Simultaneous Patent Blue Dye Injections Aid in the Preoperative CT-Guided Localization of Multiple Pulmonary Nodules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
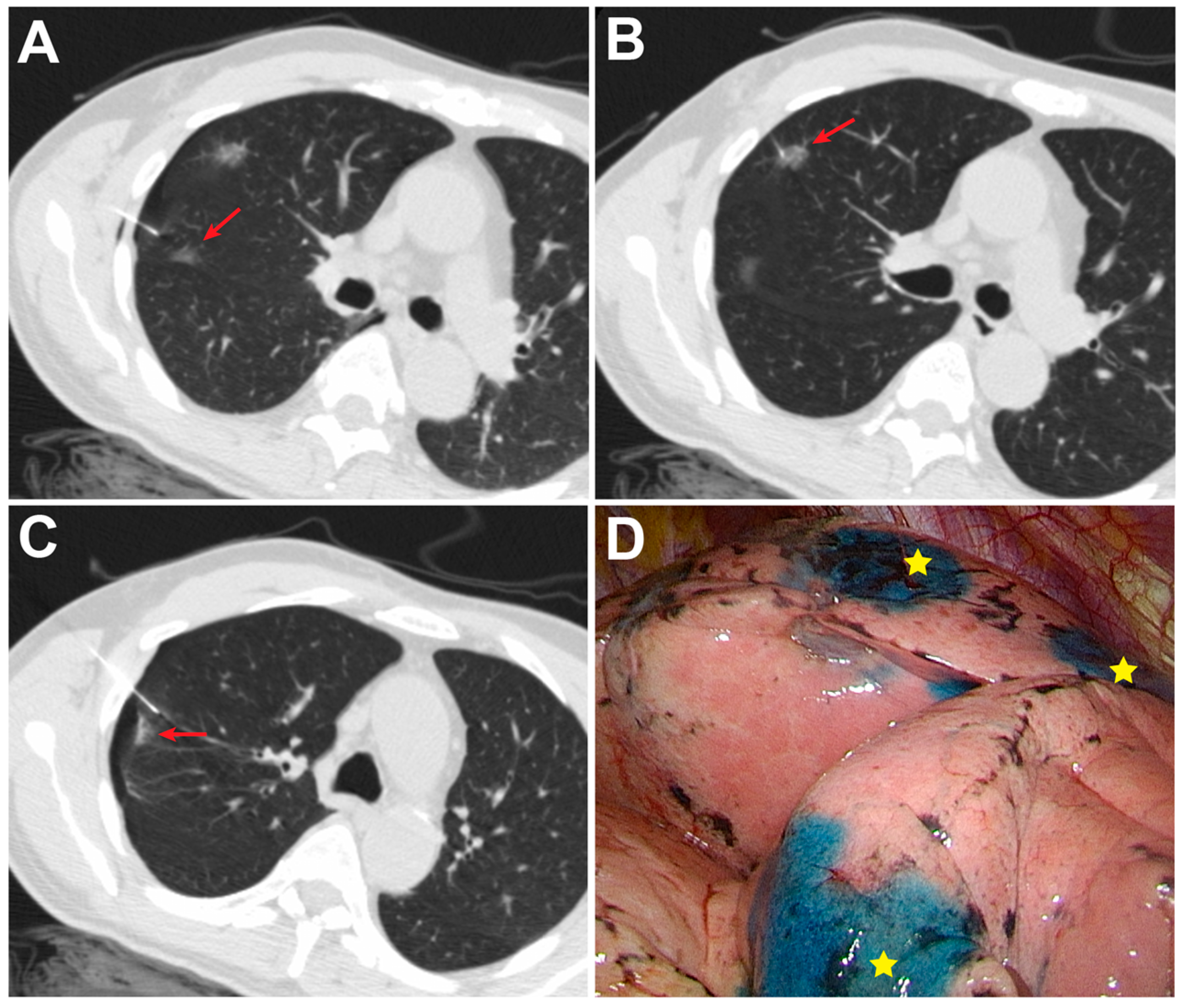
2.2. CT-Guided PBD Localization
2.3. Success Rate and Complications
2.4. VATS after Localization
2.5. Statistical Analysis
3. Results
3.1. Patient and Nodule Characteristics
3.2. Preoperative Results and Complications Related to CT-Guided Localization
3.3. Operative and Pathological Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Brawley, O.W.; Wender, R.C. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2017, 67, 100–121. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Heuvelmans, M.A.; Walter, J.E.; Peters, R.B.; Bock, G.H.; Yousaf-Khan, U.; Aalst, C.M.V.; Groen, H.J.M.; Nackaerts, K.; Ooijen, P.M.V.; Koning, H.J.; et al. Relationship between nodule count and lung cancer probability in baseline CT lung cancer screening: The NELSON study. Lung Cancer 2017, 113, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Congregado, M.; Merchan, R.J.; Gallardo, G.; Ayarra, J.; Loscertales, J. Video-assisted thoracic surgery (VATS) lobectomy: 13 years’ experience. Surg. Endosc. 2008, 22, 1852–1857. [Google Scholar] [CrossRef]
- Suzuki, K.; Nagai, K.; Yoshida, J.; Ohmatsu, H.; Takahashi, K.; Nishimura, M.; Nishiwaki, Y. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: Indications for preoperative marking. Chest 1999, 115, 563–568. [Google Scholar] [CrossRef]
- Chen, J.R.; Tseng, Y.H.; Lin, M.W.; Chen, H.M.; Chen, Y.C.; Chen, M.C.; Lee, Y.F.; Chen, J.S.; Chang, Y.C. Safety and efficacy of computed tomography-guided dye localization using patent blue V for single lung nodule for video-assisted thoracoscopic surgery: A retrospective study. Ann. Transl. Med. 2019, 7, 28. [Google Scholar] [CrossRef]
- Lin, M.W.; Tseng, Y.H.; Lee, Y.F.; Hsieh, M.S.; Ko, W.C.; Chen, J.Y.; Hsu, H.H.; Chang, Y.C.; Chen, J.S. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J. Thorac. Cardiovasc. Surg. 2016, 152, 535–544. [Google Scholar] [CrossRef]
- McDermott, S.; Fintelmann, F.J.; Bierhals, A.J.; Silin, D.D.; Price, M.C.; Ott, H.C.; Shepard, J.O.; Mayo, J.R.; Sharma, A. Image-guided preoperative localization of pulmonary nodules for video-assisted and robotically assisted surgery. Radiographics 2019, 39, 1264–1279. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, L.; Sun, H.; Huang, Z.; Zhang, Z.; Xiao, F.; Ma, Q.; Lin, J.; Xie, S. The utility of simultaneous CT-guided localization for multiple pulmonary nodules using microcoil before video-assisted thoracic surgery. BMC Pulm. Med. 2021, 21, 39. [Google Scholar] [CrossRef]
