Acute Kidney Injury: Biomarker-Guided Diagnosis and Management
Abstract
:1. Introduction
2. Diagnosis of AKI
2.1. Definition and Diagnostic Criteria
2.1.1. Definition of AKI and Types of Biomarkers
2.1.2. Biomarkers for Diagnosis
2.2. Risk Stratification for AKI Assessment and Prevention
2.2.1. Causes and Risk Factors
2.2.2. Risk-Stratification Models
2.2.3. Biomarkers for AKI Risk Assessment, Prediction, and Prevention
3. Management of AKI
3.1. Conventional Management of AKI
3.1.1. Hemodynamic Management
3.1.2. Drug Stewardship and Use of Biomarkers
3.2. RRT after Failure of Conventional Management
3.2.1. Timing of RRT Initiation and Follow-Up after RRT
3.2.2. Biomarkers for Assessing AKI Progression and Reversal
4. Limitations of Novel Biomarkers and Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, F.B.; Bruetto, R.G.; Torres, U.S.; Otaviano, A.P.; Zanetta, D.M.; Burdmann, E.A. Incidence and mortality of acute kidney injury after myocardial infarction: A comparison between KDIGO and RIFLE criteria. PLoS ONE 2013, 8, e69998. [Google Scholar] [CrossRef]
- Gameiro, J.; Fonseca, J.A.; Outerelo, C.; Lopes, J.A. Acute Kidney Injury: From Diagnosis to Prevention and Treatment Strategies. J. Clin. Med. 2020, 9, 1704. [Google Scholar] [CrossRef]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.J.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef]
- Siew, E.D.; Ware, L.B.; Ikizler, T.A. Biological markers of acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 810–820. [Google Scholar] [CrossRef]
- Ostermann, M.; Karsten, E.; Lumlertgul, N. Biomarker-Based Management of AKI: Fact or Fantasy? Nephron 2021, 26, 1–7. [Google Scholar] [CrossRef]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers from the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef]
- Kane-Gill, S.L.; Meersch, M.; Bell, M. Biomarker-guided management of acute kidney injury. Curr. Opin. Crit. Care 2020, 26, 556–562. [Google Scholar] [CrossRef]
- Coca, S.G.; Yalavarthy, R.; Concato, J.; Parikh, C.R. Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int. 2008, 73, 1008–1016. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.; Tangri, N.; Komenda, P.; Kaushal, A.; Sood, M.; Brar, R.; Gill, K.; Walker, S.; MacDonald, K.; Hiebert, B.M.; et al. Urinary, Plasma, and Serum Biomarkers’ Utility for Predicting Acute Kidney Injury Associated with Cardiac Surgery in Adults: A Meta-analysis. Am. J. Kidney Dis. 2015, 66, 993–1005. [Google Scholar] [CrossRef] [Green Version]
- Charlton, J.R.; Portilla, D.; Okusa, M.D. A basic science view of acute kidney injury biomarkers. Nephrol. Dial. Transpl. 2014, 29, 1301–1311. [Google Scholar] [CrossRef] [Green Version]
- Ostermann, M.; McCullough, P.A.; Forni, L.G.; Bagshaw, S.M.; Joannidis, M.; Shi, J.; Kashani, K.; Honore, P.M.; Chawla, L.S.; Kellum, J.A.; et al. Kinetics of Urinary Cell Cycle Arrest Markers for Acute Kidney Injury Following Exposure to Potential Renal Insults. Crit. Care Med. 2018, 46, 375–383. [Google Scholar] [CrossRef]
