Impact of Hospitalization in an Endocrinology Department on Vaccination Coverage in People Living with Diabetes: A Real-Life Study
Abstract
1. Introduction
2. Materials and Methods
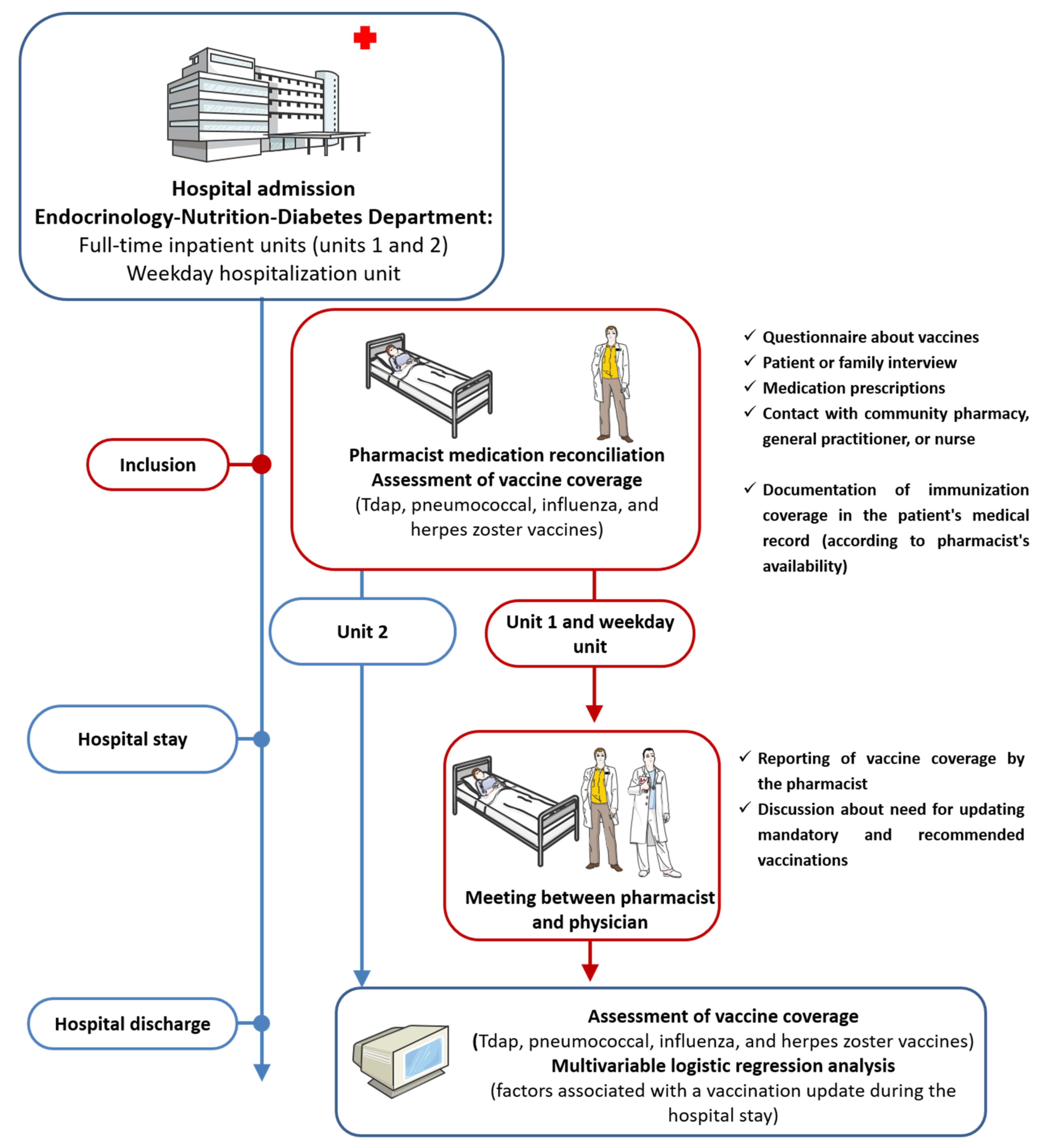
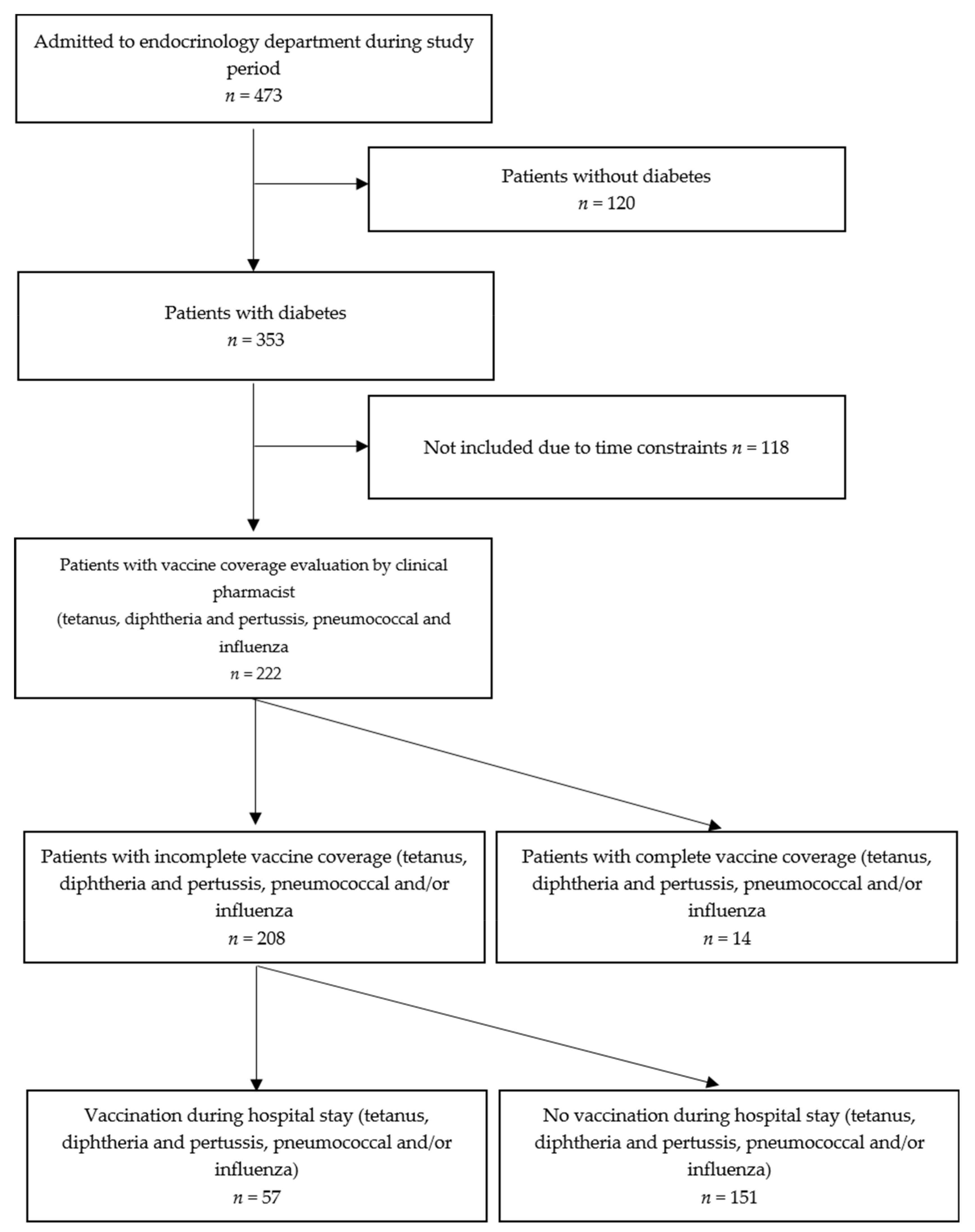
2.1. Study Design and Participants
2.2. Interventions
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Vaccination during Hospitalization
3.3. Knowledge and Feeling about Vaccines
3.4. Factors Associated with Vaccination Coverage at Hospital Admission and Vaccination during Hospitalization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carey, I.M.; Critchley, J.A.; DeWilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of Infection in Type 1 and Type 2 Diabetes Compared With the General Population: A Matched Cohort Study. Diabetes Care 2018, 41, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Harding, J.L.; Cohen, K.; Huxley, R.R.; Davis, W.A.; Shaw, J.E. Excess Risk of Dying From Infectious Causes in Those With Type 1 and Type 2 Diabetes. Diabetes Care 2015, 38, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.M.; Edelsberg, J.; Weycker, D.; Farkouh, R.A.; Strutton, D.R.; Pelton, S.I. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect. Dis. 2014, 1, ofu024. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S34–S45. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Tetanus surveillance—United States, 2001–2008. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 365–369. [Google Scholar]
- Lu, P.J.; O’Halloran, A.; Ding, H.; Srivastav, A.; Williams, W.W. Uptake of Influenza Vaccination and Missed Opportunities Among Adults with High-Risk Conditions, United States, 2013. Am. J. Med. 2016, 129, 636.e1–636.e11. [Google Scholar] [CrossRef]
