Muscle-MRI and Functional Levels for the Evaluation of Upper Limbs in Duchenne Muscular Dystrophy: A Critical Review of the Literature
Abstract
:1. Introduction
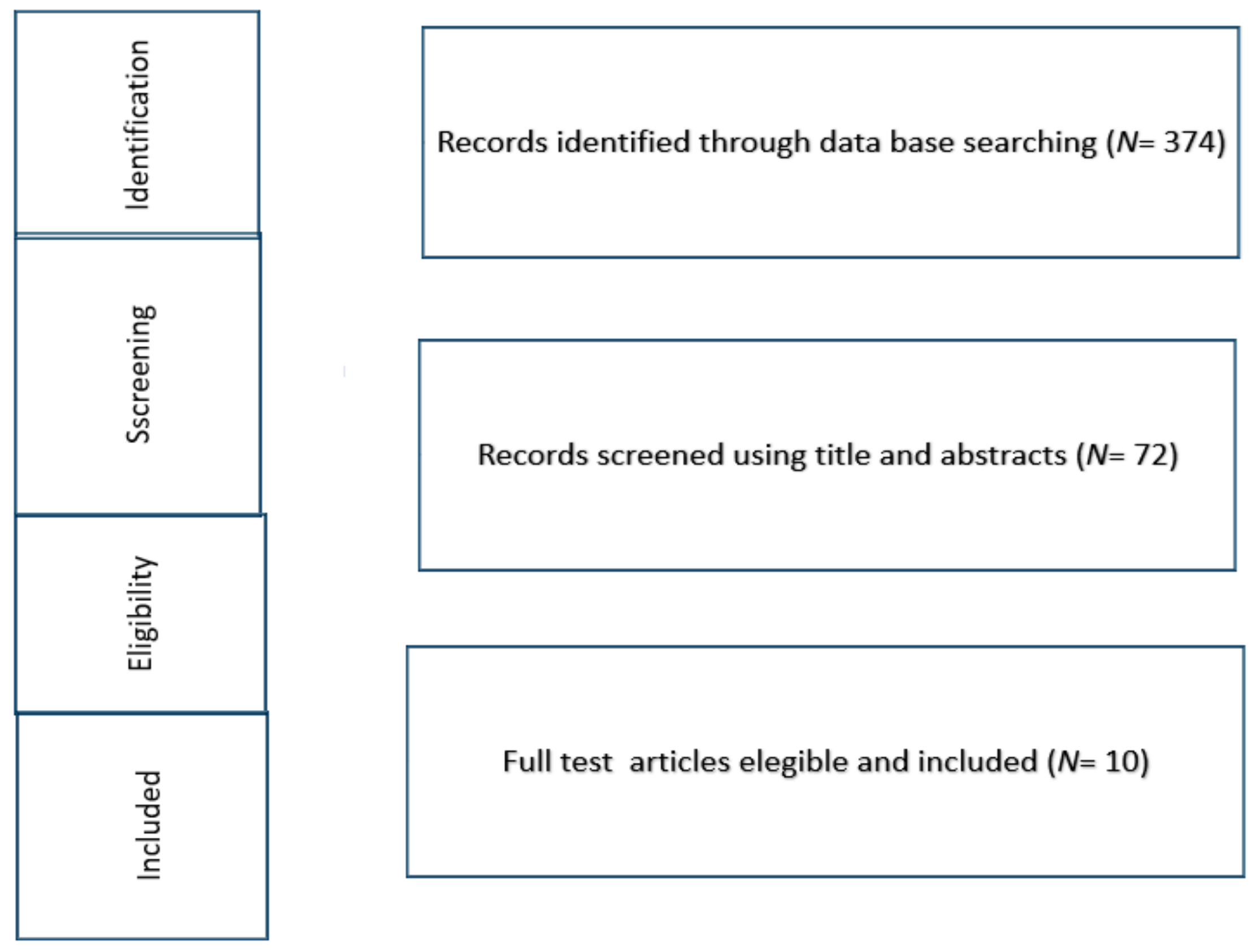
2. Material and Methods
3. Results
3.1. Muscle MRI and Functional Measures
3.2. Cross-Sectional Studies
3.3. Longitudinal Prospective Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cirak, S.; Arechavala-Gomeza, V.; Guglieri, M.; Feng, L.; Torelli, S.; Anthony, K.; Abbs, S.; Garralda, M.E.; Bourke, J.; Wells, D.J.; et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: An open-label, phase 2, dose-escalation study. Lancet 2011, 378, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Goemans, N.M.; Tulinius, M.; van den Akker, J.T.; Burm, B.E.; Ekhart, P.F.; Heuvelmans, N.; Holling, T.; Janson, A.A.; Platenburg, G.J.; Sipkens, J.A.; et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2011, 364, 1513–1522. [Google Scholar] [CrossRef]
- Willcocks, R.J.; Forbes, S.C.; Walter, G.A.; Sweeney, L.; Rodino-Klapac, L.R.; Mendell, J.R.; Vandenborne, K. Assessment of rAAVrh.74.MHCK7.micro-dystrophin Gene Therapy Using Magnetic Resonance Imaging in Children With Duchenne Muscular Dystrophy. JAMA Netw. Open. 2021, 4, e2031851. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Sahenk, Z.; Lehman, K.; Nease, C.; Lowes, L.P.; Miller, N.F.; Iammarino, M.A.; Alfano, L.N.; Nicholl, A.; Al-Zaidy, S.; et al. Assessment of Systemic Delivery of rAAVrh74.MHCK7.micro-dystrophin in Children With Duchenne Muscular Dystrophy: A Nonrandomized Controlled Trial. JAMA Neurol. 2020, 77, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Campbell, C.; Torricelli, R.E.; Finkel, R.S.; Flanigan, K.M.; Goemans, N.; Heydemann, P.; Kaminska, A.; Kirschner, J.; Muntoni, F.; et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1489–1498. [Google Scholar]
- Mendell, J.R.; Goemans, N.; Lowes, L.P.; Alfano, L.N.; Berry, K.; Shao, J.; Kaye, E.M.; Mercuri, E. Eteplirsen Study Group and Telethon Foundation DMD Italian Network. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol. 2016, 79, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Willcocks, R.J.; Rooney, W.D.; Triplett, W.T.; Forbes, S.C.; Lott, D.J.; Senesac, C.R.; Daniels, M.J.; Wang, D.J.; Harrington, A.T.; Tennekoon, G.I.; et al. Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large duchenne muscular dystrophy cohort. Ann. Neurol. 2016, 79, 535–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, S.C.; Willcocks, R.J.; Triplett, W.T.; Rooney, W.D.; Lott, D.J.; Wang, D.J.; Pollaro, J.; Senesac, C.R.; Daniels, M.J.; Finkel, R.S.; et al. Magnetic resonance imaging and spectroscopy assessment of lower extremity skeletal muscles in boys with Duchenne muscular dystrophy: A multicenter cross sectional study. PLoS ONE 2014, 9, e106435. [Google Scholar] [CrossRef] [Green Version]