- Hoste, E.; Bihorac, A.; Al-Khafaji, A.; Ortega, L.M.; Ostermann, M.; Haase, M.; Zacharowski, K.; Wunderink, R.; Heung, M.; Lissauer, M.; et al. Identification and validation of biomarkers of persistent acute kidney injury: The RUBY study. Intensive Care Med. 2020, 46, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, V.S.; Waikar, S.S.; Ferguson, M.A.; Collings, F.B.; Sunderland, K.; Gioules, C.; Bradwin, G.; Matsouaka, R.; Betensky, R.A.; Curhan, G.C.; et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin. Transl. Sci. 2008, 1, 200–208. [Google Scholar] [CrossRef]
- Moledina, D.G.; Isguven, S.; McArthur, E.; Thiessen-Philbrook, H.; Garg, A.X.; Shlipak, M.; Whitlock, R.; Kavsak, P.A.; Coca, S.G.; Parikh, C.R.; et al. Plasma Monocyte Chemotactic Protein-1 Is Associated with Acute Kidney Injury and Death After Cardiac Operations. Ann. Thorac. Surg. 2017, 104, 613–620. [Google Scholar] [CrossRef] [Green Version]
- American Society of Nephrology. American Society of Nephrology Renal Research Report. J. Am. Soc. Nephrol. 2005, 16, 1886–1903. [Google Scholar]
- Ostermann, M.; Bellomo, R.; Burdmann, E.A.; Doi, K.; Endre, Z.H.; Goldstein, S.L.; Kane-Gill, S.L.; Liu, K.D.; Prowle, J.R.; Shaw, A.D.; et al. Controversies in acute kidney injury: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2020, 98, 294–309. [Google Scholar] [CrossRef]
- Brown, J.R.; Kramer, R.S.; Coca, S.G.; Parikh, C.R. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann. Thorac. Surg. 2010, 90, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Eckardt, K.U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.; Du, L.; Wan, J.; Li, X. Serum Cystatin C Predicts AKI and the Prognosis of Patients in Coronary Care Unit: A Prospective, Observational Study. Kidney Blood Press. Res. 2017, 42, 961–973. [Google Scholar] [CrossRef] [Green Version]
- Ortega, L.M.; Heung, M. The use of cell cycle arrest biomarkers in the early detection of acute kidney injury. Is this the new renal troponin? Nefrologia 2018, 38, 361–367. [Google Scholar] [CrossRef]
- Hirooka, Y.; Nozaki, Y. Interleukin-18 in Inflammatory Kidney Disease. Front. Med. 2021, 8, 639103. [Google Scholar] [CrossRef]
- Geng, J.; Qiu, Y.; Qin, Z.; Su, B. The value of kidney injury molecule 1 in predicting acute kidney injury in adult patients: A systematic review and Bayesian meta-analysis. J. Transl. Med. 2021, 19, 105. [Google Scholar] [CrossRef]
- Yi, A.; Lee, C.H.; Yun, Y.M.; Kim, H.; Moon, H.W.; Hur, M. Effectiveness of Plasma and Urine Neutrophil Gelatinase-Associated Lipocalin for Predicting Acute Kidney Injury in High-Risk Patients. Ann. Lab. Med. 2021, 41, 60–67. [Google Scholar] [CrossRef]
- Yang, H.S.; Hur, M.; Lee, K.R.; Kim, H.; Kim, H.Y.; Kim, J.W.; Chua, M.T.; Kuan, W.S.; Chua, H.R.; Kitiyakara, C.; et al. Biomarker Rule-in or Rule-out in Patients with Acute Diseases for Validation of Acute Kidney Injury in the Emergency Department (BRAVA): A Multicenter Study Evaluating Urinary TIMP-2/IGFBP7. Ann. Lab. Med. 2022, 42, 178–187. [Google Scholar] [CrossRef]
- Legrand, M.; Hollinger, A.; Vieillard-Baron, A.; Depret, F.; Cariou, A.; Deye, N.; Fournier, M.C.; Jaber, S.; Damoisel, C.; Lu, Q.; et al. One-Year Prognosis of Kidney Injury at Discharge From the ICU: A Multicenter Observational Study. Crit. Care Med. 2019, 47, e953–e961. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [Green Version]
