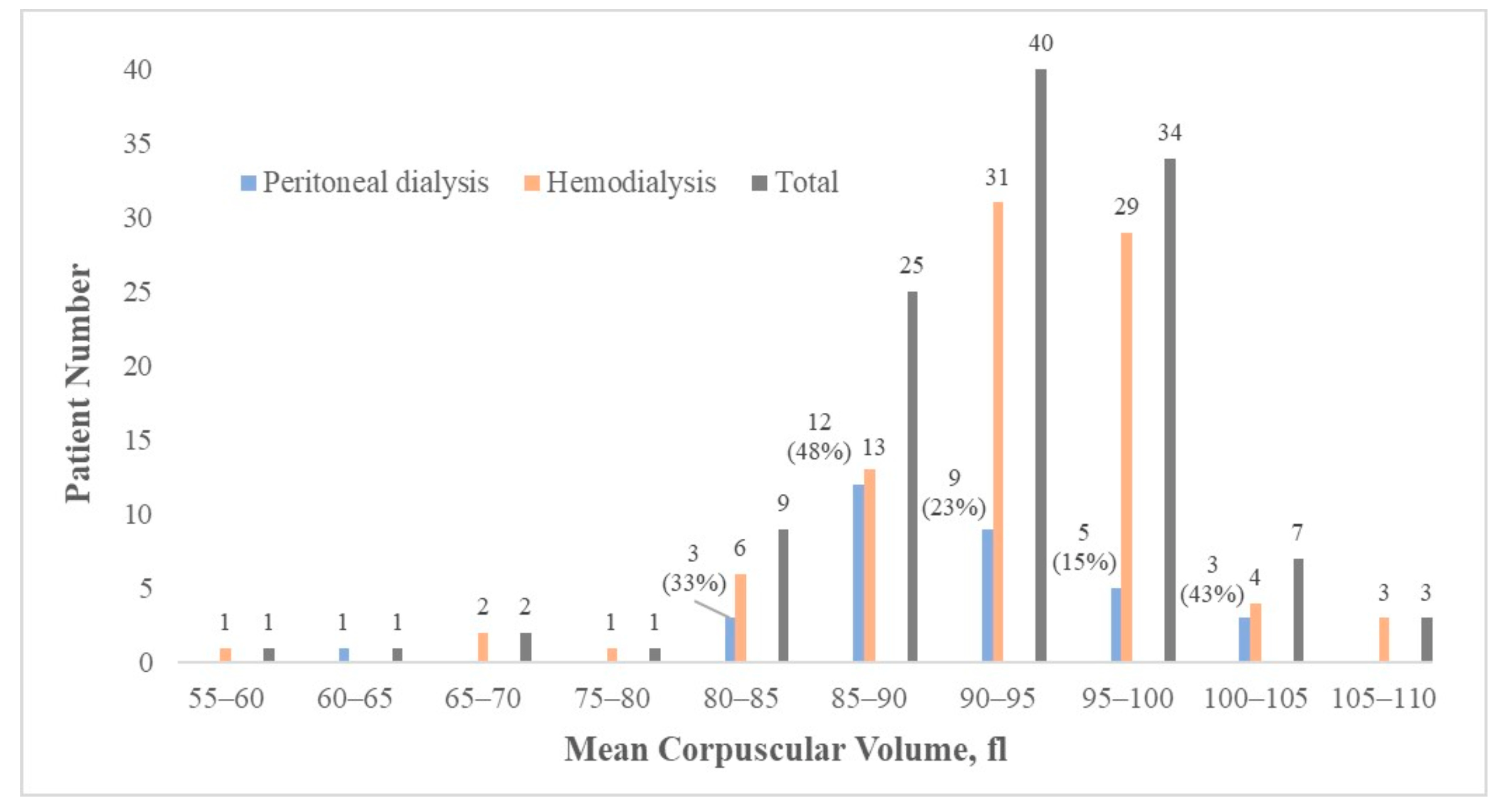
| Clinical Characteristics (n = 123) | Mean ± SD/Number (Percentage) | HD (n = 90) | PD (n = 33) | p-Value between HD and PD Groups |
|---|---|---|---|---|
| Age | 65 ± 11 | 66 ± 10 | 62 ± 12 | 0.05 |
| Gender | 1.0 | |||
| Male | 61 (49.6%) | 45 (50%) | 16 (48%) | |
| Female | 62 (50.4%) | 45 (50%) | 17 (52%) | |
| BMI | 24 ± 4.1 | 24 ± 4.3 | 25 ± 3.6 | 0.5 |
| Mean duration of dialysis (months) | 59 ± 37 | 62 ± 38 | 50 ± 33 | 0.1 |
| Types of dialysis | NA | NA | ||
| Hemodialysis | 90 (73%) | |||
| Peritoneal dialysis | 33 (27%) | |||
| Erythropoeisis-stimulating agent usage | 120 (98%) | |||
| Underlying comorbidities | ||||
| Hypertensions | 99 (80%) | 75 (83%) | 24 (73%) | 0.2 |
| Diabetes mellitus | 63 (51%) | 51 (57%) | 12 (36%) | 0.07 |
| Dyslipidemia | 41 (33%) | 30 (33%) | 11 (33%) | 1.0 |
| Gastrointestinal tract ulcer history | 21 (17%) | 17 (19%) | 3 (9.1%) | 0.3 |
| Smoking history | 0.4 | |||
| Quitted | 16 (13%) | 8 (9%) | 2 (6.1%) | |
| Current smoker | 10 (8%) | 9 (10%) | 6 (18%) | |
| Laboratory parameters | ||||
| Hemoglobin, g/dL | 10 ± 1.0 | 10 ± 1.0 | 10 ± 1.0 | 0.2 |
| RBC ×106/μL | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.3 ± 0.4 | 0.4 |
| MCV, fl | 92 ± 7.3 | 93 ± 7.4 | 91 ± 6.8 | 0.2 |
| RDW, % | 15 ± 2.3 | 15 ± 2.5 | 15 ± 1.5 | 0.6 |
| WBC count ×103/L | 6.6 ± 2.0 | 6.4 ± 1.7 | 7.1 ± 2.6 | 0.11 |
| Albumin, g/dL | 3.8 ± 0.3 | 3.8 ± 0.3 | 3.6 ± 0.4 | <0.001 |
| Phosphate, mg/dL | 5.0 ± 1.6 | 5.1 ± 1.7 | 4.7 ± 1.3 | 0.3 |
| Ca, mg/dL | 9.1 ± 0.66 | 9.1 ± 0.7 | 9.3 ± 0.6 | 0.2 |
| Corrected Ca, mg/dL | 9.3 ± 0.74 | 9.2 ± 0.8 | 9.6 ± 0.6 | 0.02 |
