The Risk of Trigeminal Neuralgia Following Osteoporosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Study Population
2.3. Outcome and Comorbidities
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Subjects with and without Osteoporosis
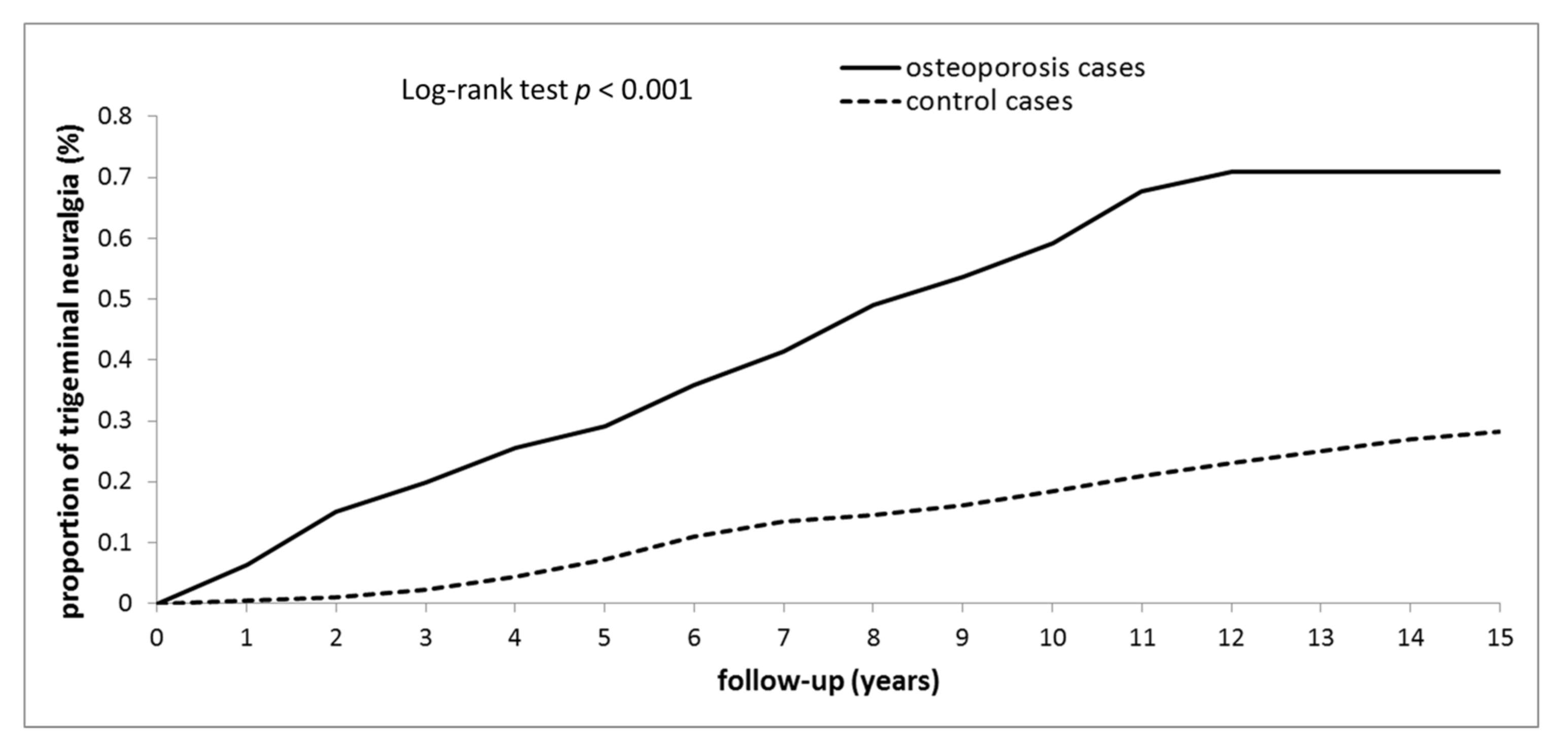
3.2. Incidence and Risk of TN
3.3. Risk Factors for TN in Osteoporosis Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, F. The Extant Works of Aretæus, The Cappadocian; Sydenham Society: London, UK, 1856. [Google Scholar]
- Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988, 8 (Suppl. S7), 1–96.
