Abstract
Background and objectives: Adenocarcinoma of the esophagogastric junction (AEG) has a complicated surgical anatomy, due to which it sometimes induces excessive intraoperative blood loss that necessitates intraoperative blood transfusion (BTF). However, few reports have focused on the impact of BTF on the survival outcomes of patients with AEG. We aimed to evaluate the impact of BTF on AEG prognosis. Materials andMethods: We included 63 patients who underwent surgical resection for AEG at our hospital between January 2010 and September 2020. Clinicopathological characteristics and survival outcomes were compared between patients with (n = 12) and without (n = 51) BTF. Multivariate analysis was performed to identify the independent prognostic factors for overall survival. Results: None of the patients who underwent minimally invasive surgery received BTF. Patients who received BTF had a significantly worse 5-year survival rate than those who did not (67.8% vs. 28.3%, p = 0.001). BTF was an independent risk factor for overall survival (hazard ratio: 3.90, 95% confidence interval 1.30–11.7), even after patients who underwent minimally invasive surgery were excluded. Conclusions: BTF adversely affected the survival outcomes of patients with AEG who underwent curative surgery. To avoid BTF, surgeons should strive to minimize intraoperative bleeding.
1. Introduction
Gastric cancer is one of the most common malignancies, and several prognostic factors have been proposed. The adverse effects of intraoperative blood loss (IBL) and intraoperative blood transfusion (BTF) on survival outcomes following curative gastrectomy have been reported, but some of them failed to prove the negative impact of IBL and BTF on survival outcomes [1,2,3,4,5,6].
The incidence of adenocarcinoma of the esophagogastric junction (AEG) has been increasing in East Asia [7,8,9]. The complicated surgical anatomy around the esophagogastric junction makes surgery for AEG difficult. Compared to surgery for gastric cancers without esophageal infiltration, surgery for AEG has a longer duration and a higher incidence of postoperative complications. Additionally, excessive IBL that necessitates BTF may occur during surgery for AEG. However, few studies have focused on the effects of IBL and BTF in patients with AEG, and information regarding their impact on survival outcomes is limited [10,11,12,13,14,15].
Therefore, the objective of this study was to clarify the impact of IBL and BTF on the survival outcomes of patients with AEG undergoing curative surgery.
2. Material and Methods
This study included 81 consecutive patients with AEG who had undergone surgical resection at the Tokyo Medical and Dental University between January 2010 and September 2020. Patients who had undergone non-curative resection (eight patients), those who had undergone preoperative endoscopic submucosal dissection (nine patients), and those who had remnant stomach cancer (one patient) were excluded. The remaining 63 patients were included in the final analysis. Data regarding the patients’ characteristics, surgical and pathological findings, and clinical course were collected from our prospectively maintained database, and we referred to individual patient electronic medical records when necessary.
Pathological tumor depth, nodal status, and surgical curability were assessed based on the International Union Against Cancer TNM Classification of Malignant Tumors, eighth edition. The tumor epicenter was assessed through pathological examination, and the Siewert classification was used for AEG categorization.
This retrospective study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the institutional review board of the Tokyo Medical and Dental University (No. M2020-279; approved date, December 16, 2020). Written informed consent was waived in this retrospective study.
2.1. Comparison between Patients with and without BTF
Clinicopathological characteristics and survival outcomes were compared between patients who required BTF (BTF group, n = 12) and those who did not (non-BTF group, n = 51).
2.2. Statistical Analysis
Continuous data are presented as medians (ranges), and they were compared using the Mann–Whitney U test. Categorical data were compared using Fisher’s exact test. Overall survival (OS) was calculated as the time from the date of surgery to the date of the last observation or death. Survival curves were derived from Kaplan–Meier estimates, and the curves were compared using the log-rank test. As all patients who had undergone minimally invasive surgery were in the non-BTF group, survival curves were also compared after excluding the patients who had undergone minimally invasive surgery. Prognostic factors were identified using the multivariable Cox proportional hazards model. Covariates with p < 0.1 in the univariate analysis were used as covariates in the subsequent multivariable analysis. Statistical significance was set at p < 0.05. All statistical analyses were performed using R statistics version 4.0.3 (R development core team, Vienna, Austria) [16].
