Do Tumour Size, Type and Localisation Affect Resection Rate in Patients with Spinal Schwannoma?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Management
2.3. Surgery
2.4. Endpoints
2.5. Statistics
3. Results
3.1. Cohort Characteristics
3.2. Surgical Approach and Complications
3.3. Predictors of GTR
3.4. Factors Influencing the Surgical Approach
3.5. Predictors of New Postoperative Deficits
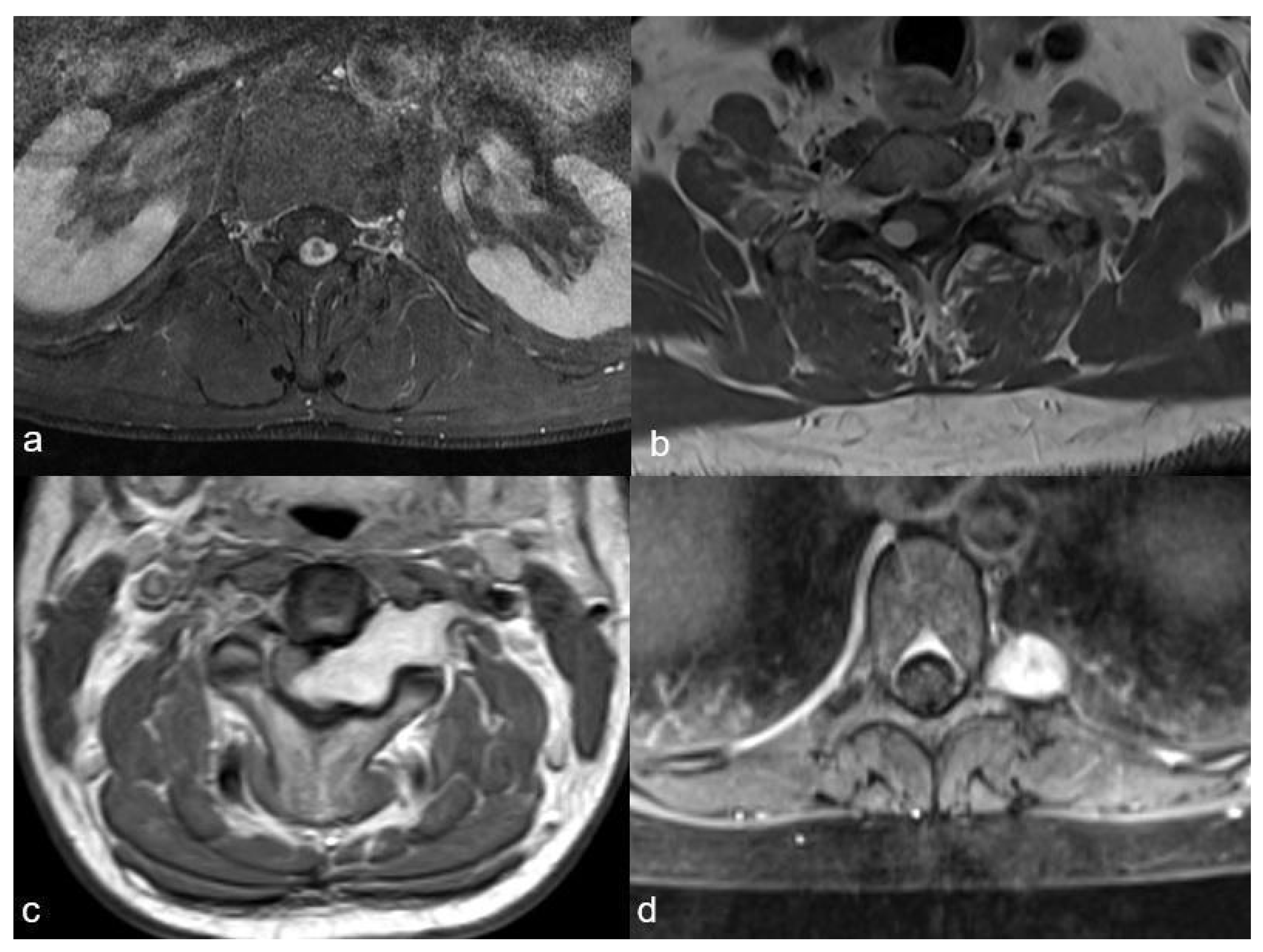
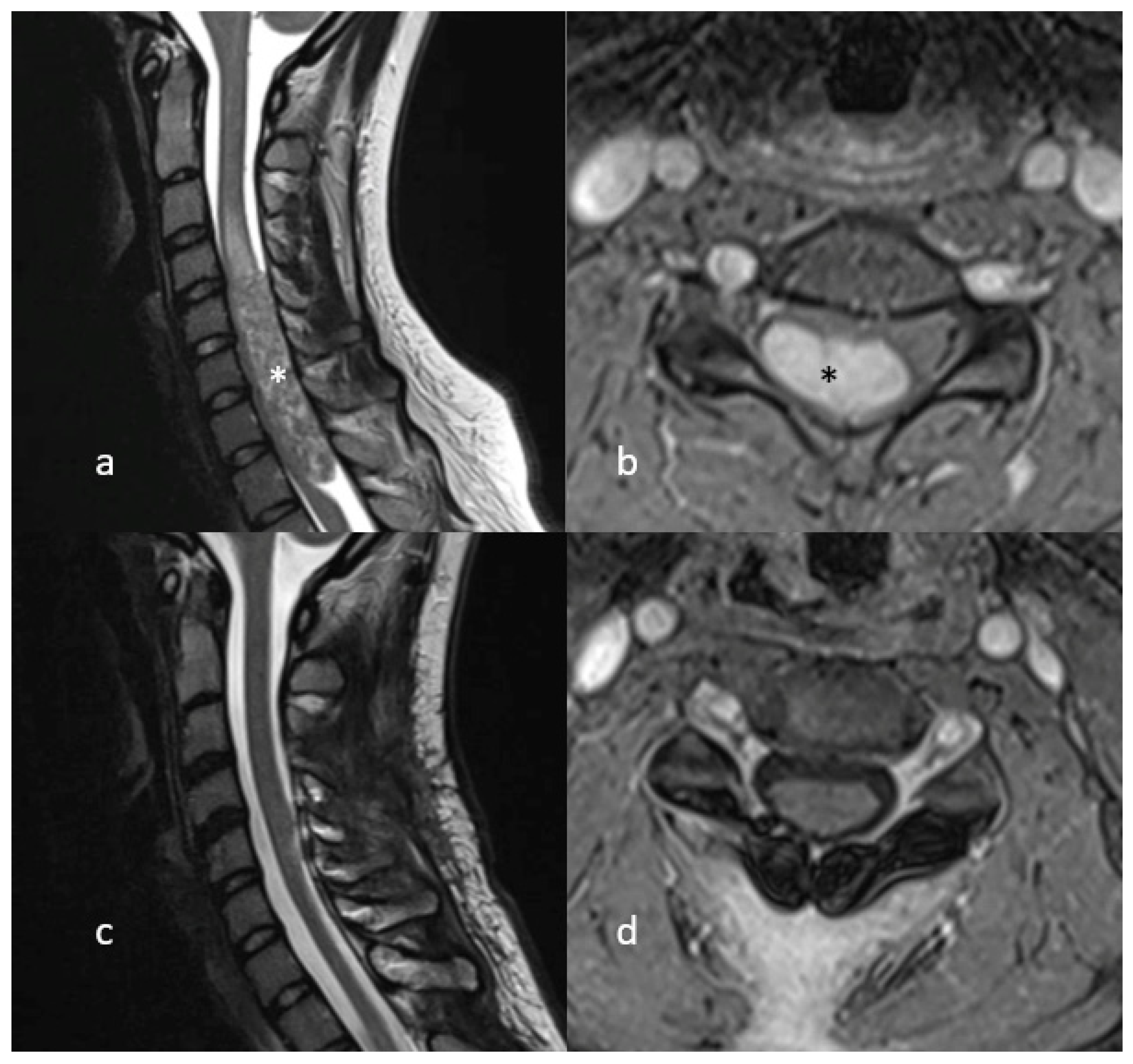
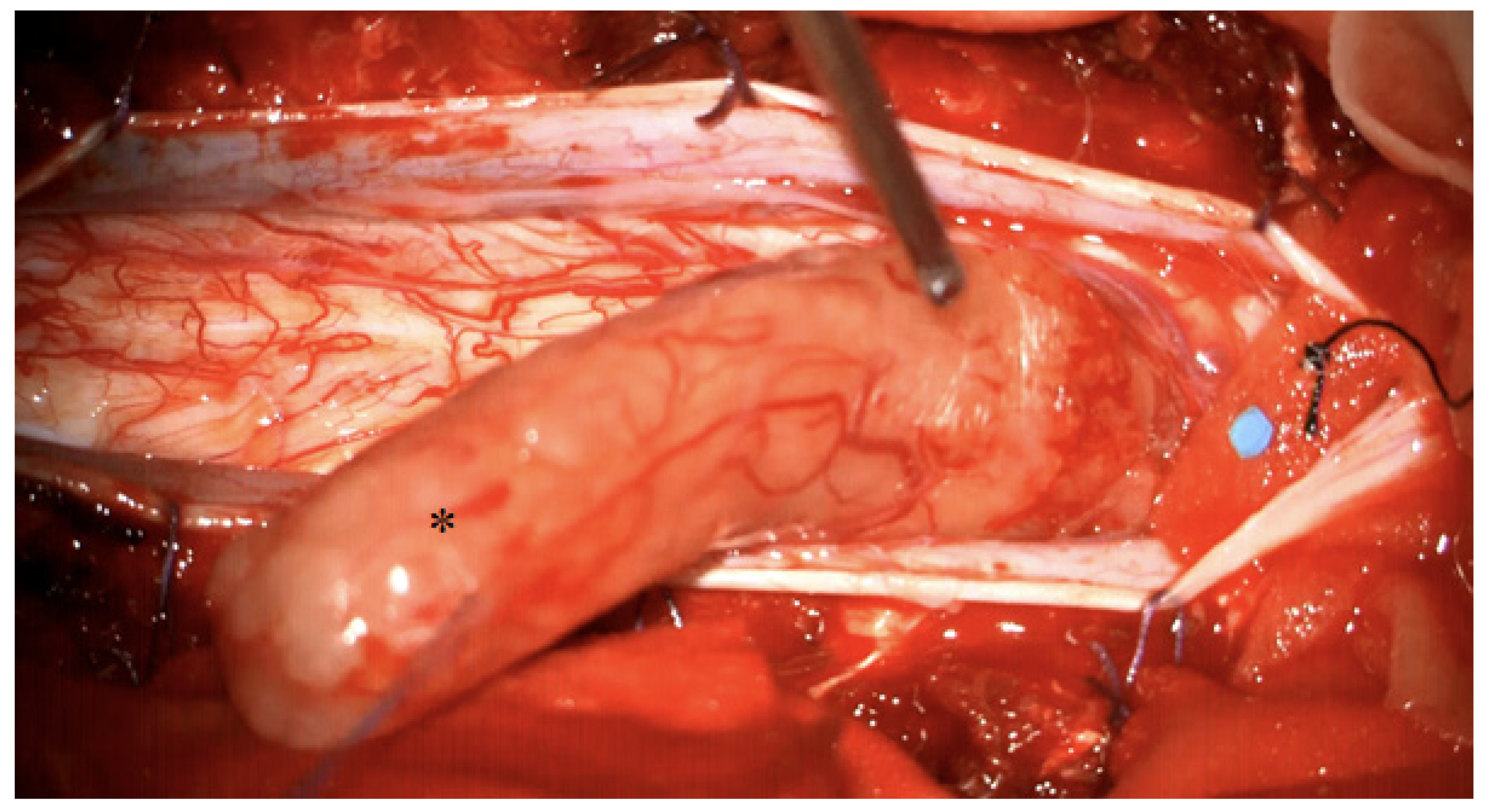
4. Illustrative Case
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Gross total resection | GTR |
| Magnetic resonance imaging | MRI |
| Neurofibromatosis type II | NF2 |
| Subtotal resection | STR |
| Intraoperative neuromonitoring | IONM |
| MEP | motor evoked potentials |
| SSEP | somatosensory evoked potentials |
References
- Tish, S.; Habboub, G.; Lang, M.; Ostrom, Q.T.; Kruchko, C.; Barnholtz-Sloan, J.S.; Recinos, P.F.; Kshettry, V.R. The epidemiology of spinal schwannoma in the United States between 2006 and 2014. J. Neurosurg. 2019, 32, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Imagama, S.; Ito, Z.; Kobayashi, K.; Yagi, H.; Hida, T.; Ito, K.; Tsushima, M.; Ishikawa, Y.; Ishiguro, N. How do spinal schwannomas progress? The natural progression of spinal schwannomas on MRI. J. Neurosurg. 2016, 24, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Seppälä, M.T.; Haltia, M.J.; Sankila, R.J.; Jääskeläinen, J.E.; Heiskanen, O. Long-term outcome after removal of spinal schwannoma: A clinicopathological study of 187 cases. J. Neurosurg. 1995, 83, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Antinheimo, J.; Sallinen, S.