| Iron, ug/dL | 66 ± 2 | 61 ± 25 | 80 ± 31 | 0.001 |
| Ferritin, ng/mL | 474 ± 369 | 455 ± 285 | 551 ± 544 | 0.2 |
| Cholesterol, mg/dl | 153 ± 37 | 152 ± 40 | 155 ± 27 | 0.7 |
| GOT, U/L | 17 ± 7.1 | 17 ± 7.4 | 17 ± 6 | 0.5 |
| GPT, U/ | 14 ± 8.2 | 14 ± 7.8 | 14 ± 9.5 | 0.9 |
| Parathyroid hormone level (pg/mL) | 418 ± 494 | 381 ± 519 | 514 ± 414 | 0.2 |
| Clinical Characteristics | Mean ± SD/Number (Percentage) | HD (n = 90) | PD (n = 33) | p-Value between HD and PD Groups | p-Value of Correlation with MCV | Pearson Correlation Index |
|---|---|---|---|---|---|---|
| T-score (n = 123, the lowest T-score of the 5 sites) | −2.5 ± 1.2 | −2.5 ± 1.3 | −2.4 ± 0.9 | 0.8 | 0.003 | −0.27 |
| Femoral neck BMD | ||||||
| Right side (n = 120) | 0.75 ± 0.16 | 0.75 ± 0.16 | 0.77 ± 0.14 | 0.5 | 0.008 | −0.24 |
| Left side (n = 118) | 0.74 ± 0.14 | 0.74 ± 0.15 | 0.74 ± 0.11 | 0.8 | 0.02 | −0.21 |
| Total hip BMD | ||||||
| Right side (n = 120) | 0.82 ± 0.17 | 0.81 ± 0.18 | 0.84 ± 0.13 | 0.4 | 0.1 | −0.15 |
| Left side (n = 118) | 0.82 ± 0.18 | 0.82 ± 0.17 | 0.81 ± 0.20 | 0.7 | 0.08 | −0.16 |
| Lumbar spine 1–4 BMD (n = 122) | 1.14 ± 0.23 | 1.15 ± 0.25 | 1.11 ± 0.16 | 0.3 | 0.038 | −0.19 |
| Fracture Risk Assessment Tool | ||||||
| 10-year probability of major osteoporotic fracture, % | 11 ± 9.1 | 11.7 ± 10 | 9.0 ± 6.1 | 0.15 | 0.3 | |
| 10-year probability of hip fracture, % | 5.4 ± 7.4 | 5.8 ± 8.1 | 4.2 ± 4.1 | 0.3 | 0.6 |
| Total Patients (n = 123) | Variables | β | p-Value |
|---|---|---|---|
| Model 1 (MCV was set as the dependent variable) | T-score | −0.96 | 0.037 |
| Age | 0.1 | 0.06 | |
| Phosphate | −0.4 | 0.2 | |
| RDW | −1.6 | <0.001 | |