3. Results
3.1. Clinicopathological Characteristics
Leukocyte-reduced red blood cell concentrates were transfused in all 12 patients of the BTF group, with a median transfusion amount of 560 mL (280–1120 mL). Fresh frozen leukocyte-reduced plasma was transfused in four patients, with a median transfusion amount of 480 mL (240–960 mL). The comparisons of the patient characteristics, surgical and pathological findings, and short-term postoperative results between the BTF and non-BTF groups are shown in Table 1, Table 2, Table 3 and Table 4. There were no between-group differences in sex or age. The preoperative hemoglobin level was significantly lower in the BTF group than in the non-BTF group (Table 1). The transthoracic approach was more frequently used in the BTF group than in the non-BTF group. All BTF patients underwent open surgery. On the other hand, 29 patients in the non-BTF group underwent minimally invasive surgery, and 22 of them received open surgery (Table 2). The tumor size was larger and the length of esophageal invasion was longer in the BTF group than in the non-BTF group (Table 3). The incidence of Clavien–Dindo grade IIIA postoperative complications was 58.3% and 17.6% in the BTF and non-BTF groups, respectively (p = 0.117, Table 4). More patients received adjuvant chemotherapy in the BTF group (60%) than in the non-BTF group (42%), although this difference was not statistically significant (p = 0.332, Table 1).

Table 1.
Patient characteristics.

Table 2.
Surgical findings.

Table 3.
Pathological findings.

Table 4.
Short-term postoperative results.
3.2. Survival Data and Prognostic Factors
The Kaplan–Meier curves for OS are shown in Figure 1 and Figure 2. The median observation period for survivors was 29.6 months. The non-BTF group had a better 5-year survival rate than the BTF group (67.8% vs. 28.3%, p = 0.001). Even after excluding the patients who had undergone minimally invasive surgery, the non-BTF group had a better 5-year survival rate than the BTF group (73.3% vs. 28.3%, p = 0.005).
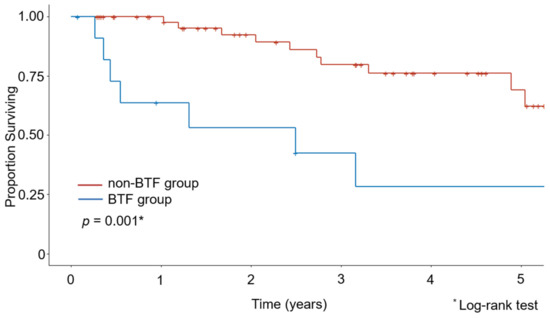
Figure 1.
Kaplan–Meier estimates of overall survival in the intraoperative blood transfusion (BTF) and non-BTF groups.
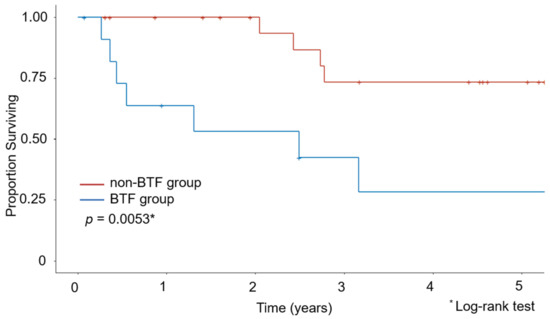
Figure 2.
Kaplan–Meier estimates of overall survival after excluding patients who underwent minimally invasive surgery.
3.3. Univariate and Multivariate Analysis of Prognostic Factors
Table 5 shows the results of the Cox proportional hazards model for OS. In the univariate analysis, pathologic stage, BTF, and vascular invasion were found to be potential prognostic factors. Multivariate analysis using these three covariates identified only BTF as an independent prognostic factor (hazard ratio: 3.90, 95% confidence interval 1.30–11.7). After excluding the patients who had undergone minimally invasive surgery, duration of surgery and BTF were identified as independent prognostic factors in univariate analysis (Table 6). The subsequent multivariable analysis using these two covariates identified only BTF as an independent prognostic factor (hazard ratio, 5.04, 95% confidence interval 1.42–18.0).

Table 5.
Cox proportional hazards model for overall survival.

Table 6.
Cox proportional hazards model for overall survival (excluding patients who had undergone minimally invasive surgery).
4. Discussion
Perioperative blood transfusion has been reported to be associated with poor postoperative survival outcomes in patients with gastric cancer and those with colorectal cancer [2,3,4,17]. We believe that this is the first report that only included patients with AEG and investigated the relationship between BTF and survival outcomes [10,11,12,13,14,15]. Our findings demonstrate the negative impact of BTF on the survival outcomes of patients with AEG.