- Finanger, E.L.; Russman, B.; Forbes, S.C.; Rooney, W.D.; Walter, G.A.; Vandenborne, K. Use of skeletal muscle MRI in diagnosis and monitoring disease progression in Duchenne muscular dystrophy. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rooney, W.D.; Berlow, Y.A.; Triplett, W.T.; Forbes, S.C.; Willcocks, R.J.; Wang, D.J.; Arpan, I.; Arora, H.; Senesac, C.; Lott, D.J.; et al. Modeling disease trajectory in Duchenne muscular dystrophy. Neurology 2020, 94, e1622–e1633. [Google Scholar] [CrossRef]
- Tasca, G.; Iannaccone, E.; Monforte, M.; Masciullo, M.; Bianco, F.; Laschena, F.; Ottaviani, P.; Pelliccioni, M.; Pane, M.; Mercuri, E.; et al. Muscle MRI in Becker muscular dystrophy. Neuromuscul. Disord. 2012, 22 (Suppl. S2), 100–106. [Google Scholar] [CrossRef]
- Tasca, G.; Monforte, M.; Iannaccone, E.; Laschena, F.; Ottaviani, P.; Silvestri, G.; Masciullo, M.; Mirabella, M.; Servidei, S.; Ricci, E. Muscle MRI in female carriers of dystrophinopathy. Eur. J. Neurol. 2012, 19, 1256–1260. [Google Scholar] [CrossRef]
- Mercuri, E.; Pichiecchio, A.; Counsell, S.; Allsop, J.; Cini, C.; Jungbluth, H.; Uggetti, C.; Bydder, G. A short protocol for muscle MRI in children with muscular dystrophies. Eur. J. Paediatr. Neurol. 2002, 6, 305–307. [Google Scholar] [CrossRef]
- Wary, C.; Azzabou, N.; Giraudeau, C.; Le Louer, J.; Montus, M.; Voit, T.; Setrvais, L.; Carlier, P. Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR Biomed. 2015, 28, 1150–1162. [Google Scholar] [CrossRef]
- Willcocks, R.J.; Triplett, W.T.; Forbes, S.C.; Arora, H.; Senesac, C.R.; Lott, D.J.; Nicholson, T.R.; Roonety, W.D.; Walter, G.A.; Vandenborne, K. Magnetic resonance imaging of the proximal upper extremity musculature in boys with Duchenne muscular dystrophy. J. Neurol. 2017, 264, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Brogna, C.; Cristiano, L.; Tartaglione, T.; Verdolotti, T.; Fanelli, L.; Ficociello, L.; Tasca, G.; Battini, R.; Coratti, G.; Forcina, N.; et al. Functional levels and MRI patterns of muscle involvement in upper limbs in Duchenne muscular dystrophy. PLoS ONE 2018, 13, e0199222. [Google Scholar] [CrossRef]
- Tartaglione, T.; Brogna, C.; Cristiano, L.; Verdolotti, T.; Pane, M.; Ficociello, L.; Fanelli, L.; Colosimo, C.; Mercuri, E. Early involvement of the supinator muscle in Duchenne muscular dystrophy. Neuromuscul. Disord. 2018, 28, 62–63. [Google Scholar] [CrossRef]