-L.; Sallinen, P.; Haapasalo, H.; Helin, H.; Horelli-Kuitunen, N.; Wessman, M.; Sainio, M.; Jääskeläinen, J.; Carpen, O. Genetic aberrations in sporadic and neurofibromatosis 2 (NF2)-associated schwannomas studied by comparative genomic hybridization (CGH). Acta Neurochir. 2000, 142, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Abbasi, S.; Senoglu, M.; Theodore, N.; Workman, R.K.; Gharabaghi, A.; Feiz-Erfan, I.; Spetzler, R.F.; Sonntag, V.K. Microsurgical management of spinal schwannomas: Evaluation of 128 cases. J. Neurosurg. 2008, 9, 40–47. [Google Scholar] [CrossRef]
- Moses, Z.B.; Barzilai, O.; O’Toole, J.E. Benign Intradural and Paraspinal nerve sheath tumors: Advanced surgical techniques. Neurosurg. Clin. 2020, 31, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Nater, A.; Zamorano, J.J.; Tetreault, L.A.; Varga, P.P.; Gokaslan, Z.L.; Boriani, S.; Fisher, C.G.; Rhines, L.; Bettegowda, C.J.S. Risk factors for recurrence of surgically treated conventional spinal schwannomas: Analysis of 169 patients from a multicenter international database. Spine 2016, 41, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowash, M.; Barzilai, O.; Kahn, S.; McLaughlin, L.; Boland, P.; Bilsky, M.H.; Laufer, I. Clinical outcomes following resection of giant spinal schwannomas: A case series of 32 patients. Spine 2017, 26, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Hohenberger, C.; Hinterleitner, J.; Schmidt, N.-O.; Doenitz, C.; Zeman, F.; Schebesch, K.-M. Neurological outcome after resection of spinal schwannoma. Neurosurgery 2020, 198, 106127. [Google Scholar] [CrossRef]
- Kahraman, S.; Gocmen, S.; Gokmen, M.H.A.; Acka, G.; Pusat, S. Intraoperative neurophysiologic monitoring for lumbar intradural schwannomas: Does it affect clinical outcome? World Neurosurg. 2019, 124, e789–e792. [Google Scholar] [CrossRef]