- Hu, L.; Gao, J.; Hong, N.; Liu, H.; Chen, C.; Zhi, X.; Sui, X. Simultaneous preoperative computed tomography-guided microcoil localizations of multiple pulmonary nodules. Eur. Radiol. 2021, 31, 6539–6546. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, C.C.; Huang, L.T.; Chung, T.J.; Liu, Y.S.; Yen, Y.T.; Tseng, Y.L. Computed tomography-guided methylene blue localization: Single vs. multiple lung nodules. Front. Med. 2021, 8, 661956. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Wu, A.L.; Yang, S.; Lin, J.; Xian, Y.T.; Fu, Y.F. Preoperative computed tomography-guided coil localization for multiple lung nodules. Ther. Adv. Respir. Dis. 2020, 14, 1753466620909762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, Y.H.; Lee, Y.F.; Hsieh, M.S.; Chien, N.; Ko, W.C.; Chen, J.Y.; Lee, J.M.; Huang, P.M.; Lin, M.W.; Chen, J.S.; et al. Preoperative computed tomography-guided dye injection to localize multiple lung nodules for video-assisted thoracoscopic surgery. J. Thorac. Dis. 2016, 8, S666–S671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, K.H.; Huang, T.W.; Lee, S.C.; Chang, W.C.; Gao, H.W.; Hsu, H.H. A simple and efficient method to perform preoperative pulmonary nodule localization: CT-guided patent blue dye injection. Clin. Imaging 2019, 58, 74–79. [Google Scholar] [CrossRef]
- Chao, Y.K.; Fang, H.Y.; Pan, K.T.; Wen, C.T.; Hsieh, M.J. Preoperative versus intraoperative image-guided localization of multiple ipsilateral lung nodules. Eur. J. Cardiothorac. Surg. 2020, 57, 488–495. [Google Scholar] [CrossRef]
- McCollough, C.; Edyvean, S.; Geise, R.; Gould, B.; Keat, N.; Huda, W.; Judy, P.; Kalender, W.; McNitt-Gray, M.; Morin, R.; et al. The Measurement, Reporting, and Management of Radiation Dose in CT; American Association of Physicists in Medicine: College Park, MD, USA, 2008. [Google Scholar]
- Tai, R.; Dunne, R.M.; Trotman-Dickenson, B.; Jacobson, F.L.; Madan, R.; Kumamaru, K.K.; Hunsaker, A.R. Frequency and severity of pulmonary hemorrhage in patients undergoing percutaneous CT-guided transthoracic lung biopsy: Single-institution experience of 1175 cases. Radiology 2016, 279, 287–296. [Google Scholar] [CrossRef]
- Kato, H.; Oizumi, H.; Suzuki, J.; Hamada, A.; Watarai, H.; Nakahashi, K.; Sadahiro, M. Thoracoscopic wedge resection and segmentectomy for small-sized pulmonary nodules. J. Vis. Surg. 2017, 3, 66. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Xu, X.Q.; Pan, X.L.; Zhang, W.; Xu, H.; Yuan, M.; Kong, L.Y.; Pu, X.H.; Chen, L.; Yu, T.F. Retrospective evaluation of safety, efficacy and risk factors for pneumothorax in simultaneous localizations of multiple pulmonary nodules using hook wire system. Cardiovasc. Interv. Radiol. 2017, 40, 1408–1414. [Google Scholar] [CrossRef]