- Chawla, L.S.; Davison, D.L.; Brasha-Mitchell, E.; Koyner, J.L.; Arthur, J.M.; Shaw, A.D.; Tumlin, J.A.; Trevino, S.A.; Kimmel, P.L.; Seneff, M.G. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit. Care 2013, 17, R207. [Google Scholar] [CrossRef] [Green Version]
- Basu, R.K.; Kaddourah, A.; Goldstein, S.L.; Investigators, A.S. Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: A multicentre, multinational, prospective observational study. Lancet Child Adolesc. Health 2018, 2, 112–120. [Google Scholar] [CrossRef]
- Koyner, J.L.; Carey, K.A.; Edelson, D.P.; Churpek, M.M. The Development of a Machine Learning Inpatient Acute Kidney Injury Prediction Model. Crit. Care Med. 2018, 46, 1070–1077. [Google Scholar] [CrossRef]
- Song, X.; Liu, X.; Liu, F.; Wang, C. Comparison of machine learning and logistic regression models in predicting acute kidney injury: A systematic review and meta-analysis. Int. J. Med. Inform. 2021, 151, 104484. [Google Scholar] [CrossRef]
- Al-Jaghbeer, M.; Dealmeida, D.; Bilderback, A.; Ambrosino, R.; Kellum, J.A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. 2018, 29, 654–660. [Google Scholar] [CrossRef]
- Park, S.; Cho, H.; Park, S.; Lee, S.; Kim, K.; Yoon, H.J.; Park, J.; Choi, Y.; Lee, S.; Kim, J.H.; et al. Simple Postoperative AKI Risk (SPARK) Classification before Noncardiac Surgery: A Prediction Index Development Study with External Validation. J. Am. Soc. Nephrol. 2019, 30, 170–181. [Google Scholar] [CrossRef] [Green Version]
- McBride, W.T.; Kurth, M.J.; McLean, G.; Domanska, A.; Lamont, J.V.; Maguire, D.; Watt, J.; Fitzgerald, P.; Young, I.; Joseph, J.; et al. Stratifying risk of acute kidney injury in pre and post cardiac surgery patients using a novel biomarker-based algorithm and clinical risk score. Sci. Rep. 2019, 9, 16963. [Google Scholar] [CrossRef]
- Schunk, S.J.; Zarbock, A.; Meersch, M.; Kullmar, M.; Kellum, J.A.; Schmit, D.; Wagner, M.; Triem, S.; Wagenpfeil, S.; Grone, H.J.; et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: An observational cohort study. Lancet 2019, 394, 488–496. [Google Scholar] [CrossRef]
- Pickkers, P.; Ostermann, M.; Joannidis, M.; Zarbock, A.; Hoste, E.; Bellomo, R.; Prowle, J.; Darmon, M.; Bonventre, J.V.; Forni, L.; et al. The intensive care medicine agenda on acute kidney injury. Intensive Care Med. 2017, 43, 1198–1209. [Google Scholar] [CrossRef]
- Brienza, N.; Giglio, M.T.; Marucci, M.; Fiore, T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit. Care Med. 2009, 37, 2079–2090. [Google Scholar] [CrossRef]
- Hjortrup, P.B.; Haase, N.; Bundgaard, H.; Thomsen, S.L.; Winding, R.; Pettila, V.; Aaen, A.; Lodahl, D.; Berthelsen, R.E.; Christensen, H.; et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: The CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016, 42, 1695–1705. [Google Scholar] [CrossRef] [Green Version]
- Bellomo, R.; Kellum, J.A.; Wisniewski, S.R.; Pinsky, M.R. Effects of norepinephrine on the renal vasculature in normal and endotoxemic dogs. Am. J. Respir. Crit. Care Med. 1999, 159, 1186–1192. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Story, D.; Letis, A.; Klemz, K.; Matalanis, G.; Seevanayagam, S.; Dragun, D.; Seeliger, E.; Mertens, P.R.; et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol. Dial. Transpl. 2012, 27, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Gordon, A.C.; Russell, J.A.; Walley, K.R.; Singer, J.; Ayers, D.; Storms, M.M.; Holmes, C.L.; Hebert, P.C.; Cooper, D.J.; Mehta, S.; et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 2010, 36, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef]