- Taha, M.K.; Weil-Olivier, C.; Bouee, S.; Emery, C.; Nachbaur, G.; Pribil, C.; Loncle-Provot, V. Risk factors for invasive meningococcal disease: A retrospective analysis of the French national public health insurance database. Hum. Vaccin. Immunother. 2021, 17, 1858–1866. [Google Scholar] [CrossRef]
- Colquhoun, A.J.; Nicholson, K.G.; Botha, J.L.; Raymond, N.T. Effectiveness of influenza vaccine in reducing hospital admissions in people with diabetes. Epidemiol. Infect. 1997, 119, 335–341. [Google Scholar] [CrossRef]
- Looijmans-Van den Akker, I.; Verheij, T.J.; Buskens, E.; Nichol, K.L.; Rutten, G.E.; Hak, E. Clinical effectiveness of first and repeat influenza vaccination in adult and elderly diabetic patients. Diabetes Care 2006, 29, 1771–1776. [Google Scholar] [CrossRef][Green Version]
- Rondy, M.; Larrauri, A.; Casado, I.; Alfonsi, V.; Pitigoi, D.; Launay, O.; Syrjanen, R.K.; Gefenaite, G.; Machado, A.; Vucina, V.V.; et al. 2015/16 seasonal vaccine effectiveness against hospitalisation with influenza A(H1N1)pdm09 and B among elderly people in Europe: Results from the I-MOVE+ project. Eurosurveillance 2017, 22, 30580. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Chung, J.R.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; McLean, H.Q.; Gaglani, M.; Murthy, K.; et al. Influenza vaccine effectiveness among patients with high-risk medical conditions in the United States, 2012–2016. Vaccine 2018, 36, 8047–8053. [Google Scholar] [CrossRef] [PubMed]
- Selvais, P.L.; Hermans, M.P.; Donckier, J.E.; Buysschaert, M. Reported rates, incentives, and effectiveness of major vaccinations in 501 attendees at two diabetes clinics. Diabetes Care 1997, 20, 1212–1213. [Google Scholar] [CrossRef] [PubMed]
- French Ministry of Solidarity and Health. Vaccination Schedule and Recommendations. 2021. Available online: https://solidarites-sante.gouv.fr/prevention-en-sante/preserver-sa-sante/vaccination/calendrier-vaccinal (accessed on 15 October 2021).