- Eden, K.J.B.J.O.S. The dumb-bell tumours of the spine. Br. J. Surg. 1941, 28, 549–570. [Google Scholar] [CrossRef]
- Asazuma, T.; Toyama, Y.; Maruiwa, H.; Fujimura, Y.; Hirabayashi, K.J.S. Surgical strategy for cervical dumbbell tumors based on a three-dimensional classification. Spine 2004, 29, E10–E14. [Google Scholar] [CrossRef]
- Sridhar, K.; Ramamurthi, R.; Vasudevan, M.; Ramamurthi, B. Giant invasive spinal schwannomas: Definition and surgical management. J. Neurosurg. Spine 2001, 94, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.; Pamir, M.N. Non-syndromic spinal schwannomas: A novel classification. Front. Neurol. 2017, 8, 318. [Google Scholar] [CrossRef] [Green Version]
- Halvorsen, C.M.; Rønning, P.; Hald, J.; Johannesen, T.B.; Kolstad, F.; Langmoen, I.A.; Lied, B.; Skaar Holme, S.; Helseth, E.J.N. The long-term outcome after resection of intraspinal nerve sheath tumors: Report of 131 consecutive cases. Neurosurgery 2015, 77, 585–593. [Google Scholar] [CrossRef]
- Guha, D.; Davidson, B.; Nadi, M.; Alotaibi, N.M.; Fehlings, M.G.; Gentili, F.; Valiante, T.A.; Tator, C.H.; Tymianski, M.; Guha, A.J.J.O.N. Management of peripheral nerve sheath tumors: 17 years of experience at Toronto Western Hospital. J. Neurosurg. 2018, 128, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Safaee, M.M.; Lyon, R.; Barbaro, N.M.; Chou, D.; Mummaneni, P.V.; Weinstein, P.R.; Chin, C.T.; Tihan, T.; Ames, C.P. Neurological outcomes and surgical complications in 221 spinal nerve sheath tumors. J. Neurosurg. Spine 2017, 26, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Li, J.; Miao, W.; Zhao, Y.; Jiao, J.; Wu, Z.; Yang, X.; Wei, H.; Xiao, J. Prognostic analysis of clinical and immunohistochemical factors for patients with spinal schwannoma. World Neurosurg. 2018, 120, e617–e627. [Google Scholar] [CrossRef]
