A Case of Stevens–Johnson Syndrome Complicated with Multimatrix System Mesalamine in Ulcerative Colitis
Abstract
:1. Introduction
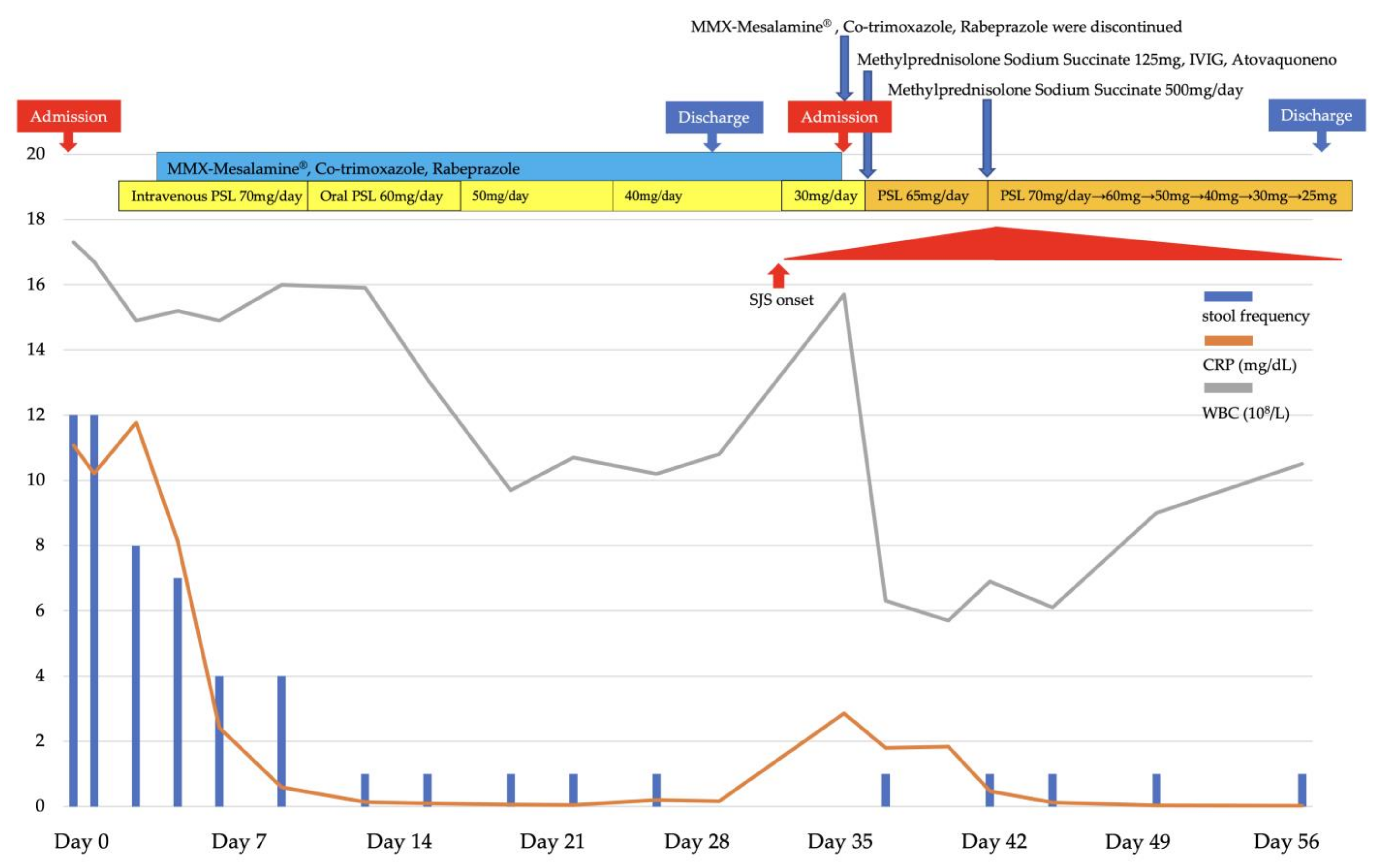
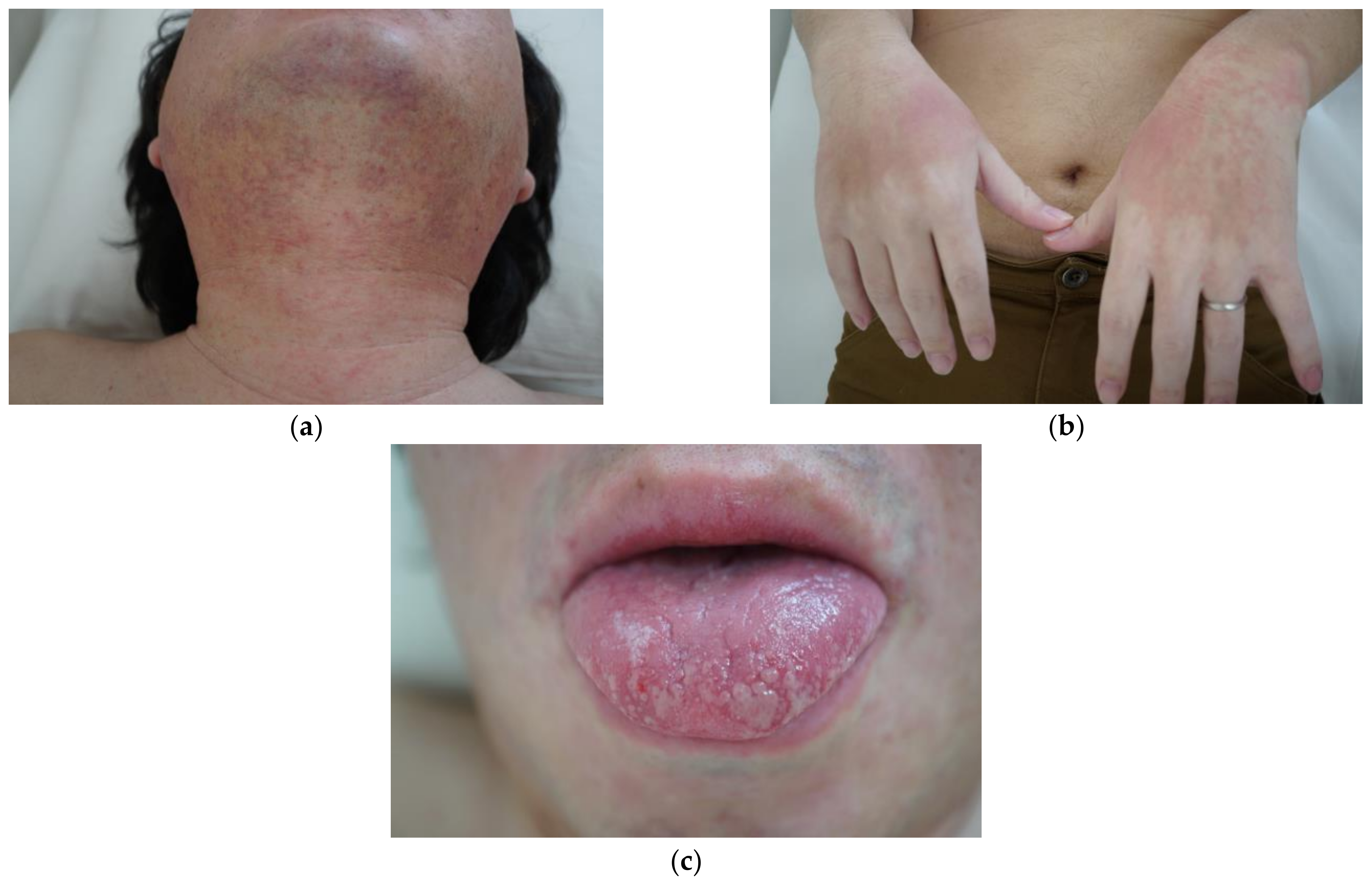
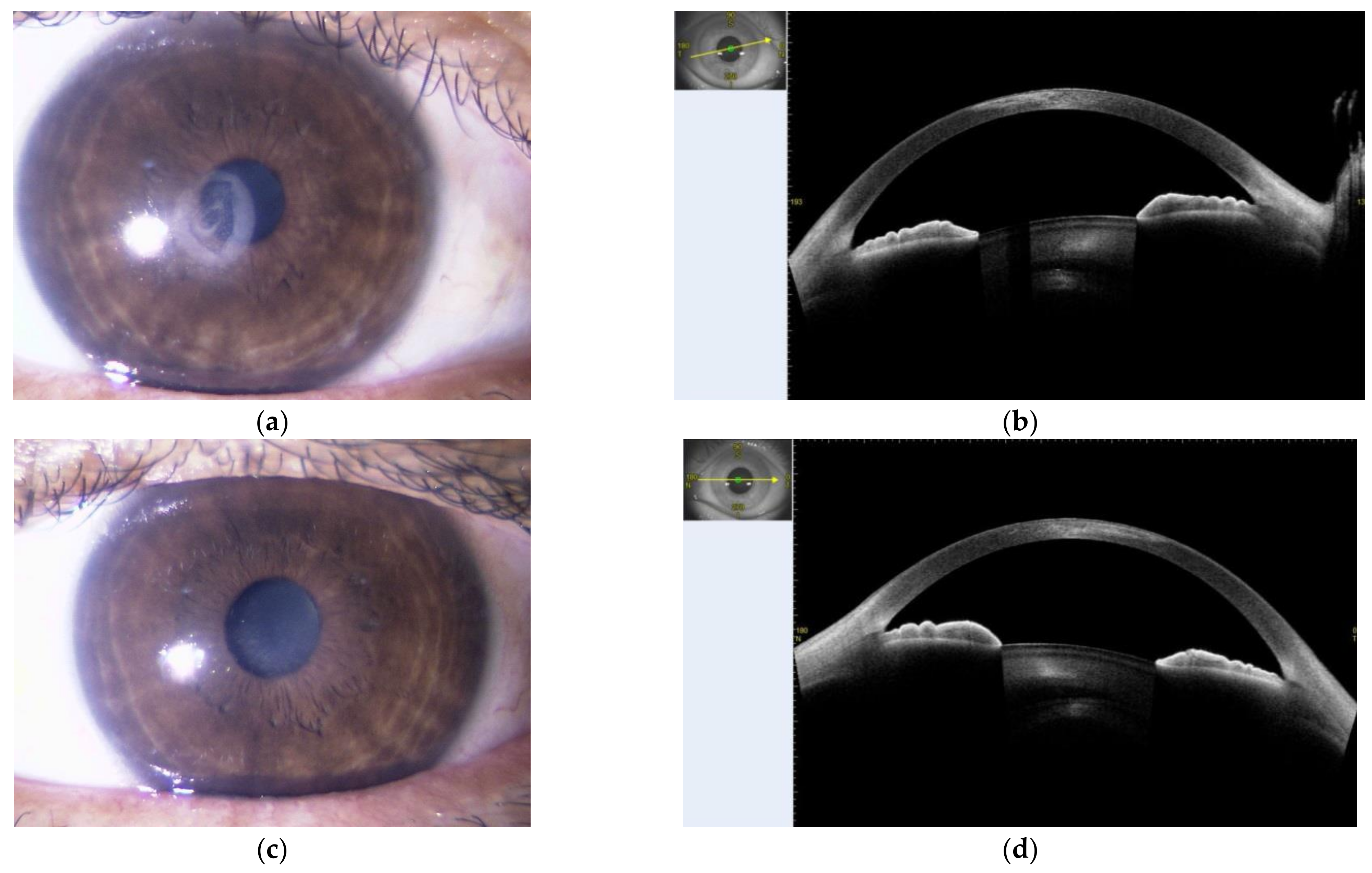
2. Presentation of Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lichtiger, S.; Present, D.H. Preliminary report: Cyclosporin in treatment of severe active ulcerative colitis. Lancet 1990, 336, 16–19. [Google Scholar] [CrossRef]
- Roujeau, J.C.; Stern, R.S. Severe adverse cutaneous reactions to drugs. N. Engl. J. Med. 1994, 331, 1272–1285. [Google Scholar] [CrossRef]
- Assier, H.; Bastuji-Garin, S.; Revuz, J.; Roujeau, J.C. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. Arch. Dermatol. 1995, 131, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Creamer, D.; Walsh, S.A.; Dziewulski, P.; Exton, L.S.; Lee, H.Y.; Dart, J.K.; Setterfield, J.; Bunker, C.B.; Ardern-Jones, M.R.; Watson, K.M.; et al. U.K. guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br. J. Dermatol. 2016, 174, 1194–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrándiz-Pulido, C.; García-Fernández, D.; Gómez-Morell, P.; Palao, R.; García-Patos, V. Síndrome de Stevens-Johnson y necrólisis epidérmica tóxica: Revisión de la experiencia clínica en un Hospital Universitario (1989–2008) [Stevens-Johnson syndrome and toxic epidermal necrolysis: A review of the clinical experience of a University Hospital (1989–2008)]. Med. Clin. 2011, 136, 583–587. [Google Scholar] [CrossRef]
- Revuz, J.; Penso, D.; Roujeau, J.C.; Guillaume, J.C.; Payne, C.R.; Wechsler, J.; Touraine, R. Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch. Dermatol. 1987, 123, 1160–1165. [Google Scholar] [CrossRef]
- Power, W.J.; Ghoraishi, M.; Merayo-Lloves, J.; Neves, R.A.; Foster, C.S. Analysis of the acute ophthalmic manifestations of the erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis disease spectrum. Ophthalmology 1995, 102, 1669–1676. [Google Scholar] [CrossRef]