BTF-induced anti-tumor immunosuppression may explain the adverse effect of BTF on survival outcomes. In 1981, Gantt reported that perioperative allogeneic blood transfusion-induced immunosuppression might promote tumor growth [18]. Since then, many studies have demonstrated that BTF can cause a wide range of cytokine-mediated immune responses and suppress cellular and humoral immunity [19,20,21,22]. BTF-induced immunomodulatory effects include a reduction in the levels of interferon-gamma [19] and T-lymphocyte subsets (CD3+, CD4+, and CD4+/CD8+) [19,21,22], and suppression of interleukin-2 production [20], which may worsen survival outcomes.
There were some between-group differences in clinicopathological characteristics. The BTF group included patients with large and advanced-stage tumors; further, the duration of surgery was longer and the incidence of postoperative complications was higher in the BTF group than in the non-BTF group. These factors can affect survival outcomes; therefore, to identify independent prognostic factors, we conducted multivariate analysis using the possible prognostic factors identified by univariate analysis. However, our study sample was relatively small, and another study with a larger sample should be conducted to confirm our results.
In this study, none of the patients who had undergone minimally invasive surgery, including robotic or laparoscopic surgery, required BTF. Although minimally invasive surgeries have a longer duration of surgery than open surgeries, they are associated with less intraoperative blood loss, resulting in a decreased requirement for perioperative blood transfusion [23]. To eliminate the effect of the surgical approach, we compared the survival curves and identified independent prognostic factors after excluding the patients who had undergone minimally invasive surgery; we found that patients with BTF had poor survival outcomes, and BTF was identified as an independent prognostic factor.
This study has some limitations. First, it was a single-center, retrospective study. The sample size was small, and due to insufficient power, we could not assess the impact of BTF according to Siewert type. Second, some patients were followed for less than five years. To validate the results of this study, a well-designed prospective, multicenter study is warranted. Significant differences in clinicopathologic characteristics exist between the non-BTF and BTF groups. To eliminate potential bias between the groups, we conducted subgroup and multivariate analyses. However, the differences could not be completely adjusted for, and therefore a well-designed multicenter study with a larger number of patients is warranted.
5. Conclusions
BTF was significantly associated with poor OS in patients with AEG undergoing curative surgery. Surgeons should strive to minimize IBL to avoid BTF, which may adversely affect survival outcomes.
Author Contributions
Study conception and design: K.N., M.T. and Y.K.; acquisition of data: K.N., M.T., K.O., K.S., N.F., Y.S., A.H. and T.M.; analysis and interpretation of data: K.N. and M.T.; drafting of manuscript: K.N. and M.T.; critical revision of manuscript: K.N., M.T., K.O., K.S., N.F., Y.S., A.H., T.M. and Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors have not received grant support or any other form of assistance.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the institutional review board of the Tokyo Medical and Dental University (protocol code No. M2020-279; approved date, 16 December 2020).
Informed Consent Statement
Patient consent was waived because this study was not an interventional study, and the study used data obtained from databases and medical charts.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nakanishi, K.; Kanda, M.; Kodera, Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J. Gastroenterol. 2019, 25, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Kobayashi, D.; Tanaka, C.; Iwata, N.; Yamada, S.; Fujii, T.; Nakayama, G.; Sugimoto, H.; Koike, M.; Nomoto, S.; et al. Adverse prognostic impact of perioperative allogeneic transfusion on patients with stage II/III gastric cancer. Gastric Cancer 2016, 19, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ojima, T.; Iwahashi, M.; Nakamori, M.; Nakamura, M.; Naka, T.; Katsuda, M.; Iida, T.; Hayata, K.; Yamaue, H. Association of allogeneic blood transfusions and long-term survival of patients with gastric cancer after curative gastrectomy. J. Gastrointest. Surg. 2009, 13, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Squires, M.H.; Kooby, D.A.; Poultsides, G.A.; Weber, S.M.; Bloomston, M.; Fields, R.C.; Pawlik, T.M.; Votanopoulos, K.I.; Schmidt, C.R.; Ejaz, A.; et al. Effect of perioperative transfusion on recurrence and survival after gastric cancer resection: A 7-institution analysis of 765 patients from the US Gastric Cancer Collaborative. J. Am. Coll. Surg. 2015, 221, 767–777. [Google Scholar] [CrossRef]
