Flow Cytometry and Molecular Techniques Could Complement Morphological Detection of Leukemic Infiltration in Ascitic Fluids: A Case Report
Abstract
:1. Introduction
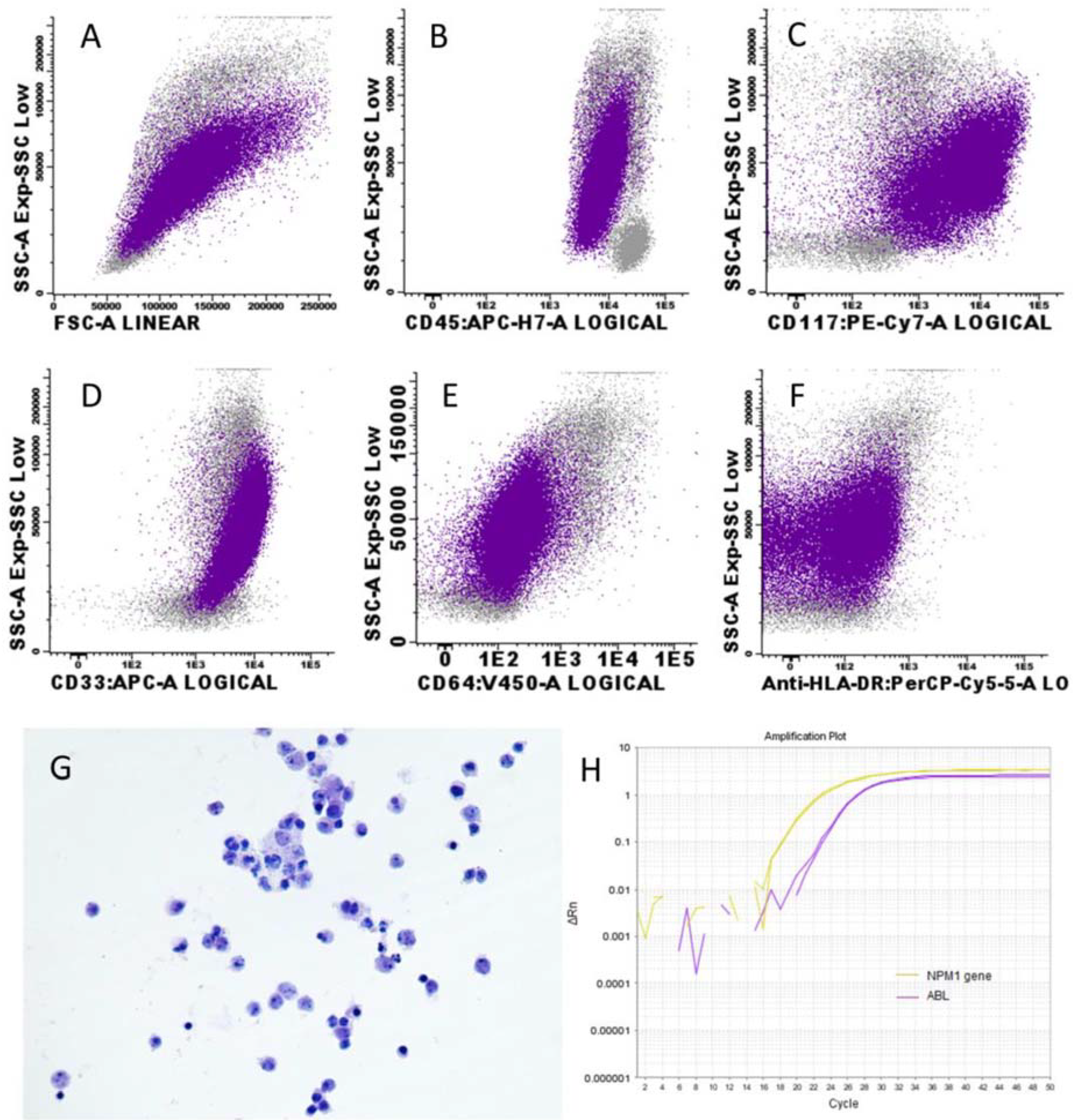
2. Case Report
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- De Kouchkovsky, I.; Abdul-Hay, M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef] [PubMed]
- Liesveld, J.L.; Lichtman, M.A. Acute Myelogenous Leukemia. In Williams Hematology; Lichtman, M.A., Kipps, T.J., Seligsohn, U., Kaushansky, K., Prchal, J.T., Eds.; The McGraw-Hill Companies: New York, NY, USA, 2016; p. 1373. [Google Scholar]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlap, J.B.; Leonard, J.; Rosenberg, M.; Cook, R.; Press, R.; Fan, G.; Raess, P.W.; Druker, B.J.; Traer, E. The combination of NPM1, DNMT3A, and IDH1/2 mutations leads to inferior overall survival in AML. Am. J. Hematol. 2019, 94, 913–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, E.F.; Kuo, F.C.; Hasserjian, R.P.; Seegmiller, A.C.; Pozdnyakova, O. A distinct immunophenotype identifies a subset of NPM1-mutated AML with TET2 or IDH1/2 mutations and improved outcome. Am. J. Hematol. 2018, 93, 504–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, S.Y.; Park, S.H.; Kim, I.S.; Yi, J.; Kim, H.H.; Chang, C.L.; Lee, E.Y.; Cho, Y.U.; Jang, S.; Park, C.J.; et al. Correlation of NPM1 Type A Mutation Burden With Clinical Status and Outcomes in Acute Myeloid Leukemia Patients With Mutated NPM1 Type A. Ann. Lab. Med. 2016, 36, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Jayson, G.C. The current and future management of malignant ascites. Clin. Oncol. 2003, 15, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Campidelli, C.; Agostinelli, C.; Stitson, R.; Pileri, S.A. Myeloid sarcoma: Extramedullary manifestation of myeloid disorders. Am. J. Clin. Pathol. 2009, 132, 426–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prolla, J.C.; Kirsner, J.B. The Gastrointestinal Lesions and Complications of the Leukemias. Ann. Intern. Med. 1964, 61, 1084–1103. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, C.G. Cytology of ascitic fluid in a patient with granulocytic sarcoma (extramedullary myeloid tumor). A case report. Acta Cytol. 