- Katusic, S.; Beard, C.M.; Bergstralth, E.; Kurland, L.T. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann. Neurol. 1990, 27, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.L.; Yen, M.F.; Chiu, Y.H.; Chen, L.S.; Chen, H.H. Increased risk of trigeminal neuralgia after hypertension: A population-based study. Neurology 2011, 77, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Chen, Y.T.; Fuh, J.L.; Wang, S.J. Increased risk of trigeminal neuralgia in patients with migraine: A nationwide population-based study. Cephalalgia 2016, 36, 1218–1227. [Google Scholar] [CrossRef]
- Hu, X.; Ma, S.; Yang, C.; Wang, W.; Chen, L. Relationship between senile osteoporosis and cardiovascular and cerebrovascular diseases. Exp. Ther. Med. 2019, 17, 4417–4420. [Google Scholar] [CrossRef]
- Bouquot, J.; Roberts, A.; Person, P.; Christian, J. Neuralgia-inducing cavitational osteonecrosis (NICO). Osteomyelitis in 224 jawbone samples from patients with facial neuralgia. Oral Surg. Oral Med. Oral Pathol. 1992, 73, 307–319; discussion 319–320. [Google Scholar] [CrossRef]
- Bouquot, J.E.; Christian, J. Long-term effects of jawbone curettage on the pain of facial neuralgia. J. Oral Maxillofac. Surg. 1995, 53, 387–397; discussion 397–389. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [CrossRef]
- Tolle, T.; Dukes, E.; Sadosky, A. Patient burden of trigeminal neuralgia: Results from a cross-sectional survey of health state impairment and treatment patterns in six European countries. Pain Pract. 2006, 6, 153–160. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Manzoni, G.C.; Torelli, P. Epidemiology of typical and atypical craniofacial neuralgias. Neurol. Sci. 2005, 26 (Suppl. S2), S65–S67. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, P.; Cooper, C. Osteoporosis. Lancet 2006, 367, 2010–2018. [Google Scholar] [CrossRef]
- Warburton, D.E.; Nicol, C.W.; Gatto, S.N.; Bredin, S.S. Cardiovascular disease and osteoporosis: Balancing risk management. Vasc. Health Risk Manag. 2007, 3, 673–689. [Google Scholar] [PubMed]
- Wu, C.Y.; Lu, Y.Y.; Lu, C.C.; Su, Y.F.; Tsai, T.H.; Wu, C.H. Osteoporosis in adult patients with atopic dermatitis: A nationwide population-based study. PLoS ONE 2017, 12, e0171667. [Google Scholar] [CrossRef]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef]
- Yoshida, T.; Stern, P.H. How vitamin D works on bone. Endocrinol. Metab. Clin. N. Am. 2012, 41, 557–569. [Google Scholar] [CrossRef]
- Yagci, N.; Aslan, D.; Durmus, M. The effect of vitamin D deficiency in patients with trigeminal neuralgia: A case control study. Med. Sci. 2021, 10, 5. [Google Scholar] [CrossRef]
- Guiglia, R.; Di Fede, O.; Lo Russo, L.; Sprini, D.; Rini, G.B.; Campisi, G. Osteoporosis, jawbones and periodontal disease. Med. Oral Patol. Oral Cirugía Bucal 2013, 18, e93–e99. [Google Scholar] [CrossRef]
- Gulsahi, A. Osteoporosis and jawbones in women. J. Int. Soc. Prev. Community Dent. 2015, 5, 263–267. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Liu, N.; Yang, Q.; Luo, E. Osteoporosis in the jawbones: A correlative factor of primary trigeminal neuralgia? Med. Sci. Monit. 2014, 20, 1481–1485. [Google Scholar]