- Turel, M.K.; D’Souza, W.P.; Rajshekhar, V. Hemilaminectomy approach for intradural extramedullary spinal tumors: An analysis of 164 patients. Neurosurg. Focus 2015, 39, E9. [Google Scholar] [CrossRef] [Green Version]
- Ponce, F.A.; Killory, B.D.; Wait, S.D.; Theodore, N.; Dickman, C.A. Endoscopic resection of intrathoracic tumors: Experience with and long-term results for 26 patients. J. Neurosurg. Spine 2011, 14, 377–381. [Google Scholar] [CrossRef]
- Wong, A.P.; Lall, R.R.; Dahdaleh, N.S.; Lawton, C.D.; Smith, Z.A.; Wong, R.H.; Harvey, M.J.; Lam, S.; Koski, T.R.; Fessler, R.G. Comparison of open and minimally invasive surgery for intradural-extramedullary spine tumors. Neurosurg. Focus 2015, 39, E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutter, M.; Deletis, V.; Dvorak, J.; Eggspuehler, A.; Grob, D.; MacDonald, D.; Mueller, A.; Sala, F.; Tamaki, T.J.E.S.J. Current opinions and recommendations on multimodal intraoperative monitoring during spine surgeries. Eur. Spine J. 2007, 16, 232–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, K.; Kobayashi, K.; Nakashima, H.; Machino, M.; Ito, S.; Kanbara, S.; Inoue, T.; Segi, N.; Koshimizu, H.; Imagama, S. Surgical outcomes and factors related to postoperative motor and sensory deficits in resection for 244 cases of spinal schwannoma. J. Clin. Neurosci. 2020, 81, 6–11. [Google Scholar] [CrossRef] [PubMed]
| n | % | ||
|---|---|---|---|
| Gender | Female | 18 | 36 |
| Age (years) a | 46 (±14) | ||
| Clinical presentation | Symptoms related to tumor: | 46 | 92 |
| Radiculopathy b | 44 | 88 | |
| Spinal deficit | 11 | 22 | |
