St. Thomas Modified Cardioplegia Effects on Myoblasts’ Viability and Morphology
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Culturing with Cold Cardioplegia
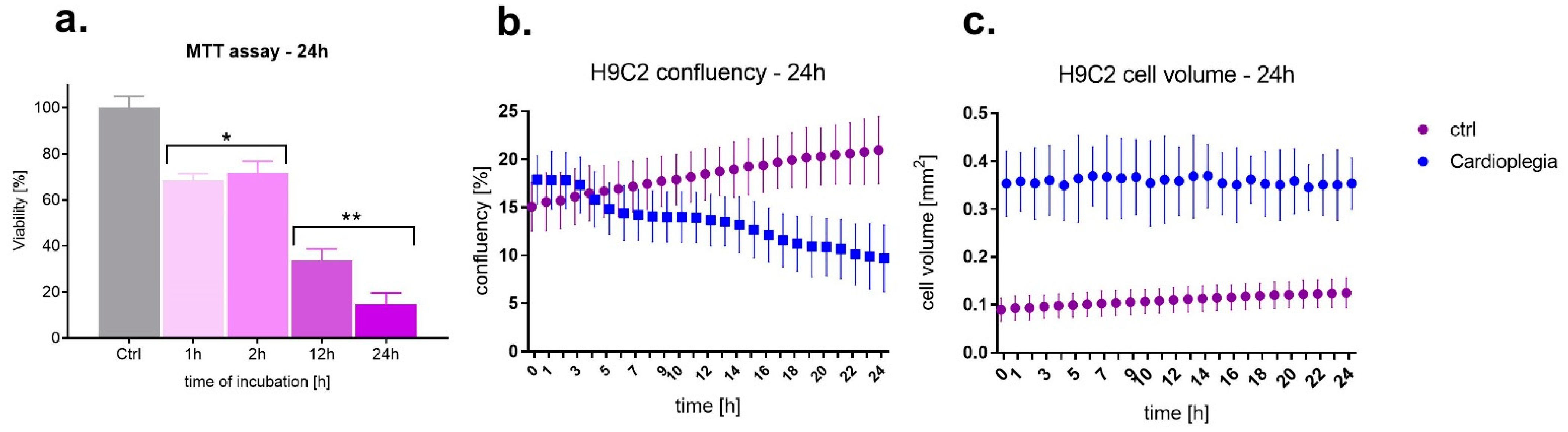
2.3. Cell Viability
2.4. Cells’ Confluency and Volume
2.5. Holographic Tomography Microscopy (HTM)
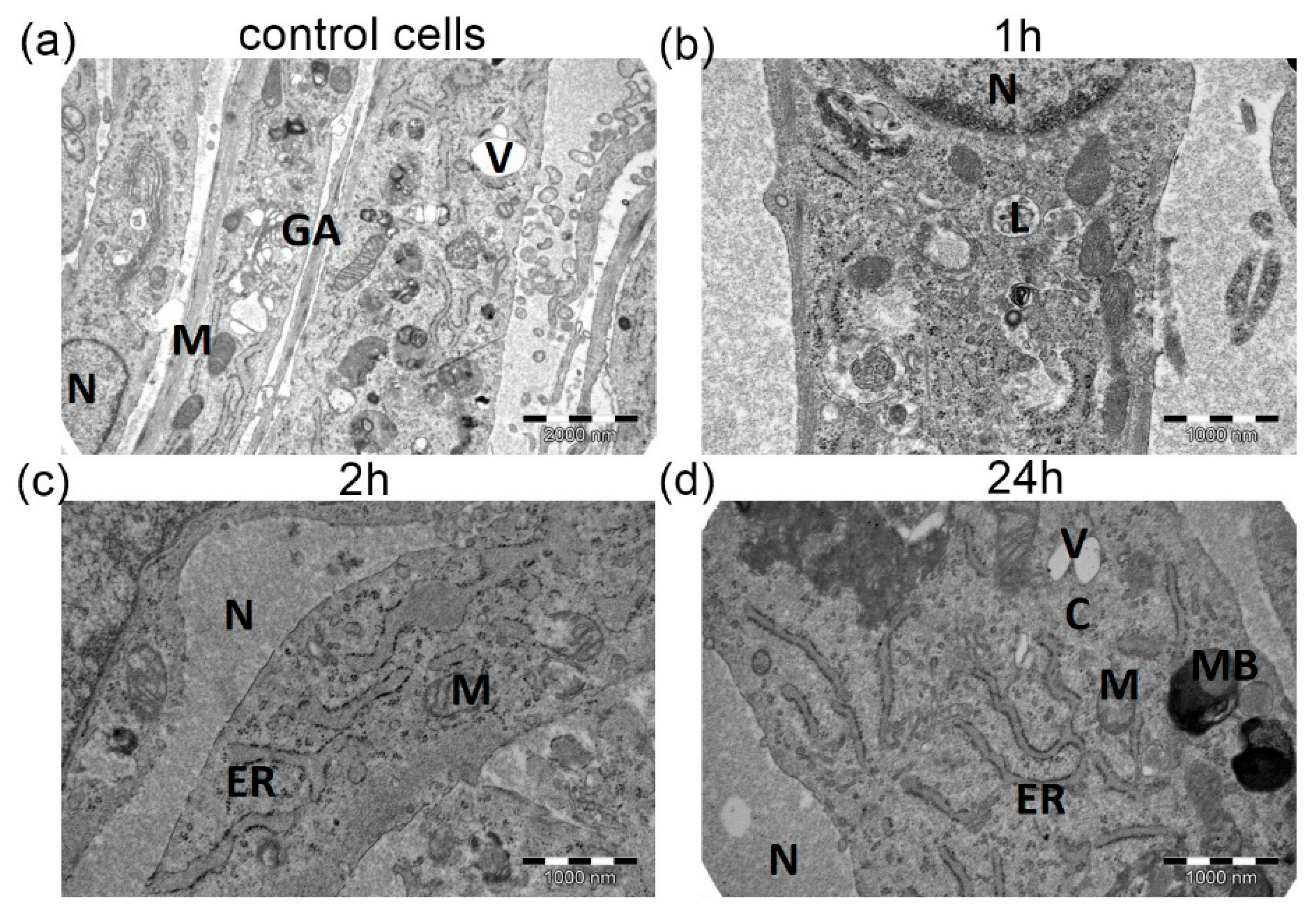
2.6. Transmission Electron Microscopy (TEM)
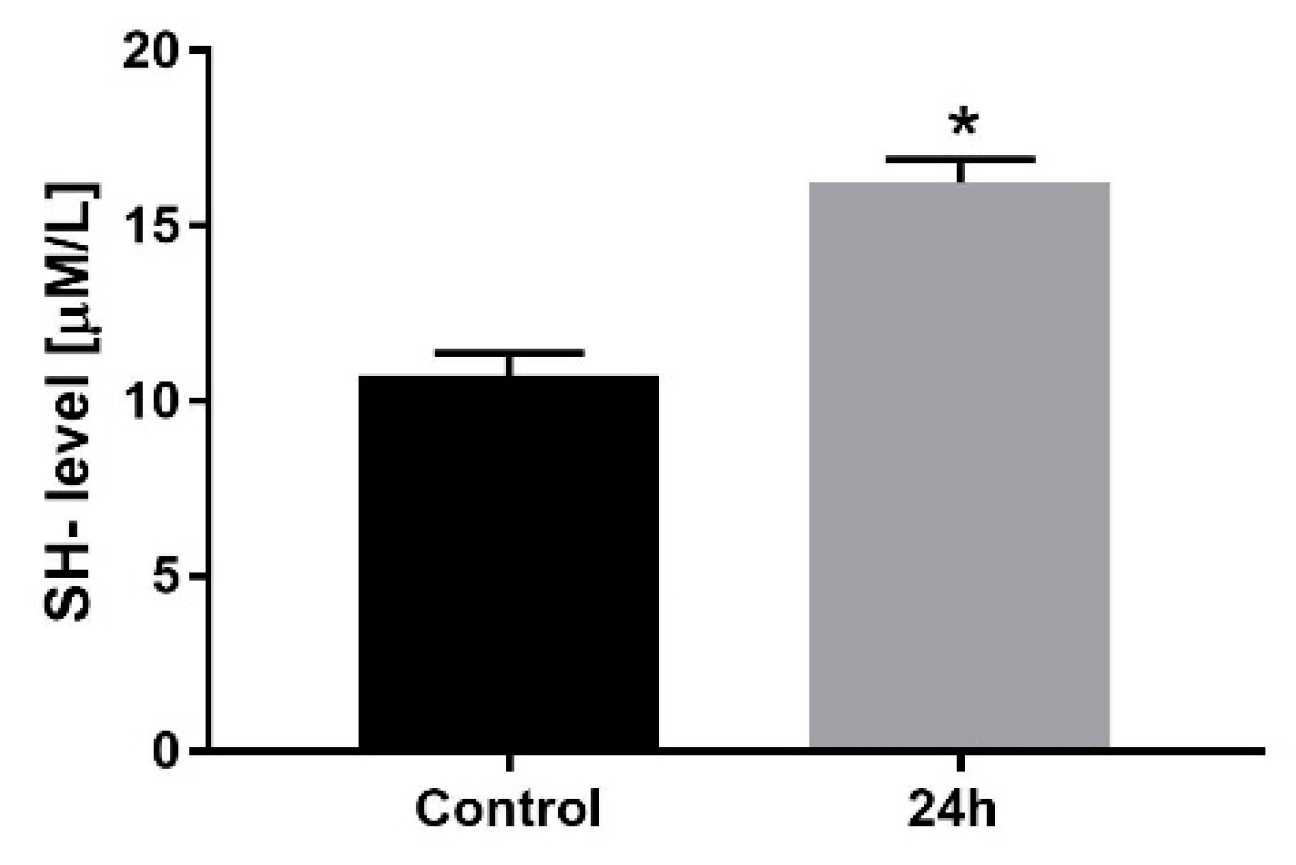
2.7. Thiol Groups Level
2.8. Immunocytochemistry
2.9. Statistical Analysis
3. Results
3.1. Cell Viability, Confluency and Volume
3.2. Cardioplegia Effects on Myocytes’ Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beyersdorf, F. The use of controlled reperfusion strategies in cardiac surgery to minimize ischaemia/reperfusion damage. Cardiovasc. Res. 2009, 83, 262–268. [Google Scholar] [CrossRef]
- Carvajal, C.; Goyal, A.; Tadi, P. Cardioplegia; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Yang, F.; Wang, J.; Zhai, B. The myocardial protective effect of monosodium phosphate cardioplegia in cardiopulmonary bypass in infants with an atrial septal defect. Medicine 2020, 99, e20934. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, L.M.; Noirhomme, P.H.; Van Dyck, M.J.; El Khoury, G.A.; Matta, A.J.; Goenen, M.J.; Dion, R.A. Randomized trial of intermittent antegrade warm blood versus cold crystalloid cardioplegia. Ann. Thorac. Surg. 1999, 67, 471–477. [Google Scholar] [CrossRef]
- Elwatidy, A.M.F.; Fadalah, M.A.; Bukhari, E.A.; Aljubair, K.A.; Syed, A.; Ashmeg, A.K.; Alfagih, M.R. Antegrade crystalloid cardioplegia vs antegrade/retrograde cold and tepid blood cardioplegia in CABG. Ann. Thorac. Surg. 1999, 68, 447–453. [Google Scholar] [CrossRef]