- Keller, J.J.; Sheu, J.J.; Lin, H.C. Chronic periodontitis and the subsequent risk of trigeminal neuralgia: A 5-year follow-up study. J. Clin. Periodontol. 2012, 39, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Lambru, G.; Zakrzewska, J.; Matharu, M. Trigeminal neuralgia: A practical guide. Pract. Neurol. 2021, 21, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Zhang, Z.H.; Wu, M.K.; Wang, C.H.; Lu, Y.Y.; Lin, C.L. Increased migraine risk in osteoporosis patients: A nationwide population-based study. Springerplus 2016, 5, 1378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Young, E.W.; Morris, C.D.; McCarron, D.A. Urinary calcium excretion in essential hypertension. J. Lab. Clin. Med. 1992, 120, 624–632. [Google Scholar] [PubMed]
- MacGregor, G.A.; Cappuccio, F.P. The kidney and essential hypertension: A link to osteoporosis? J. Hypertens. 1993, 11, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Gadallah, M.; Massry, S.G.; Bigazzi, R.; Horst, R.L.; Eggena, P.; Campese, V.M. Intestinal absorption of calcium and calcium metabolism in patients with essential hypertension and normal renal function. Am. J. Hypertens. 1991, 4, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Perez-Castrillon, J.L.; Justo, I.; Silva, J.; Sanz, A.; Igea, R.; Escudero, P.; Pueyo, C.; Diaz, C.; Hernandez, G.; Duenas, A. Bone mass and bone modelling markers in hypertensive postmenopausal women. J. Hum. Hypertens. 2003, 17, 107–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Izawa, Y.; Sagara, K.; Kadota, T.; Makita, T. Bone disorders in spontaneously hypertensive rat. Calcif. Tissue Res. 1985, 37, 605–607. [Google Scholar] [CrossRef]
- Cirillo, M.; Galletti, F.; Strazzullo, P.; Torielli, L.; Melloni, M.C. On the pathogenetic mechanism of hypercalciuria in genetically hypertensive rats of the milan strain. Am. J. Hypertens. 1989, 2, 741–746. [Google Scholar] [CrossRef]
- Samsam, M.; Covenas, R.; Csillik, B.; Ahangari, R.; Yajeya, J.; Riquelme, R.; Narvaez, J.A.; Tramu, G. Depletion of substance p, neurokinin a and calcitonin gene-related peptide from the contralateral and ipsilateral caudal trigeminal nucleus following unilateral electrical stimulation of the trigeminal ganglion; a possible neurophysiological and neuroanatomical link to generalized head pain. J. Chem. Neuroanat. 2001, 21, 161–169. [Google Scholar] [PubMed]
- Qin, Z.L.; Yang, L.Q.; Li, N.; Yue, J.N.; Wu, B.S.; Tang, Y.Z.; Guo, Y.N.; Lai, G.H.; Ni, J.X. Clinical study of cerebrospinal fluid neuropeptides in patients with primary trigeminal neuralgia. Clin. Neurol. Neurosurg. 2016, 143, 111–115. [Google Scholar] [CrossRef]
- Silver, W.L.; Finger, T.E. The anatomical and electrophysiological basis of peripheral nasal trigeminal chemoreception. Ann. N. Y. Acad. Sci. 2009, 1170, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, K.; Gutow, A.P.; Troiano, N.; Gundberg, C.; Gilligan, J.P.; Vignery, A. Effects of calcitonin gene-related peptide on bone turnover in ovariectomized rats. Bone 1997, 21, 269–274. [Google Scholar] [CrossRef]
- Lin, J.; Lu, C.; Gao, L. Study on the level of plasma calcitonin gene-related peptide and adrenomedullin in subjects with primary osteoporosis. Zhonghua Yi Xue Za Zhi 2001, 81, 841–843. [Google Scholar] [PubMed]
- Wu, T.H.; Hu, L.Y.; Lu, T.; Chen, P.M.; Chen, H.J.; Shen, C.C.; Wen, C.H. Risk of psychiatric disorders following trigeminal neuralgia: A nationwide population-based retrospective cohort study. J. Headache Pain 2015, 16, 64. [Google Scholar] [CrossRef]
- Holle, D.; Heber, A.; Naegel, S.; Diener, H.C.; Katsarava, Z.; Obermann, M. Influences of smoking and caffeine consumption on trigeminal pain processing. J. Headache Pain 2014, 15, 39. [Google Scholar] [CrossRef]

| Variables | Osteoporosis | p-Value | |
|---|---|---|---|
| Yes (n = 45,393) | No (n = 45,393) | ||