| Unrelated symptom(s) leading to diagnosis | 4 | 8 | |
| Time between first symptoms and diagnosis (months) | 16 (35) | ||
| Tumour characteristics (MR imaging) | |||
| Tumour localisation | Cervical | 15 | 30 |
| Thoracal | 6 | 12 | |
| Lumbosacral | 29 | 58 | |
| Central | 9 | 18 | |
| Lateral: | 41 | 82 | |
| Right | 21 | 42 | |
| Left | 20 | 40 | |
| Volume of tumour (cm3) | Mean volume a | 9.7 (18.8) | |
| Size A (0–2) | 22 | 44 | |
| Size B (2–6) | 12 | 24 | |
| Size C (>6) | 16 | 32 | |
| Localisation type | I | 17 | 34 |
| II | 11 | 22 | |
| III | 18 | 36 | |
| IV | 4 | 8 | |
| Presence of myelopathy | yes | 6 | 12 |
| Primary Endpoints | |||||
|---|---|---|---|---|---|
| GTR | STR | p-Value | OR | 95% CI | |
| Tumour size | |||||
| A | 21 | 1 | 0.004 | 13.59 | 1.59–116.03 |
| B | 11 | 1 | 0.248 | 4.48 | 0.52–39.01 |
| C | 6 | 10 | <0.001 | 0.04 | 0.01–0.22 |
| Localisation type | |||||
| I | 17 | 0 | 0.004 | 1.81 | 1.36–2.41 |
| II | 11 | 0 | 0.046 | 1.41 | 1.15–1.72 |
| III | 7 | 11 | >0.001 | 0.21 | 0.02–0.19 |
| IV | 3 | 1 | >0.99 | 0.94 | 0.09–10.01 |
| Secondary endpoints | |||||
| Laminotomy | Hemilaminectomy | ||||
| Tumour size | |||||
| A | 16 | 6 | 0.919 | 1.07 | 0.31––3.71 |
| B | 9 | 3 | >0.99 | 1.22 | 0.28–5.38 |
| C | 11 | 5 | 0.746 | 0.79 | 0.22–2.92 |
| Localisation type | |||||
| I | 16 | 1 | 0.018 | 10.40 | 1.23–88.18 |
| II | 9 | 2 | 0.705 | 2.00 | 0.37–10.69 |
| III | 10 | 8 | 0.052 | 0.29 | 0.08–1.04 |
| IV | 1 | 3 | 0.061 | 0.11 | 0.01–1.11 |
| Myelopathy | 5 | 1 | 0.663 | 2.01 | 0.22–19.75 |
| Persisting deficits (n = 6) | No new deficits (n = 44) | ||||
| IONM | 2 | 20 | 0.683 | 0.60 | 0.10–3.62 |
| IONM alteration | 0 | 3 | >0.99 | 1.00 | 0.04–25.69 |