- Francica, A.; Tonelli, F.; Rossetti, C.; Tropea, I.; Luciani, G.B.; Faggian, G.; Dobson, G.P.; Onorati, F. Cardioplegia between Evolution and Revolution: From Depolarized to Polarized Cardiac Arrest in Adult Cardiac Surgery. J. Clin. Med. 2021, 10, 4485. [Google Scholar] [CrossRef] [PubMed]
- Günday, M.; Bingöl, H. Is crystalloid cardioplegia a strong predictor of intra-operative hemodilution? J. Cardiothorac. Surg. 2014, 9, 23. [Google Scholar] [CrossRef]
- Sanetra, K.; Pawlak, I.; Cisowski, M. Del Nido cardioplegia-What is the current evidence? Kardiochir. I Torakochir. Pol. 2018, 15, 114–118. [Google Scholar] [CrossRef]
- Nowicki, R.; Saczko, J.; Kulbacka, J.; Choromanska, A.; Dumanska, M.; Krajewska, B.; Daczewska, M.; Dumanski, A.; Kustrzycki, W. The estimation of oxidative stress markers and apoptosis in right atrium auricles cardiomyocytes of patients undergoing surgical heart revascularisation with the use of warm blood cardioplegia. Folia Histochem. Cytobiol. 2010, 48, 202–207. [Google Scholar] [CrossRef][Green Version]
- Marczak, J.; Nowicki, R.; Kulbacka, J.; Saczko, J. Is remote ischaemic preconditioning of benefit to patients undergoing cardiac surgery? Interact. Cardiovasc. Thorac. Surg. 2012, 14, 634–639. [Google Scholar] [CrossRef]
- Sá, M.P.B.O.; Rueda, F.G.; Ferraz, P.E.; Chalegre, S.T.; Vasconcelos, F.P.; Lima, R.C. Is there any difference between blood and crystalloid cardioplegia for myocardial protection during cardiac surgery? A meta-analysis of 5576 patients from 36 randomized trials. Perfusion 2012, 27, 535–546. [Google Scholar] [CrossRef]