| Trigeminal neuralgia patients, n (%) | 205 (0.45) | 128 (0.28) | <0.001 |
| Period of developing trigeminal neuralgia median (IQR), years | 3.8 (1.8–7.0) | 7.5 (4.9–11.0) | <0.001 |
| Mean age at diagnosis of trigeminal neuralgia, years | 64.3 (8.9) | 67.2 (10.3) | <0.001 |
| Age group, n (%) | |||
| 50–60 | 16,782 (36.97) | 16,782 (36.97) | |
| 60–70 | 14,598 (32.16) | 14,598 (32.16) | |
| >70 | 14,013 (30.87) | 14,013 (30.87) | 1.000 |
| Gender, n (%) | |||
| Men | 9112 (20.07) | 9112 (20.07) | |
| Women | 36,281 (79.93) | 36,281 (79.93) | 1.000 |
| Charlson Comorbidity Index, n (%) | |||
| 0 | 1801 (3.97) | 8160 (17.98) | |
| 1–2 | 9943 (21.90) | 16,099 (35.47) | |
| 3–4 | 13,169 (29.01) | 11,273 (24.83) | |
| ≥5 | 20,480 (45.12) | 9861 (21.72) | <0.001 |
| Comorbidity, n (%) | |||
| Hypertension | 34,327 (75.62) | 25,583 (56.36) | <0.001 |
| Diabetes mellitus | 18,670 (41.13) | 13,099 (28.86) | <0.001 |
| Hyperlipidemia | 28,693 (69.21) | 21,053 (46.38) | <0.001 |
| Migraine | 3011 (6.63) | 1358 (2.99) | <0.001 |
| Chronic periodontitis | 18,656 (41.10) | 16,225 (35.74) | <0.001 |
| Chronic kidney disease | 10,471 (23.07) | 5592 (12.32) | <0.001 |
| Chronic pulmonary disease | 21,199 (46.70) | 12,386 (27.29) | <0.001 |
| Chronic liver disease | 19,053 (41.97) | 12,934 (28.49) | <0.001 |
| Coronary artery syndrome | 6700 (14.76) | 3206 (7.06) | <0.001 |
| Alcohol-attributed disease | 1163 (2.56) | 993 (2.19) | <0.001 |
| Humeral fracture | 2343 (5.16) | 1094 (2.41) | <0.001 |
| Wrist fracture | 4097 (9.03) | 1624 (3.58) | <0.001 |
| Vertebral fracture | 10,212 (22.5) | 1355 (2.99) | <0.001 |
| Hip fracture | 3580 (7.89) | 676 (1.49) | <0.001 |
| Variables | People with Osteoporosis | People without Osteoporosis | Compared to Non-Osteoporosis Group | |||
|---|---|---|---|---|---|---|
| Trigeminal Neuralgia | Rate | Trigeminal Neuralgia | Rate | Crude HR (95% CI) | Adjusted HR * (95% CI) | |
| Overall | 205 | 0.60 | 128 | 0.18 | 3.12 (2.46–3.96) # | 1.80 (1.38–2.34) # |
| Gender | ||||||
| Men | 32 | 0.57 | 25 | 0.18 | 3.27 (1.84–5.83) # | 1.90 (1.03–3.53) # |
| Women | 173 | 0.61 | 103 | 0.20 | 3.11 (2.39–4.04) # | 1.83 (1.38–2.42) # |
| Stratify by age | ||||||
| 50–60 | 77 | 0.56 | 36 | 0.14 | 3.81 (2.54–5.72) # | 1.87 (1.23–2.85) # |
| 60–70 | 66 | 0.57 | 38 | 0.17 | 3.25 (2.16–4.89) # | 1.84 (1.21–2.79) # |
| >70 | 62 | 0.70 | 54 | 0.26 | 2.67 (1.83–3.89) # | 1.78 (1.21–2.63) # |
| Comorbidity & | ||||||
| No | 8 | 0.23 | 11 | 0.05 | 4.20 (1.68–10.49) # | 3.09 (1.23–7.75) # |
| Yes | 197 | 0.64 | 117 | 0.23 | 2.56 (2.01–3.28) # | 1.76 (1.35–2.28) # |
| Variables | Adjusted HR * (95% CI) |
|---|---|
| Migraine | 4.91 (3.62–6.67) # |
| Hypertension | 1.55 (1.03–2.34) # |
| Charlson Comorbidity Index | 1.28 (1.05–1.56) # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-F.; Wu, C.-H.; Wang, W.-T.; Lieu, A.-S. The Risk of Trigeminal Neuralgia Following Osteoporosis. Medicina 2022, 58, 447. https://doi.org/10.3390/medicina58030447
Su Y-F, Wu C-H, Wang W-T, Lieu A-S. The Risk of Trigeminal Neuralgia Following Osteoporosis. Medicina. 2022; 58(3):447. https://doi.org/10.3390/medicina58030447
Chicago/Turabian StyleSu, Yu-Feng, Chieh-Hsin Wu, Wei-Ting Wang, and Ann-Shung Lieu. 2022. "The Risk of Trigeminal Neuralgia Following Osteoporosis" Medicina 58, no. 3: 447. https://doi.org/10.3390/medicina58030447
APA StyleSu, Y.-F., Wu, C.-H., Wang, W.-T., & Lieu, A.-S. (2022). The Risk of Trigeminal Neuralgia Following Osteoporosis. Medicina, 58(3), 447. https://doi.org/10.3390/medicina58030447





