Appropriate Method of Administering Vasopressors for Maternal Hypotension Associated with Combined Spinal Epidural Anesthesia in Elective Cesarean Section: Impact on Postnatal Respiratory Support for Newborns
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
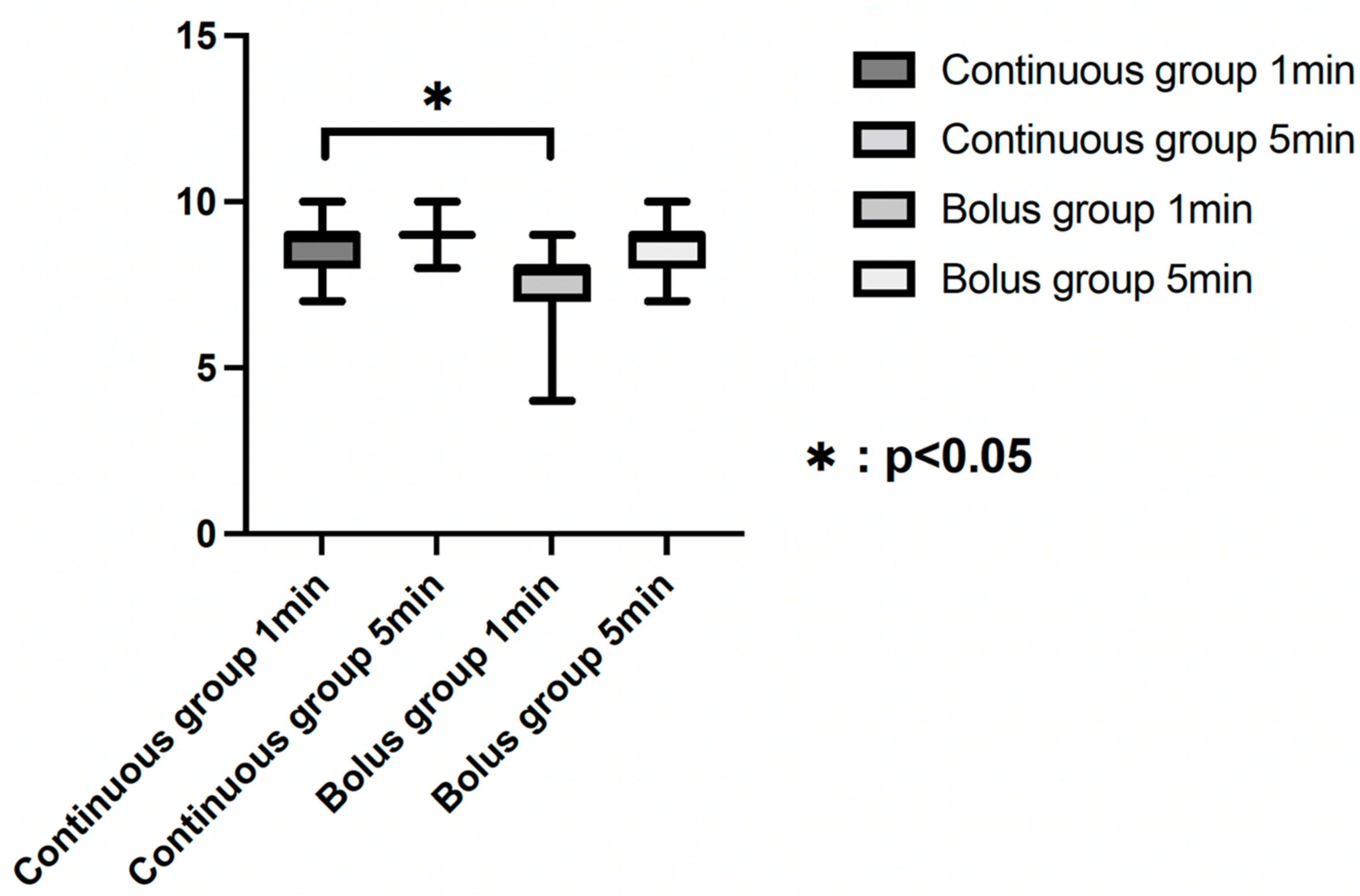
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzgerald, J.P.; Fedoruk, K.A.; Jadin, S.M.; Carvalho, B.; Halpern, S.H. Prevention of hypotension after spinal anaesthesia for caesarean section: A systematic review and network meta-analysis of randomised controlled trials. Anaesthesia 2019, 75, 109–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omaygenc, D.O.; Dogu, T.; Omaygenc, M.O.; Ozmen, F.; Albayrak, M.D.; Güler, G.B.; Gür, E.K.; Özenç, E. Type of anesthesia affects neonatal wellbeing and frequency of transient tachypnea in elective cesarean sections. J. Matern. Fetal Neonatal Med. 2014, 28, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Knigin, D.; Avidan, A.; Weiniger, C.F. The effect of spinal hypotension and anesthesia-to-delivery time interval on neonatal outcomes in planned cesarean delivery. Am. J. Obstet. Gynecol. 2020, 223, 747.e1–747.e13. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.; Bennett, A.; Wilkes, M.; Lewis, M. Spinal anaesthesia for Caesarean section: Comparison of infusions of phenylephrine and ephedrine. Br. J. Anaesth. 1994, 73, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Kee, W.D.N.; Khaw, K.S. Vasopressors in obstetrics: What should we be using? Curr. Opin. Anaesthesiol. 2006, 19, 238–243. [Google Scholar] [CrossRef]
- Veeser, M.; Hofmann, T.; Roth, R.; Klöhr, S.; Rossaint, R.; Heesen, M. Vasopressors for the management of hypotension after spinal anesthesia for elective caesarean section. Systematic review and cumulative meta-analysis. Acta Anaesthesiol. Scand. 2012, 56, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Buthelezi, A.S.; Bishop, D.G.; Rodseth, R.N.; Dyer, R.A. Prophylactic phenylephrine and fluid co-administration to reduce spinal hypotension during elective caesarean section in a resource-limited setting: A prospective alternating intervention study. Anaesthesia 2020, 75, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.S.C.; Boys, P.R.J.; Rodeck, F.C.; Morgan, F.B. Maternal and fetal haemodynamic effects of spinal and extradural anaesthesia for elective caesarean section. Br. J. Anaesth. 1992, 68, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Antoine, C.; Young, B.K. Fetal lactic acidosis with epidural anesthesia. Am. J. Obstet. Gynecol. 1982, 142, 55–59. [Google Scholar] [CrossRef]
- Datta, S.; Kitzmiller, J.L.; Naulty, J.S.; Ostheimer, G.W.; Weiss, J.B. Acid-Base Status of Diabetic Mothers and Their Infants following Spinal Anesthesia for Cesarean Section. Anesthesia Analg. 1982, 61, 662–665. [Google Scholar] [CrossRef]