- Sotozono, C.; Ang, L.P.; Koizumi, N.; Higashihara, H.; Ueta, M.; Inatomi, T.; Yokoi, N.; Kaido, M.; Dogru, M.; Shimazaki, J.; et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology 2007, 114, 1294–1302. [Google Scholar] [CrossRef]
- Roujeau, J.C.; Huynh, T.N.; Bracq, C.; Guillaume, J.C.; Revuz, J.; Touraine, R. Genetic susceptibility to toxic epidermal necrolysis. Arch. Dermatol. 1987, 123, 1171–1173. [Google Scholar] [CrossRef]
- Ueta, M.; Kaniwa, N.; Sotozono, C.; Tokunaga, K.; Saito, Y.; Sawai, H.; Miyadera, H.; Sugiyama, E.; Maekawa, K.; Nakamura, R.; et al. Independent strong association of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related Stevens-Johnson syndrome with severe mucosal involvement. Sci. Rep. 2014, 4, 4862. [Google Scholar] [CrossRef] [Green Version]
- Ueta, M.; Kannabiran, C.; Wakamatsu, T.H.; Kim, M.K.; Yoon, K.C.; Seo, K.Y.; Joo, C.K.; Sangwan, V.; Rathi, V.; Basu, S.; et al. Trans-ethnic study confirmed independent associations of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related Stevens-Johnson syndrome with severe ocular surface complications. Sci. Rep. 2014, 4, 5981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, D.; Hayashida, M.; Sato, T.; Minowa, S.; Ikezaki, O.; Mitsui, T.; Miura, M.; Sakuraba, A.; Hisamatsu, T. Evaluation of the drug-induced lymphocyte stimulation test for diagnosing mesalazine allergy. Intest. Res. 2018, 16, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Maddocks, J.L.; Slater, D.N. Toxic epidermal necrolysis, agranulocytosis and erythroid hypoplasia associated with sulphasalazine. J. R. Soc. Med. 1980, 73, 587–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolia, V. Sulfasalazine desensitization in children and adolescents with chronic inflammatory bowel disease. Am. J. Gastroenterol. 1992, 87, 1029–1032. [Google Scholar]