| Resection intralesional | 4 | 29 | >0.99 | 1.03 | 0.17–6.31 |
| Myelopathy | 0 | 6 | >0.99 | 0.52 | 0.02–9.10 |
| GTR | 4 | 34 | 0.621 | 0.588 | 0.09–3.67 |
| Tumour size | |||||
| A | 2 | 20 | 0.683 | 0.60 | 0.10–3.62 |
| B | 2 | 10 | 0.621 | 1.70 | 0.27–10.68 |
| C | 2 | 14 | >0.99 | 1.07 | 0.18–6.56 |
| Tumour localisation | |||||
| I | 2 | 9 | 0.601 | 1.94 | 0.31–12.35 |
| II | 3 | 15 | 0.654 | 1.93 | 0.35–10.77 |
| III | 0 | 4 | >0.99 | 0.69 | 0.03–14.43 |
| IV | 2 | 9 | 0.601 | 1.94 | 0.31–12.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parlak, A.; Oppong, M.D.; Jabbarli, R.; Gembruch, O.; Dammann, P.; Wrede, K.; Rauschenbach, L.; Sure, U.; Özkan, N. Do Tumour Size, Type and Localisation Affect Resection Rate in Patients with Spinal Schwannoma? Medicina 2022, 58, 357. https://doi.org/10.3390/medicina58030357
Parlak A, Oppong MD, Jabbarli R, Gembruch O, Dammann P, Wrede K, Rauschenbach L, Sure U, Özkan N. Do Tumour Size, Type and Localisation Affect Resection Rate in Patients with Spinal Schwannoma? Medicina. 2022; 58(3):357. https://doi.org/10.3390/medicina58030357
Chicago/Turabian StyleParlak, Ahmet, Marvin Darkwah Oppong, Ramazan Jabbarli, Oliver Gembruch, Philipp Dammann, Karsten Wrede, Laurèl Rauschenbach, Ulrich Sure, and Neriman Özkan. 2022. "Do Tumour Size, Type and Localisation Affect Resection Rate in Patients with Spinal Schwannoma?" Medicina 58, no. 3: 357. https://doi.org/10.3390/medicina58030357
APA StyleParlak, A., Oppong, M. D., Jabbarli, R., Gembruch, O., Dammann, P., Wrede, K., Rauschenbach, L., Sure, U., & Özkan, N. (2022). Do Tumour Size, Type and Localisation Affect Resection Rate in Patients with Spinal Schwannoma? Medicina, 58(3), 357. https://doi.org/10.3390/medicina58030357