- Richardson, B.S.; Ruttinger, S.; Brown, H.K.; Regnault, T.R.; de Vrijer, B. Maternal body mass index impacts fetal-placental size at birth and umbilical cord oxygen values with implications for regulatory mechanisms. Early Hum. Dev. 2017, 112, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Temming, L.A.; Stout, M.J.; Macones, G.A.; Cahill, A.G.; Tuuli, M.G.; Raghuraman, N. Umbilical Cord Oxygen Content and Neonatal Morbidity at Term. Am. J. Perinatol. 2018, 35, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, G.; Siristatidis, C.; Salamalekis, E.; Creatsas, G. Spinal and epidural versus general anesthesia for elective cesarean section at term: Effect on the acid–base status of the mother and newborn. J. Matern. Fetal Neonatal Med. 2003, 13, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.T.; Dawson, J.D.; Toth, P.P.; Bowdler, N. Predictors of neonatal resuscitation, low Apgar scores, and umbilical artery pH among growth-restricted neonates. Obstet. Gynecol. 1998, 91, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Sabol, B.A.; Caughey, A.B. Acidemia in neonates with a 5-minute Apgar score of 7 or greater—What are the outcomes? Am. J. Obs. Gynecol. 2016, 215, 486.e1–486.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegyi, T.; Carbone, T.; Anwar, M.; Ostfeld, B.; Hiatt, M.; Koons, A.; Pinto-Martin, J.; Paneth, N. The Apgar score and its components in the preterm infant. Pediatrics 1998, 101, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, V. Association of Apgar scores with death and neurologic disability. Clin. Epidemiol. 2009, 1, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Person, M.K.; Esposito, D.H.; Holman, R.C.; Mehal, J.M.; Stoll, B.J. Risk factors for infectious disease death among infants in the United States. Pediatr. Infect. Dis. J. 2014, 33, e280–e285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalili, H.; Nili, F.; Sheikh, M.; Hardani, A.K.; Shariat, M.; Nayeri, F. Comparison of the four proposed Apgar scoring systems in the assessment of birth asphyxia and adverse early neurologic outcomes. PLoS ONE 2015, 10, e0122116. [Google Scholar] [CrossRef] [PubMed]
- Leybovitz-Haleluya, N.; Wainstock, T.; Sheiner, E.; Segal, I.; Landau, D.; Walfisch, A. Low Apgar scores in term newborns and long-term gastro-intestinal morbidity: A population-based cohort study with up to 18 years of follow-up. J. Matern. Fetal. Neonatal. Med. 2019, 32, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
| Continuous Group | Bolus Group | p Score | |
|---|---|---|---|
| age | 34.58 ± 5.40 | 34.27 ± 4.93 | 0.665 |
| para | 1.85 ± 0.93 | 1.97 ± 0.74 | 0.296 |
| gestational weeks | 38.30 ± 0.92 | 38.27 ± 0.62 | 0.781 |
| BMI before pregnancy | 23.04 ± 4.53 | 23.16 ± 4.98 | 0.855 |
| BMI on delivery | 26.55 ± 4.47 | 26.87 ± 5.13 | 0.627 |
| Continuous Group | Bolus Group | p Score | |
|---|---|---|---|
| Max SBP | 135.7 ± 14.1 | 143.3 ± 23.5 | 0.003 |
| Mini SBP | 109.3 ± 12.6 | 85.3 ± 12.2 | <0.001 |
| SBP deference | 26.3 ± 11.9 | 58.0 ± 24.5 | <0.001 |
| Max DBP | 80.8 ± 11.2 | 83.2 ± 14.9 | 0.174 |
| Mini DBP | 57.4 ± 13.7 | 39.8 ± 10.8 | <0.001 |
| DBP deference | 23.4 ± 10.6 | 43.4 ± 17.9 | <0.001 |
| time | 25.9 ± 6.5 | 27.1 ± 5.4 | 0.160 |
| Continuous Group | Bolus Group | p Score | |
|---|---|---|---|
| pH | 7.27 ± 0.05 | 7.25 ± 0.07 | 0.089 |
| pCO2 | 49.1 ± 8.9 | 49.9 ± 8.7 | 0.499 |
| pO2 | 20.3 ± 7.2 | 14.5 ± 5.7 | <0.001 |
| BE | −4.3 ± 2.6 | −4.2 ± 2.3 | 0.411 |