- Perazella, M.A.; Markowitz, G.S. Drug-induced acute interstitial nephritis. Nat. Rev. Nephrol. 2010, 6, 461–470. [Google Scholar] [CrossRef]
- Perazella, M.A. Drug use and nephrotoxicity in the intensive care unit. Kidney Int. 2012, 81, 1172–1178. [Google Scholar] [CrossRef] [Green Version]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Barreto, E.F.; Rule, A.D.; Voils, S.A.; Kane-Gill, S.L. Innovative Use of Novel Biomarkers to Improve the Safety of Renally Eliminated and Nephrotoxic Medications. Pharmacotherapy 2018, 38, 794–803. [Google Scholar] [CrossRef]
- Kane-Gill, S.L.; Smithburger, P.L.; Kashani, K.; Kellum, J.A.; Frazee, E. Clinical Relevance and Predictive Value of Damage Biomarkers of Drug-Induced Kidney Injury. Drug Saf. 2017, 40, 1049–1074. [Google Scholar] [CrossRef]
- Udawatte, N.S.; Kang, S.W.; Wang, Y.; Arumugam, T.V.; Seneviratne, C.J. Predictive Nephrotoxicity Profiling of a Novel Antifungal Small Molecule in Comparison to Amphotericin B and Voriconazole. Front. Pharmacol. 2020, 11, 511. [Google Scholar] [CrossRef]
- Rocha, P.N.; Macedo, M.N.; Kobayashi, C.D.; Moreno, L.; Guimaraes, L.H.; Machado, P.R.; Badaro, R.; Carvalho, E.M.; Glesby, M.J. Role of urine neutrophil gelatinase-associated lipocalin in the early diagnosis of amphotericin B-induced acute kidney injury. Antimicrob. Agents Chemother. 2015, 59, 6913–6921. [Google Scholar] [CrossRef] [Green Version]
- Tajima, S.; Yamamoto, N.; Masuda, S. Clinical prospects of biomarkers for the early detection and/or prediction of organ injury associated with pharmacotherapy. Biochem. Pharmacol. 2019, 170, 113664. [Google Scholar] [CrossRef]
- Gaudry, S.; Hajage, D.; Martin-Lefevre, L.; Lebbah, S.; Louis, G.; Moschietto, S.; Titeca-Beauport, D.; Combe, B.; Pons, B.; de Prost, N.; et al. Comparison of two delayed strategies for renal replacement therapy initiation for severe acute kidney injury (AKIKI 2): A multicentre, open-label, randomised, controlled trial. Lancet 2021, 397, 1293–1300. [Google Scholar] [CrossRef]
- STARRT-AKI Investigators. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N. Engl. J. Med. 2020, 383, 240–251. [Google Scholar] [CrossRef]
- Barbar, S.D.; Clere-Jehl, R.; Bourredjem, A.; Hernu, R.; Montini, F.; Bruyere, R.; Lebert, C.; Bohe, J.; Badie, J.; Eraldi, J.P.; et al. Timing of Renal-Replacement Therapy in Patients with Acute Kidney Injury and Sepsis. N. Engl. J. Med. 2018, 379, 1431–1442. [Google Scholar] [CrossRef]
- Chen, J.J.; Chang, C.H.; Huang, Y.T.; Kuo, G. Furosemide stress test as a predictive marker of acute kidney injury progression or renal replacement therapy: A systemic review and meta-analysis. Crit. Care 2020, 24, 202. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Mao, Z.; Li, Q.; Zhou, F. Timing of renal replacement therapy initiation for acute kidney injury in critically ill patients: A systematic review of randomized clinical trials with meta-analysis and trial sequential analysis. Crit. Care 2021, 25, 15. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Wald, R. Strategies for the optimal timing to start renal replacement therapy in critically ill patients with acute kidney injury. Kidney Int. 2017, 91, 1022–1032. [Google Scholar] [CrossRef]