1999, 43, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Y.; Hussein, K.K.; Walter, M.G.; Hasan, M.K.; Kern, W.; Kharfan-Dabaja, M.A. Granulocytic sarcoma presenting with malignant anasarca in a patient with secondary acute myeloid leukemia. Int. J. Hematol. 2004, 79, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L.; Steingart, R.; Miller, K.B.; Kruskal, J.B.; Pihan, G. Leukemic ascites. Arch. Pathol. Lab. Med. 2005, 129, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Simel, D.L.; Weinberg, J.B. Leukemic ascites complicating acute myelomonoblastic leukemia. Arch. Pathol. Lab. Med. 1985, 109, 365–367. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Alfonzo, I.; Láinez-González, D.; Solán-Blanco, L.; Franganillo-Suarez, A.; Cornejo, J.I.; Garcia-Lopez, A.; Martín-Herrero, S.; Castaño-Bonilla, T.; Salgado-Sánchez, R.; Arquero-Portero, T.; et al. Flow Cytometry and Molecular Techniques Could Complement Morphological Detection of Leukemic Infiltration in Ascitic Fluids: A Case Report. Medicina 2022, 58, 264. https://doi.org/10.3390/medicina58020264
Martínez-Alfonzo I, Láinez-González D, Solán-Blanco L, Franganillo-Suarez A, Cornejo JI, Garcia-Lopez A, Martín-Herrero S, Castaño-Bonilla T, Salgado-Sánchez R, Arquero-Portero T, et al. Flow Cytometry and Molecular Techniques Could Complement Morphological Detection of Leukemic Infiltration in Ascitic Fluids: A Case Report. Medicina. 2022; 58(2):264. https://doi.org/10.3390/medicina58020264
Chicago/Turabian StyleMartínez-Alfonzo, Inés, Daniel Láinez-González, Laura Solán-Blanco, Aida Franganillo-Suarez, José I. Cornejo, Amanda Garcia-Lopez, Sara Martín-Herrero, Tamara Castaño-Bonilla, Rocío Salgado-Sánchez, Teresa Arquero-Portero, and et al. 2022. "Flow Cytometry and Molecular Techniques Could Complement Morphological Detection of Leukemic Infiltration in Ascitic Fluids: A Case Report" Medicina 58, no. 2: 264. https://doi.org/10.3390/medicina58020264
APA StyleMartínez-Alfonzo, I., Láinez-González, D., Solán-Blanco, L., Franganillo-Suarez, A., Cornejo, J. I., Garcia-Lopez, A., Martín-Herrero, S., Castaño-Bonilla, T., Salgado-Sánchez, R., Arquero-Portero, T., Cortti-Ferrari, M. J., Llamas-Sillero, P., & Alonso-Dominguez, J. M. (2022). Flow Cytometry and Molecular Techniques Could Complement Morphological Detection of Leukemic Infiltration in Ascitic Fluids: A Case Report. Medicina, 58(2), 264. https://doi.org/10.3390/medicina58020264