- Chen, C.L.; Zheng, H.; Guo, H. Comparison of the cardioprotection between crystalloid and blood cardioplegia in adult patients undergoing cardiac surgery: A meta-analysis. Zhonghua Wai Ke Za Zhi 2013, 51, 71–76. [Google Scholar]
- Mishra, P.; Jadhav, R.B.; Mohapatra, C.K.R.; Khandekar, J.; Raut, C.; Ammannaya, G.K.; Seth, H.S.; Singh, J.; Shah, V. Comparison of del Nido cardioplegia and St. Thomas Hospital solution–two types of cardioplegia in adult cardiac surgery. Kardiochir. I Torakochir. Pol. = Pol. J. Cardio-Thorac. Surg. 2016, 13, 295. [Google Scholar] [CrossRef]
- Arbustini, E.; Diegqli, M.; Grasso, M.; Fasani, R.; D’Armini, A.; Martinelli, L.; Goggi, C.; Campana, C.; Gavazzi, A.; Vigano’, M. Expression of proliferating cell markers in normal and diseased human hearts. Am. J. Cardiol. 1993, 72, 608–614. [Google Scholar] [CrossRef]
- Gunaydin, S.; Akbay, E.; Gunertem, O.; McCusker, K.; Onur, M.; Ozisik, K. Long-Term Protective Effects of Single-Dose Cardioplegic Solutions in Cell Culture Models. J. Extra. Corpor. Technol. 2020, 52, 279–288. [Google Scholar] [PubMed]
- Stephenson, E.; Jayawant, A.; Baumgarten, C.; Damiano, R. Cardioplegia-induced cell swelling: Prevention by normothermic infusion. Ann. Thorac. Surg. 2000, 69, 1393–1398. [Google Scholar] [CrossRef]
- Sun, X.; Ducko, C.; Hoenicke, E.; Reigle, K.; Damiano, R. Mechanisms responsible for cell volume regulation during hyperkalemic cardioplegic arrest. Ann. Thorac. Surg. 2000, 70, 633–638. [Google Scholar] [CrossRef]