- Fu, Y.F.; Gao, Y.G.; Zhang, M.; Wang, T.; Shi, Y.B.; Huang, Y.Y. Computed tomography-guided simultaneous coil localization as a bridge to one-stage surgery for multiple lung nodules: A retrospective study. J. Cardiothorac. Surg. 2019, 14, 43. [Google Scholar] [CrossRef]
- Sun, S.H.; Gao, J.; Zeng, X.M.; Zhang, Y.F. Computed tomography-guided localization for lung nodules: Methylene-blue versus coil localization. Minim. Invasive Ther. Allied Technol. 2021, 30, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Boskovic, T.; Stanic, J.; Pena-Karan, S.; Zarogoulidis, P.; Drevelegas, K.; Katsikogiannis, N.; Machairiotis, N.; Mpakas, A.; Tsakiridis, K.; Kesisis, G.; et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J. Thorac. Dis. 2014, 6 (Suppl. 1), S99–S107. [Google Scholar] [CrossRef] [PubMed]
- Dennie, C.J.; Matzinger, F.R.; Marriner, J.R.; Maziak, D.E. Transthoracic needle biopsy of the lung: Results of early discharge in 506 outpatients. Radiology 2001, 219, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Lempel, J.K.; Raymond, D.P. Intraoperative percutaneous microcoil localization of small peripheral pulmonary nodules using cone-beam CT in a hybrid operating room. AJR Am. J. Roentgenol. 2019, 213, 778–781. [Google Scholar] [CrossRef]
- Yang, S.M.; Ko, W.C.; Lin, M.W.; Hsu, H.H.; Chan, C.Y.; Wu, I.H.; Chang, Y.C.; Chen, J.S. Image-guided thoracoscopic surgery with dye localization in a hybrid operating room. J. Thorac. Dis. 2016, 8, S681–S689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, Y.K.; Pan, K.T.; Wen, C.T.; Fang, H.Y.; Hsieh, M.J. A comparison of efficacy and safety of preoperative versus intraoperative computed tomography-guided thoracoscopic lung resection. J. Thorac. Cardiovasc. Surg. 2018, 156, 1974–1983. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.H.; Hsu, H.H.; Yang, S.M.; Tsai, T.M.; Tsou, K.C.; Liao, H.C.; Lin, M.W.; Chen, J.S. Preoperative dye localization for thoracoscopic lung surgery: Hybrid versus computed tomography room. Ann. Thorac. Surg. 2018, 106, 1661–1667. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Fang, H.Y.; Lin, C.C.; Wen, C.T.; Chen, H.W.; Chao, Y.K. Single-stage localization and removal of small lung nodules through image-guided video-assisted thoracoscopic surgery. Eur. J. Cardiothorac. Surg. 2018, 53, 353–358. [Google Scholar] [CrossRef]
- Lenglinger, F.X.; Schwarz, C.D.; Artmann, W. Localization of pulmonary nodules before thoracoscopic surgery: Value of percutaneous staining with methylene blue. AJR Am. J. Roentgenol. 1994, 163, 297–300. [Google Scholar] [CrossRef]
- Starnes, S.L.; Wolujewicz, M.; Guitron, J.; Williams, V.; Scheler, J.; Ristagno, R. Radiotracer localization of nonpalpable pulmonary nodules: A single-center experience. J. Thorac. Cardiovasc. Surg. 2018, 156, 1986–1992. [Google Scholar] [CrossRef]