- Liang, Y.X.; Guo, H.H.; Deng, J.Y.; Wang, B.G.; Ding, X.W.; Wang, X.N.; Zhang, L.; Liang, H. Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J. Gastroenterol. 2013, 19, 5542–5550. [Google Scholar] [CrossRef]
- Rausei, S.; Ruspi, L.; Galli, F.; Tirotta, F.; Inversini, D.; Frattini, F.; Chiappa, C.; Rovera, F.; Boni, L.; Dionigi, G.; et al. Peri-operative blood transfusion in gastric cancer surgery: Prognostic or confounding factor? Int. J. Surg. 2013, 11, S100–S103. [Google Scholar] [CrossRef][Green Version]
- Shibata, A.; Matsuda, T.; Ajiki, W.; Sobue, T. Trend in incidence of adenocarcinoma of the esophagus in Japan, 1993–2001. Jpn. J. Clin. Oncol. 2008, 38, 464–468. [Google Scholar] [CrossRef]
- Hasegawa, S.; Yoshikawa, T.; Cho, H.; Tsuburaya, A.; Kobayashi, O. Is adenocarcinoma of the esophagogastric junction different between Japan and western countries? The incidence and clinicopathological features at a Japanese high-volume cancer center. World J. Surg. 2009, 33, 95–103. [Google Scholar] [CrossRef]
- Hatta, W.; Tong, D.; Lee, Y.Y.; Ichihara, S.; Uedo, N.; Gotoda, T. Different time trend and management of esophagogastric junction adenocarcinoma in three Asian countries. Dig. Endosc. 2017, 29, 18–25. [Google Scholar] [CrossRef]
- Fukuchi, M.; Mochiki, E.; Ishiguro, T.; Saito, K.; Naitoh, H.; Kumagai, Y.; Ishibashi, K.; Ishida, H. Prognostic impact of splenectomy in patients with esophagogastric junction carcinoma. In Vivo 2018, 32, 145–149. [Google Scholar] [CrossRef]
- Weitz, J.; D’Angelica, M.; Gonen, M.; Klimstra, D.; Coit, D.G.; Brennan, M.F.; Karpeh, M.S. Interaction of splenectomy and perioperative blood transfusions on prognosis of patients with proximal gastric and gastroesophageal junction cancer. J. Clin. Oncol. 2003, 21, 4597–4603. [Google Scholar] [CrossRef] [PubMed]
- Legarde, S.M.; ten Kate, F.J.W.; Reitsma, J.B.; Busch, O.R.C.; van Lanschot, J.J.B. Prognostic factors in adenocarcinoma of the esophagus or gastroesophageal junction. J. Clin. Oncol. 2006, 24, 4347–4355. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Jiang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Chen, Q. Long-term outcomes and prognostic factor analysis of resected Siewert type II adenocarcinoma of esophagogastric junction in China: A seven-year study. BMC Surg. 2020, 20, 302. [Google Scholar] [CrossRef] [PubMed]
- Fjederholt, K.T.; Svendsen, L.B.; Mortensen, F.V. Perioperative blood transfusions increases the risk of anastomotic leakage after surgery for GEJ-cancer. Am. J. Surg. 2017, 214, 293–298. [Google Scholar] [CrossRef]
- Matsuda, T.; Kurokawa, Y.; Yoshikawa, T.; Kishi, K.; Misawa, K.; Ohi, M.; Mine, S.; Hiki, N.; Takeushi, H. Clinicopathological characteristics and prognostic factors of patients with Siewert type II esophagogastric junction carcinoma: A retrospective multicenter study. World J. Surg. 2016, 40, 1672–1679. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- Pang, Q.Y.; An, R.; Liu, H.L. Perioperative transfusion and the prognosis of colorectal cancer surgery: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 17, 7. [Google Scholar] [CrossRef]
- Gantt, C.L. Red blood cells for cancer patients. Lancet 1981, 2, 363. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, F.J.; Gong, M.; Yan, M. Effect of perioperative autologous versus allogeneic blood transfusion on the immune system in gastric cancer patients. J. Zhejiang Univ. Sci. B. 2007, 8, 560–565. [Google Scholar] [CrossRef]
- Cata, J.P.; Wang, H.; Gottumukkala, V.; Reuben, J.; Sessler, D.I. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br. J. Anaesth. 2013, 110, 690–701. [Google Scholar] [CrossRef]
- Roelen, D.; Brand, A.; Claas, F.H.J. Pretransplant blood transfusions revisited: A role for CD(4+) regulatory T cells? Transplantation 2004, 77, S26–S28. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Nascimento, J.E.; Zampieri-Filho, J.P.; Bordin, J.O. Implications of perioperative allogeneic red blood cell transfusion on the immune-inflammatory response. Hematol. Transfus. Cell Ther. 2021, 43, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Reim, D.; Borghi, F.; Nguyen, N.T.; Qi, F.; Coratti, A.; Cianchi, F.; Cesari, M.; Bazzocchi, F.; Alimoglu, O.; et al. Minimally invasive surgery for gastric cancer: A comparison between robotic, laparoscopic and open surgery. World J. Gastroenterol. 2007, 23, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).