- Pepper, J.R.; Mumby, S.; Gutteridge, J.M.C. Blood Cardioplegia Increases Plasma Iron Overload and Thiol Levels During Cardiopulmonary Bypass. Ann. Thorac. Surg. 1995, 60, 1735–1740. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P. Mitochondrial Thioredoxin Reductase and Thiol Status. Methods Enzym. 2002, 347, 307–316. [Google Scholar]
- Nardi, P.; Pisano, C.; Bertoldo, F.; Vacirca, S.R.; Saitto, G.; Costantino, A.; Bovio, E.; Pellegrino, A.; Ruvolo, G. Warm blood cardioplegia versus cold crystalloid cardioplegia for myocardial protection during coronary artery bypass grafting surgery. Cell Death Discov. 2018, 23, 1–6. [Google Scholar] [CrossRef]
- Ovrum, E.; Tangen, G.; Tølløfsrud, S.; Øystese, R.; Ringdal, M.A.L.; Istad, R. Cold blood versus cold crystalloid cardioplegia: A prospective randomised study of 345 aortic valve patients. Eur. J. Cardio-Thorac. Surg. 2010, 38, 745–749. [Google Scholar] [CrossRef]
- Ji, M.J.; Hong, J.H. A Cardioplegic Solution with an Understanding of a Cardiochannelopathy. Antioxidants 2021, 10, 1878. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; He, G.W. Effect of Cardioplegic and Organ Preservation Solutions and Their Components on Coronary Endothelium-Derived Relaxing Factors. Ann. Thorac. Surger. 2005, 80, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Fedosova, M.; Kimose, H.H.; Greisen, J.R.; Fast, P.; Gissel, M.S.; Jakobsen, C.J. Blood cardioplegia benefits only patients with a long cross-clamp time. Perfusion 2018, 34, 42–49. [Google Scholar] [CrossRef] [PubMed]