- Iemoli, E.; Piconi, S.; Ardizzone, S.; Bianchi, P.G.; Raimond, F. Erythroderma and toxic epidermal necrolysis caused by to 5-aminosalacylic acid. Inflamm. Bowel Dis. 2006, 12, 1007–1008. [Google Scholar] [CrossRef]
- Fukunaga, K.; Ohda, Y.; Inoue, T.; Kono, T.; Miwa, H.; Matsumoto, T. Toxic epidermal necrosis associated with mesalamine in a patient with ulcerative colitis. Inflamm. Bowel Dis. 2007, 13, 1055–1056. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, L.; de Chambrun, G.P.; De Vroey, B.; Lavogiez, C.; Delaporte, E.; Colombel, J.F.; Cortot, A. Stevens-Johnson syndrome with sulfasalazine treatment: Report of two cases. J. Crohns Colitis 2011, 5, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Zizi, N.; Elmrahi, A.; Dikhaye, S.; Fihmi, N.; Alami, Z. Stevens Johnson syndrome-Toxic Epidermal Necrolysis Overlap induced by sulfasalazine treatment: A case report. Tunis. Med. 2015, 93, 413–415. [Google Scholar]
- Núñez, O.A.; Trigo, S.C.; de la Cruz, R.M.D.; Herrera, J.J.M.; Leo, C.E. Topical mesalazine as a cause of Stevens-Johnson syndrome. Rev. Esp. Enferm. Dig. 2018, 110, 736–738. [Google Scholar] [CrossRef]
- Xiong, H.; Chen, S.; Luo, X. Salazosulphapyridine-related Stevens-Johnson Syndrome Caused by Sulphapyridine and Confirmed by Enzyme-linked Immunospot Assay. J. Crohns Colitis 2018, 12, 381–382. [Google Scholar] [CrossRef]
- Viola, A.; Caltagirone, A.M.; Campisi, G.; Guarneri, G.; Cappello, M. Stevens-Johnson syndrome on treatment with sulfasalazine for Crohn’s disease: Need for a multidisciplinary approach. Turk. J. Gastroenterol. 2019, 30, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, S.; Pisani, L.F.; Tontini, G.E.; Vecchi, M.; Pastorelli, L. MMX® technology and its applications in gastrointestinal diseases. Therap. Adv. Gastroenterol. 2017, 10, 545–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenstein, G.R. Budesonide Multi-matrix for the Treatment of Patients with Ulcerative Colitis. Dig. Dis. Sci. 2016, 61, 358–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherlock, M.E.; MacDonald, J.K.; Griffiths, A.M.; Steinhart, A.H.; Seow, C.H. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2015, 26, CD007698. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Travis, S.; Moro, L.; Jones, R.; Gautille, T.; Bagin, R.; Huang, M.; Yeung, P.; Ballard, E.D., 2nd. Once-daily budesonide MMX® extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: Results from the CORE I study. Gastroenterology 2012, 143, 1218–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, S.P.; Danese, S.; Kupcinskas, L.; Alexeeva, O.; D’Haens, G.; Gibson, P.R.; Moro, L.; Jones, R.; Ballard, E.D.; Masure, J.; et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: Results from the randomised CORE II study. Gut 2014, 63, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, L.; Saibeni, S.; Spina, L.; Signorelli, C.; Celasco, G.; de Franchis, R.; Vecchi, M. Oral, colonic-release low-molecular-weight heparin: An initial open study of Parnaparin-MMX for the treatment of mild-to-moderate left-sided ulcerative colitis. Aliment. Pharmacol. Ther. 2008, 28, 581–588. [Google Scholar] [CrossRef]
- DuPont, H.L.; Petersen, A.; Zhao, J.; Mundt, A.; Jiang, Z.D.; Miller, S.; Flores, J.; Shringarpure, R.; Moro, L.; Bagin, R.G.; et al. Targeting of rifamycin SV to the colon for treatment of travelers’ diarrhea: A randomized, double-blind, placebo-controlled phase 3 study. J. Travel. Med. 2014, 21, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Chung, W.H.; Hung, S.I. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J. Dermatol. Sci. 2012, 66, 190–196. [Google Scholar] [CrossRef]
- Nickoloff, B.J. Saving the skin from drug-induced detachment. Nat. Med. 2008, 14, 1311–1313. [Google Scholar] [CrossRef]