- Forbes, S.C.; Arora, H.; Willcocks, R.J.; Triplett, W.T.; Rooney, W.D.; Barnard, A.M.; AlAbasi, U.; Wang, D.-J.; Lott, D.J.; Senesac, C.R.; et al. Upper and Lower Extremities in Duchenne Muscular Dystrophy Evaluated with Quantitative MRI and Proton MR Spectroscopy in a Multicenter Cohort. Radiology 2020, 295, 616–625. [Google Scholar] [CrossRef]
- Ricotti, V.; Evans, M.R.; Sinclair, C.D.; Butler, J.W.; Ridout, D.A.; Hogrel, J.Y.; Emira, A.; Morrow, J.M.; Reilly, M.M.; Hanna, M.G.; et al. Upper Limb Evaluation in Duchenne Muscular Dystrophy: Fat-Water Quantification by MRI, Muscle Force and Function Define Endpoints for Clinical Trials. PLoS ONE 2016, 11, e0162542. [Google Scholar]
- Hogrel, J.Y.; Wary, C.; Moraux, A.; Azzabou, N.; Decostre, V.; Ollivier, G.; Canal, A.; Lilien, C.; Ledoux, I.; Annoussamy, M.; et al. Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology 2016, 86, 1022–1030. [Google Scholar] [CrossRef]
- Naarding, K.J.; van der Holst, M.; van Zwet, E.W.; van de Velde, N.M.; de Groot, I.J.M.; Verschuuren, J.J.G.M.; Kan, H.E.; Niks, E.H. Association of Elbow Flexor MRI Fat Fraction with Loss of Hand-to-Mouth Movement in Patients with Duchenne Muscular Dystrophy. Neurology 2021, 97, e1737–e1742. [Google Scholar] [CrossRef]
- Lilien, C.; Reyngoudt, H.; Seferian, A.M.; Gidaro, T.; Annoussamy, M.; Chê, V.; Decostre, V.; Ledoux, I.; Le Louër, J.; Guemas, E.; et al. Upper limb disease evolution in exon 53 skipping eligible patients with Duchenne muscular dystrophy. Ann. Clin. Transl. Neurol. 2021, 8, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Cristiano, L.; Verdolotti, T.; Norcia, G.; Ficociello, L.; Ruiz, R.; Coratti, G.; Fanelli, L.; Forcina, N.; Petracca, G.; et al. Longitudinal motor functional outcomes and Magnetic Resonance Imaging patterns of muscle involvement in upper limbs in Duchenne muscular dystrophy. Medicina 2021, 57, 1267. [Google Scholar] [CrossRef] [PubMed]
| Authors | Study Type | Genotype | Patients | Mean Age (Age in Years + SD) | Non-Ambulant Patients | Upper Limb Section Evaluated | MRI Type and Scoring | Motor Functional Tools |
|---|---|---|---|---|---|---|---|---|
| Prospective cross sectional studies | ||||||||
| Wary et al., 2015 [14] | Prospective cross-sectional | 53 skippable patients | 24 | 11.2 ± 3.7 | 14 | Forearm | quantitative FF and T2 MRI, 31P NMRS (3.0-T MRI system) | None |
| Willcocks et al., 2016 [15] | Prospective Cross-sectional | Not reported | 22 | 10.8 ± 2.5 | 2 | Shoulder, upper arm, forearm | quantitative FF and T2 MRI 1 H-MRS, (3.0-T MRI system) | PUL, Brooke Upper Extremity Scale, grip and pinch strength |
| Brogna et al., 2018 [16] | Prospective Cross-sectional | Not reported | 31 | 12.7 ± 5.5 | 14 | Shoulder, arm, forearm | T1 MRI Mercuri Score (1.5-T MRI system) | PUL |