| Intensity of ABC Reaction | [%] of Stained Cells | ||
|---|---|---|---|
| Control | - | - | |
| Incubation time | 1 h | - | - |
| 2 h | -/+ | 5 ± 2 | |
| 24 h | ++/+++ | 90 ± 9 * | |
| Intensity of ABC Reaction | [%] of Stained Cells | ||
|---|---|---|---|
| Control | ++ | 99 ± 4 | |
| Incubation time | 1 h | ++ | 98 ± 3 |
| 2 h | ++ | 87 ± 6 | |
| 24 h | ++/+++ | 95 ± 5 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicki, R.; Bieżuńska-Kusiak, K.; Kulbacka, J.; Choromanska, A.; Daczewska, M.; Potoczek, S.; Rachwalik, M.; Saczko, J. St. Thomas Modified Cardioplegia Effects on Myoblasts’ Viability and Morphology. Medicina 2022, 58, 280. https://doi.org/10.3390/medicina58020280
Nowicki R, Bieżuńska-Kusiak K, Kulbacka J, Choromanska A, Daczewska M, Potoczek S, Rachwalik M, Saczko J. St. Thomas Modified Cardioplegia Effects on Myoblasts’ Viability and Morphology. Medicina. 2022; 58(2):280. https://doi.org/10.3390/medicina58020280
Chicago/Turabian StyleNowicki, Rafał, Katarzyna Bieżuńska-Kusiak, Julita Kulbacka, Anna Choromanska, Małgorzata Daczewska, Stanisław Potoczek, Maciej Rachwalik, and Jolanta Saczko. 2022. "St. Thomas Modified Cardioplegia Effects on Myoblasts’ Viability and Morphology" Medicina 58, no. 2: 280. https://doi.org/10.3390/medicina58020280
APA StyleNowicki, R., Bieżuńska-Kusiak, K., Kulbacka, J., Choromanska, A., Daczewska, M., Potoczek, S., Rachwalik, M., & Saczko, J. (2022). St. Thomas Modified Cardioplegia Effects on Myoblasts’ Viability and Morphology. Medicina, 58(2), 280. https://doi.org/10.3390/medicina58020280