- Lizza, N.; Eucher, P.; Haxhe, J.P.; De Wispelaere, J.F.; Johnson, P.M.; Delaunois, L. Thoracoscopic resection of pulmonary nodules after computed tomographic-guided coil labeling. Ann. Thorac. Surg. 2001, 71, 986–988. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.J.; Chen, B.; Cao, J.M.; Lu, G.M.; Yuan, L.; Li, K.; Xu, J. Novel CT-guided coil localization of peripheral pulmonary nodules prior to video-assisted thoracoscopic surgery: A pilot study. Acta Radiol. 2014, 55, 699–706. [Google Scholar] [CrossRef] [PubMed]

| Variables | Simultaneous (n = 31) | Sequential (n = 30) | p Value |
|---|---|---|---|
| Number of patients with | |||
| two nodules, n | 29 | 27 | |
| three nodules, n | 2 | 3 | |
| Age, years | |||
| Median (IQR) | 65 (58–70) | 61 (50–67) | 0.103 |
| Range | [38–83] | [29–81] | |
| Sex, n (%) | 0.939 | ||
| Male | 8 (25.8) | 8 (26.7) | |
| Female | 23 (74.2) | 22 (73.3) | |
| Smoking status, n (%) | 0.955 | ||
| Never | 26 (83.9) | 25 (83.3) | |
| Current or former | 5 (16.1) | 5 (16.7) | |
| Size, mm | |||
| Median (IQR) | 7 (5–9) | 6 (4.5–8) | 0.058 |
| Range | [3–25] | [2–18] | |
| Depth, mm | |||
| Median (IQR) | 7.8 (3–14.3) | 5 (1.6–12.8) | 0.21 |
| Range | [0–34] | [0–33] | |
| Pulmonary lobe, n (%) | 0.132 | ||
| RUL | 21 (32.8) | 24 (38.1) | |
| RML | 2 (3.1) | 8 (12.7) | |
| RLL | 16 (25) | 15 (23.8) | |
| LUL | 14 (21.9) | 6 (9.5) | |
| LLL | 11 (17.2) | 10 (15.9) | |
| Nodule attenuation, n (%) | 0.164 | ||
| Ground-glass opacity | 50 (78.1) | 56 (88.9) | |
| Part solid | 2 (3.1) | 3 (4.8) | |
| Solid | 10 (15.6) | 4 (6.3) | |
| Cavitation | 2 (3.1) | 0 (0) |
| Variable | Simultaneous (n = 31) | Sequential (n = 30) | p Value |
|---|---|---|---|
| Localization success, % | 100 | 93.7 | 0.041 |
| Effective dose, mSv | 2.7 (2.43–3.19) | 3.5 (2.77–3.89) | 0.001 |
| Procedure time, min | 20.95 (16.45–22.47) | 25.28 (22.85–27.16) | 0.001 |
| Operator 1, min (n = 27) | 20.02 (17.19–24.32) | 24.42 (21.42–26.47) | 0.006 |
| Operator 2, min (n = 34) | 22.98 (18.82–24.98) | 25.75 (23.82–28.88) | 0.019 |
| Distance between the nodule and the dye, mm | 0.5 (0–4.9) | 3 (0–10) | 0.058 |
| Distance between the chest wall and the nodule, mm | 47.5 (38–65.75) | 55 (42–66) | 0.229 |
| Position, n (%) | 0.018 | ||
| Supine | 27 (42.2) | 29 (46.0) | |
| Prone | 25 (39.1) | 32 (50.8) | |
| Lateral decubitus | 12 (18.8) | 2 (3.2) | |
| Pneumothorax, n (%) | 10 (32.3) | 10 (33.3) | 0.929 |
| Pulmonary hemorrhage, n (%) | 2 (6.3) | 1 (3.0) | 1 |
| Variable | p Value | OR (95% CI) |
|---|---|---|
| Age, years | 0.858 | 1.004 (0.959–1.052) |
| Sex (ref: F) | 0.093 | 2.750 (0.843–8.967) |