- Cerda, J.; Liu, K.D.; Cruz, D.N.; Jaber, B.L.; Koyner, J.L.; Heung, M.; Okusa, M.D.; Faubel, S. Promoting Kidney Function Recovery in Patients with AKI Requiring RRT. Clin. J. Am. Soc. Nephrol. 2015, 10, 1859–1867. [Google Scholar] [CrossRef]
- Fortrie, G.; de Geus, H.R.H.; Betjes, M.G.H. The aftermath of acute kidney injury: A narrative review of long-term mortality and renal function. Crit. Care 2019, 23, 24. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.; Khan, I.; Simpson, W.; Prescott, G.; Townend, J.; Smith, W.; Macleod, A. Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J. Am. Soc. Nephrol. 2007, 18, 1292–1298. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Feng, Z.; Xia, L.; Bai, J.; Xiao, F.; Liu, J.; Tang, L.; Chen, X. Risk factors of prognosis after acute kidney injury in hospitalized patients. Front. Med. 2017, 11, 393–402. [Google Scholar] [CrossRef]
- Hoste, E.A.; McCullough, P.A.; Kashani, K.; Chawla, L.S.; Joannidis, M.; Shaw, A.D.; Feldkamp, T.; Uettwiller-Geiger, D.L.; McCarthy, P.; Shi, J.; et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol. Dial. Transpl. 2014, 29, 2054–2061. [Google Scholar] [CrossRef]
- Albert, C.; Haase, M.; Albert, A.; Zapf, A.; Braun-Dullaeus, R.C.; Haase-Fielitz, A. Biomarker-Guided Risk Assessment for Acute Kidney Injury: Time for Clinical Implementation? Ann. Lab. Med. 2021, 41, 1–15. [Google Scholar] [CrossRef]
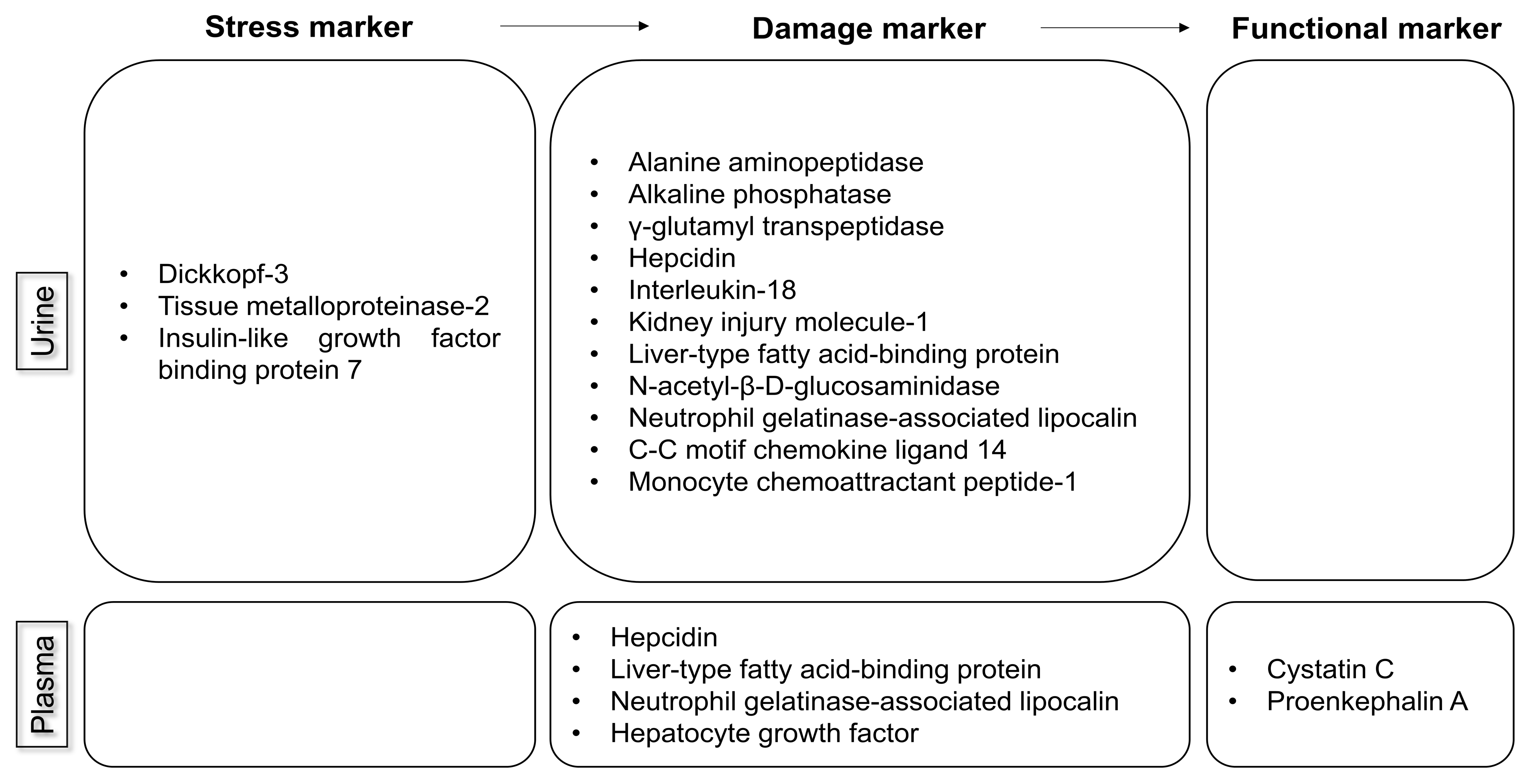
| AKI Biomarker | Biological Role (Source) | Type of Marker (Sample) | Time of Increase after Injury | Limitations (Studied Population) |
|---|---|---|---|---|
| Alanine aminopeptidase; alkaline phosphatase; γ-glutamyl transpeptidase | Located in proximal tubular cells; released into urine after tubular damage ([9]) | Damage (urine) | Elevated in UTI, cardiovascular disease, and stroke (patients in the ICU) | |
| Cystatin C | Produced by nucleated human cells; freely filtered ([8,9,10]) | Functional (plasma) | 12–24 h after injury | Confounded by age, sex, inflammatory state, diabetes, low albumin level, muscle mass, and use of high-dose steroids (patients undergoing cardiac surgery or liver transplantation; hospitalized patients) |
| Hepcidin | Predominantly produced in hepatocytes; freely filtered ([10]) | Damage (urine and plasma) | Decreased in anemia and increased in an inflammatory state (patients undergoing cardiac surgery; patients in the ICU) | |
| Tissue metalloproteinase-2; insulin-like growth factor binding protein-7 | Metalloproteinases released during cell-cycle arrest ([8,12,25]) | Stress (urine) | As early as 4 h but typically within 12 h | Elevated in diabetes (patients undergoing cardiac or noncardiac surgery; patients in the ICU; patients in the ED) |
| Interleukin-18 | Released into urine after tubular damage ([9,10]) | Damage (urine) | Elevated in an inflammatory state; lack of cutoff values (hospitalized patients; patients in the ICU or ED; patients undergoing cardiac surgery) | |