- Grijalva, C.G.; Chen, L.; Delzell, E.; Baddley, J.W.; Beukelman, T.; Winthrop, K.L.; Griffin, M.R.; Herrinton, L.J.; Liu, L.; Ouellet-Hellstrom, R.; et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA 2011, 306, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.T.; Yu, D.T.; Targoff, C.; Bluestone, R. Effect of corticosteroids on the human immune response. Suppression of mitogen-induced lymphocyte proliferation by “pulse” methylprednisolone. Transplantation 1978, 26, 266–267. [Google Scholar] [PubMed]

| Normal Range | Normal Range | ||||
|---|---|---|---|---|---|
| AST | 31 U/L | 13–30 | WBC | 1.57 × 103/μL | 3.30–8.60 |
| ALT | 65 U/L | 10–42 | RBC | 3.91 × 106/μL | 4.35–5.55 |
| ALP | 254 U/L | 106–322 | Hb | 11.2 g/dL | 13.7–16.8 |
| LD | 155 U/L | 124–222 | Plt | 385 × 109/L | 158–348 |
| γGTP | 130 U/L | 13–64 | ESR (1 hr) | 40 mm | 2–10 |
| T-Bil | 0.4 mg/dL | 0.4–1.5 | ferritin | 38.4 ng/mL | 21.8–274.6 |
| UN | 16 mg/dL | 8–20 | IgG | 752 mg/dL | 870–1700 |
| Cre | 0.59 mg/dL | 0.65–1.07 | IgE | 12.6 IU/mL | <170 |
| Alb | 3.0 mg/dL | 4.1–5.1 | HSV-IgG | 35.6 (+) | <2.0 |
| Na | 139 mmol/L | 138–145 | CMVAg | negative | |
| K | 4.4 mmol/L | 3.6–4.8 | Mycoplasma | negative | |
| Cl | 104 mmol/L | 101–108 | ASO | negative | |
| CRP | 2.85 mg/dL | <0.14 | TARC | 215 pg/mL | <450 |
| No. | Author | Published | Age | Sex | Disease | Diagnosis | Causative Drug | Time to Onset of SJS | Nikolsky’s Sign | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Maddocks JL | 1980 | 39 | M | UC | TEN | SASP | 60 days | - | intravenous methylprednisolone | death |
| 2 | Tolia V | 1992 | 17 | M | UC | SJS | SASP | - | - | - | improved |
| 3 | Tolia V | 1992 | 13 | F | UC | SJS | SASP | - | - | - | improved |
| 4 | Tolia V | 1992 | 16 | M | UC | SJS | SASP | - | - | - | improved |
| 5 | Lemoli E | 2006 | - | M | UC | TEN | mesalamine | - | - | steroids, antimycotics, antibiotics | improved |
| 6 | Fukunaga K | 2007 | 17 | M | UC | TEN | mesalamine | 19–34 days | positive | intravenous methylprednisolone | improved |
| 7 | Tremblay L | 2011 | 36 | F | UC | SJS | SASP | 11 days | positive | symptomatic treatment | improved |