| Smoking status | 0.596 | 1.458 (0.361–5.894) |
| Size, mm | 0.532 | 1.372 (0.509–3.703) |
| Depth, mm | 0.582 | 1.130 (0.731–1.746) |
| Simultaneous (ref: sequential) | 0.929 | 0.952 (0.327–2.774) |
| Operator (ref: operator 1) | 0.640 | 1.295 (0.438–3.834) |
| Pulmonary lobe (ref: RUL) | ||
| RML | 0.327 | 2.000 (0.500–7.997) |
| RLL | 0.922 | 0.952 (0.359–2.526) |
| LUL | 0.791 | 0.857 (0.274–2.679) |
| LLL | 0.706 | 1.231 (0.419–3.613) |
| Attenuation (ref: GGO) | ||
| Part solid | 0.885 | 1.145 (0.183–7.156) |
| Solid | 0.056 | 0.132 (0.017–1.049) |
| Cavitation | 0.999 | - |
| Position (ref: supine) | ||
| Prone | 0.395 | 0.717 (0.334–1.543) |
| Decubitus | 0.078 | 0.239 (0.049–1.171) |
| Variable | Simultaneous (n = 31) | Sequential (n = 30) | p Value |
|---|---|---|---|
| Surgical procedure, n (%) | 0.077 | ||
| Wedge resection | 52 (81.3) | 56 (88.9) | |
| Segmentectomy | 7 (10.9) | 7 (11.1) | |
| Lobectomy | 5 (7.8) | 0 (0) | |
| Operation time, min | |||
| Median (IQR) | 57 (42–106) | 54 (42.5–99.75) | 0.834 |
| Range | [27–357] | [24–175] | |
| Inpatient days, days | |||
| Median (IQR) | 7 (5–9) | 7 (5–8) | 0.775 |
| Range | [4–18] | [4–17] | |
| Pathology, n (%) | 0.077 | ||
| Invasive adenocarcinoma | 33 (51.6) | 35 (55.6) | |
| MIA | 13 (20.3) | 9 (14.3) | |
| AIS | 4 (6.3) | 8 (12.7) | |
| Metastasis | 6 (9.4) | 0 (0) | |
| AAH | 0 (0) | 3 (4.8) | |
| Carcinoid tumor | 1 (1.6) | 0 (0) | |
| Focal interstitial fibrosis | 4 (6.3) | 5 (8) | |
| Chronic granulomatous inflammation | 0 (0) | 1 (1.6) | |
| Hemorrhage with congestion | 1 (1.6) | 2 (3.2) | |
| Minute pulmonary meningothelial-like nodule | 2 (3.1) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Huang, T.-W.; Hsu, H.-H.; Chang, W.-C.; Ko, K.-H. Simultaneous Patent Blue Dye Injections Aid in the Preoperative CT-Guided Localization of Multiple Pulmonary Nodules. Medicina 2022, 58, 405. https://doi.org/10.3390/medicina58030405
Chen Y-C, Huang T-W, Hsu H-H, Chang W-C, Ko K-H. Simultaneous Patent Blue Dye Injections Aid in the Preoperative CT-Guided Localization of Multiple Pulmonary Nodules. Medicina. 2022; 58(3):405. https://doi.org/10.3390/medicina58030405
Chicago/Turabian StyleChen, Ya-Che, Tsai-Wang Huang, Hsian-He Hsu, Wei-Chou Chang, and Kai-Hsiung Ko. 2022. "Simultaneous Patent Blue Dye Injections Aid in the Preoperative CT-Guided Localization of Multiple Pulmonary Nodules" Medicina 58, no. 3: 405. https://doi.org/10.3390/medicina58030405
APA StyleChen, Y.-C., Huang, T.-W., Hsu, H.-H., Chang, W.-C., & Ko, K.-H. (2022). Simultaneous Patent Blue Dye Injections Aid in the Preoperative CT-Guided Localization of Multiple Pulmonary Nodules. Medicina, 58(3), 405. https://doi.org/10.3390/medicina58030405