| AS 1 min | 7.9 ± 0.5 | 7.3 ± 0.07 | <0.001 |
| AS 5 min | 8.8 ± 0.4 | 8.7 ± 0.5 | 0.029 |
| AS 1 min < 7 (cases) | 2 cases (2.0%) | 16 cases (13.1%) | 0.003 |
| Background Associated with Respiratory Support | ||
|---|---|---|
| β | p | |
| Continuous/Bolus group | 0.317 | <0.001 |
| F score | 24.438 | |
| R2 | 0.101 | |
| adjusted R2 | 0.970 | |
| Blood pressure associated with respiratory support | ||
| β | p | |
| DBP deference | 0.254 | <0.001 |
| F score | 14.993 | |
| R2 | 0.064 | |
| adjusted R2 | 0.060 | |
| MaxSBP Associated with Respiratory Support | ||
|---|---|---|
| β | p | |
| maxSBP | 0.199 | 0.005 |
| F score | 7.944 | |
| R2 | 0.035 | |
| adjusted R2 | 0.031 | |
| miniSBP associated with respiratory support | ||
| β | p | |
| miniSBP | 0.621 | <0.001 |
| F score | 137.029 | |
| R2 | 0.386 | |
| adjusted R2 | 0.383 | |
| SBP deference associated with respiratory support | ||
| β | p | |
| SBP deference | 0.317 | <0.001 |
| F score | 24.438 | |
| R2 | 0.101 | |
| adjusted R2 | 0.970 | |
| maxDBP associated with respiratory support | ||
| β | p | |
| maxDBP | 0.172 | 0.010 |
| F score | 6.664 | |
| R2 | 0.030 | |
| adjusted R2 | 0.025 | |
| miniDBP associated with respiratory support | ||
| β | p | |
| miniDBP | −0.154 | 0.022 |
| F score | 5.310 | |
| R2 | 0.024 | |
| adjusted R2 | 0.019 | |
| DBP deference associated with respiratory support | ||
| β | p | |
| DBP deference | 0.254 | <0.001 |
| F score | 14.993 | |
| R2 | 0.064 | |
| adjusted R2 | 0.060 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magawa, S.; Nii, M.; Sakakura, Y.; Enomoto, N.; Takakura, S.; Maki, S.; Tanaka, H.; Kondo, E.; Ikeda, T. Appropriate Method of Administering Vasopressors for Maternal Hypotension Associated with Combined Spinal Epidural Anesthesia in Elective Cesarean Section: Impact on Postnatal Respiratory Support for Newborns. Medicina 2022, 58, 403. https://doi.org/10.3390/medicina58030403
Magawa S, Nii M, Sakakura Y, Enomoto N, Takakura S, Maki S, Tanaka H, Kondo E, Ikeda T. Appropriate Method of Administering Vasopressors for Maternal Hypotension Associated with Combined Spinal Epidural Anesthesia in Elective Cesarean Section: Impact on Postnatal Respiratory Support for Newborns. Medicina. 2022; 58(3):403. https://doi.org/10.3390/medicina58030403
Chicago/Turabian StyleMagawa, Shoichi, Masafumi Nii, Yosuke Sakakura, Naosuke Enomoto, Sho Takakura, Shintaro Maki, Hiroaki Tanaka, Eiji Kondo, and Tomoaki Ikeda. 2022. "Appropriate Method of Administering Vasopressors for Maternal Hypotension Associated with Combined Spinal Epidural Anesthesia in Elective Cesarean Section: Impact on Postnatal Respiratory Support for Newborns" Medicina 58, no. 3: 403. https://doi.org/10.3390/medicina58030403
APA StyleMagawa, S., Nii, M., Sakakura, Y., Enomoto, N., Takakura, S., Maki, S., Tanaka, H., Kondo, E., & Ikeda, T. (2022). Appropriate Method of Administering Vasopressors for Maternal Hypotension Associated with Combined Spinal Epidural Anesthesia in Elective Cesarean Section: Impact on Postnatal Respiratory Support for Newborns. Medicina, 58(3), 403. https://doi.org/10.3390/medicina58030403