| 8 | Tremblay L | 2011 | 19 | F | UC | SJS | SASP | 21 days | - | symptomatic treatment | improved |
| 9 | Zizi N | 2015 | 33 | F | - | SJS/ TEN | SASP | 15 days | positive | symptomatic treatment | improved |
| 10 | Núñez Ortiz A | 2018 | 46 | F | UC | SJS | mesalamine | 14 days | negative | intravenous corticosteroids | improved |
| 11 | Xiong H | 2018 | 61 | F | UC | SJS | SASP | 12 days | - | - | - |
| 12 | Viola A. | 2019 | 32 | F | CD | SJS | SASP | within 30 days | - | intravenous steroid, antihistamine | improved |
| Present case | 41 | M | UC | SJS | MMX mesalamine | 28 days | positive | intravenous steroid, IVIG | improved |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanazawa, M.; Tominaga, K.; Kanamori, A.; Tanaka, T.; Masuyama, S.; Watanabe, S.; Abe, K.; Yamamiya, A.; Goda, K.; Irisawa, A. A Case of Stevens–Johnson Syndrome Complicated with Multimatrix System Mesalamine in Ulcerative Colitis. Medicina 2022, 58, 276. https://doi.org/10.3390/medicina58020276
Kanazawa M, Tominaga K, Kanamori A, Tanaka T, Masuyama S, Watanabe S, Abe K, Yamamiya A, Goda K, Irisawa A. A Case of Stevens–Johnson Syndrome Complicated with Multimatrix System Mesalamine in Ulcerative Colitis. Medicina. 2022; 58(2):276. https://doi.org/10.3390/medicina58020276
Chicago/Turabian StyleKanazawa, Mimari, Keiichi Tominaga, Akira Kanamori, Takanao Tanaka, Satoshi Masuyama, Shoko Watanabe, Keiichiro Abe, Akira Yamamiya, Kenichi Goda, and Atsushi Irisawa. 2022. "A Case of Stevens–Johnson Syndrome Complicated with Multimatrix System Mesalamine in Ulcerative Colitis" Medicina 58, no. 2: 276. https://doi.org/10.3390/medicina58020276
APA StyleKanazawa, M., Tominaga, K., Kanamori, A., Tanaka, T., Masuyama, S., Watanabe, S., Abe, K., Yamamiya, A., Goda, K., & Irisawa, A. (2022). A Case of Stevens–Johnson Syndrome Complicated with Multimatrix System Mesalamine in Ulcerative Colitis. Medicina, 58(2), 276. https://doi.org/10.3390/medicina58020276