| Hb | 0.7 | 0.2 | |
| WBC | −0.3 | 0.2 | |
| Iron | 0.007 | 0.7 | |
| Ferritin | 0.002 | 0.1 | |
| Cholesterol | 0.02 | 0.2 | |
| Model 2 (T-score was set as the dependent variable) | MCV | −0.027 | 0.048 |
| Age | −0.03 | 0.001 | |
| Phosphate | 0.07 | 0.3 | |
| BMI | 0.06 | 0.007 | |
| Parathyroid hormone level | −0.001 | 0.01 | |
| Dyslipidemia (Yes) | −0.3 | 0.5 | |
| GI tract ulcer history (Yes) | 0.5 | 0.3 | |
| Model 3 (T-score was set as the dependent variable) | MCV | −0.03 | 0.036 |
| Age | −0.03 | 0.002 | |
| WBC | −0.05 | 0.4 | |
| Albumin | 0.2 | 0.6 | |
| GOT | −0.02 | 0.3 | |
| GPT | 0.02 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, M.-H.; Yang, C.-Y.; Kuo, Y.-J.; Cheng, C.-Y.; Huang, S.-W.; Chen, Y.-P. Inverse Relationship between Mean Corpuscular Volume and T-Score in Chronic Dialysis Patients. Medicina 2022, 58, 497. https://doi.org/10.3390/medicina58040497
Chiang M-H, Yang C-Y, Kuo Y-J, Cheng C-Y, Huang S-W, Chen Y-P. Inverse Relationship between Mean Corpuscular Volume and T-Score in Chronic Dialysis Patients. Medicina. 2022; 58(4):497. https://doi.org/10.3390/medicina58040497
Chicago/Turabian StyleChiang, Ming-Hsiu, Chih-Yu Yang, Yi-Jie Kuo, Chung-Yi Cheng, Shu-Wei Huang, and Yu-Pin Chen. 2022. "Inverse Relationship between Mean Corpuscular Volume and T-Score in Chronic Dialysis Patients" Medicina 58, no. 4: 497. https://doi.org/10.3390/medicina58040497
APA StyleChiang, M.-H., Yang, C.-Y., Kuo, Y.-J., Cheng, C.-Y., Huang, S.-W., & Chen, Y.-P. (2022). Inverse Relationship between Mean Corpuscular Volume and T-Score in Chronic Dialysis Patients. Medicina, 58(4), 497. https://doi.org/10.3390/medicina58040497