| Tartaglione et al., 2018 [17] | Case series | Not reported | 4 | 5–15 | 1 | Forearm | T1 MRI Mercuri Score (1.5-T MRI system) | Distal PUL |
| Forbes et al., 2020 [18] | prospective cross-sectional | Not reported | 119 | 12 ± 3 | 35 | Shoulder, arm, forearm | Quantitative FF and T2 MRI 1 H MRS (3.0-T MRI system) | PUL, Brooke upper extremity Scale, Grip Strength, Pinch Stength |
| Prospective longitudinal studies | ||||||||
| Ricotti et al., 2016 [19] | Prospective longitudinal | Not reported | 15 | 13.2 | 15 | Forearm | quantitative MRI FF and T2 (3.0-T MRI system) | PUL, Myopinch, Myogrip, Moviplate, Egen Klassification (EK2) |
| Hogrel et al., 2016 [20] | Prospective longitudinal | 53-skippable patients | 25 | 8.2 in ambulant, 13.9 in non ambulant patients | 15 | Forearm | quantitative MRI FF and T2 and phosphorous MRS (3.0-T MRI system) | MFM, hand grip and key pinch strength, MoviPlate |
| Naarding et al., 2021 [21] | Prospective longitudinal | Not reported | 20 | 13.5 (12.5–16.4) | 20 | Forearm | quantitative MRI FF (3.0-T MRI system) | PUL |
| Lillien et al., 2021 [22] | Prospective longitudinal | 53-skippable patients | 40 | 11.7 (3.4) | 22 | Forearm | quantitative MRI FF and cross-sectional area (1.5 and 3.0-T MRI system) | Brooke score, MFM, hand grip and key pinch strength, and MoviPlate |
| Brogna et al., 2021 [23] | Prospective longitudinal | Not reported | 27 | 5–30 | 17 | shoulder arm, forearm | T1 MRI Mercuri Score (1.5-T MRI system) | PUL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristiano, L.; Brogna, C.; Tasca, G.; Verdolotti, T.; Pane, M.; Mercuri, E. Muscle-MRI and Functional Levels for the Evaluation of Upper Limbs in Duchenne Muscular Dystrophy: A Critical Review of the Literature. Medicina 2022, 58, 440. https://doi.org/10.3390/medicina58030440
Cristiano L, Brogna C, Tasca G, Verdolotti T, Pane M, Mercuri E. Muscle-MRI and Functional Levels for the Evaluation of Upper Limbs in Duchenne Muscular Dystrophy: A Critical Review of the Literature. Medicina. 2022; 58(3):440. https://doi.org/10.3390/medicina58030440
Chicago/Turabian StyleCristiano, Lara, Claudia Brogna, Giorgio Tasca, Tommaso Verdolotti, Marika Pane, and Eugenio Mercuri. 2022. "Muscle-MRI and Functional Levels for the Evaluation of Upper Limbs in Duchenne Muscular Dystrophy: A Critical Review of the Literature" Medicina 58, no. 3: 440. https://doi.org/10.3390/medicina58030440
APA StyleCristiano, L., Brogna, C., Tasca, G., Verdolotti, T., Pane, M., & Mercuri, E. (2022). Muscle-MRI and Functional Levels for the Evaluation of Upper Limbs in Duchenne Muscular Dystrophy: A Critical Review of the Literature. Medicina, 58(3), 440. https://doi.org/10.3390/medicina58030440