| Kidney injury molecule-1 | Produced by proximal tubular cells; released into urine after tubular damage ([8,9,10]) | Damage (urine) | 12–24 h after injury | Elevated in chronic proteinuria and inflammatory diseases (hospitalized patients; patients in the ED; patients undergoing cardiac surgery; patients in the ICU) |
| Liver-type fatty acid-binding protein | Freely filtered and reabsorbed in proximal tubules; released into urine after tubular cell damage ([10]) | Damage (urine and plasma) | Associated with anemia in patients without diabetes (patients undergoing cardiac surgery; patients in the ICU or ED) | |
| N-acetyl-β-D-glucosaminidase | Released into urine after tubular damage ([8,11]) | Damage (urine) | Within 2–4 h after injury | Elevated in diabetes and albuminuria (patients undergoing cardiac surgery; hospitalized patients) |
| Neutrophil gelatinase-associated lipocalin | At least three different types: (1) produced by neutrophils and epithelial tissues, including tubular cells; (2) produced by neutrophils; and (3) produced by tubular cells ([9,10,11]) | Damage (urine and plasma) | Elevated in sepsis, UTI, and CKD; lack of specific cutoff values (patients undergoing cardiac or noncardiac surgery; patients undergoing coronary angiography; patients in the ICU; post-transplantation patients; patients in the ED) | |
| Proenkephalin A | Freely filtered ([26]) | Functional (plasma) | (Patients in the ICU; patients undergoing cardiac surgery; hospitalized patients) |
| AKI Biomarker | Biological Site (Source) | Type of Marker (Sample) | Time of Increase after Injury | Limitations (Studied Population) |
|---|---|---|---|---|
| C-C motif chemokine ligand 14 | Released into urine after stress or damage to tubular cells ([8,13]) | Damage (urine) | To identify patients who will develop persistent AKI for >72 h | Variable performance in different AKI phenotypes (patients in the ICU) |
| Hepatocyte growth factor | Produced by mesenchymal cells and involved in tubular cell regeneration after AKI ([14]) | Damage (plasma) | Limited performance (hospitalized patients) | |
| Monocyte chemoattractant peptide-1 | Expressed in tubular epithelial cells, kidney mesangial cells, and podocytes ([15]) | Damage (urine) | (Patients undergoing cardiac surgery) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.-Y.; Kim, J.-S.; Jeong, K.-H.; Kim, S.-K. Acute Kidney Injury: Biomarker-Guided Diagnosis and Management. Medicina 2022, 58, 340. https://doi.org/10.3390/medicina58030340
Yoon S-Y, Kim J-S, Jeong K-H, Kim S-K. Acute Kidney Injury: Biomarker-Guided Diagnosis and Management. Medicina. 2022; 58(3):340. https://doi.org/10.3390/medicina58030340
Chicago/Turabian StyleYoon, Soo-Young, Jin-Sug Kim, Kyung-Hwan Jeong, and Su-Kang Kim. 2022. "Acute Kidney Injury: Biomarker-Guided Diagnosis and Management" Medicina 58, no. 3: 340. https://doi.org/10.3390/medicina58030340
APA StyleYoon, S.-Y., Kim, J.-S., Jeong, K.-H., & Kim, S.-K. (2022). Acute Kidney Injury: Biomarker-Guided Diagnosis and Management. Medicina, 58(3), 340. https://doi.org/10.3390/medicina58030340