- Sultan, A.; Bauduceau, B.; Baron, S.; Brunot, S.; Casanova, L.; Chaumeil, C.; Galtier, F.; Lecointre, B.; Morand, A.; Phirmis, L.; et al. Référentiel de la Société francophone du diabète (SFD): Vaccination chez la personne diabétique. Méd. Mal. Métab. 2020, 14, 12. [Google Scholar] [CrossRef]
- American Diabetes Association. Vaccination Practices for Adults with Diabetes. Available online: https://www.diabeteseducator.org/docs/default-source/practice/educator-tools/vaccination-practices-for-adults-with-diabetesv2.pdf?sfvrsn=2 (accessed on 26 March 2021).
- Andreoni, M.; Sticchi, L.; Nozza, S.; Sarmati, L.; Gori, A.; Tavio, M. Recommendations of the Italian society for infectious and tropical diseases (SIMIT) for adult vaccinations. Hum. Vaccin. Immunother. 2021, 17, 4265–4282. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Diabetes Type 1 and Type 2 and Adult Vaccination. Available online: https://www.cdc.gov/vaccines/adults/rec-vac/health-conditions/diabetes.html (accessed on 15 October 2021).
- Ministero della Salute. Persone a Rischio Per Patologia. Available online: https://www.salute.gov.it/portale/vaccinazioni/dettaglioContenutiVaccinazioni.jsp?lingua=italiano&id=4811&area=vaccinazioni&menu=fasce (accessed on 15 October 2021).
- Diabetes Austria. Living with Diabetes, Preventing Complications. Available online: https://www.diabetesaustralia.com.au/living-with-diabetes/preventing-complications/influenza/ (accessed on 15 October 2021).
- Diabetes Canada. Influenza, Pneumococcal, Hepatitis B and Herpes Zoster Vaccinations. Available online: https://guidelines.diabetes.ca/cpg/chapter19 (accessed on 15 October 2021).
- Jimenez-Trujillo, I.; Lopez-de Andres, A.; Hernandez-Barrera, V.; Carrasco-Garrido, P.; Santos-Sancho, J.M.; Jimenez-Garcia, R. Influenza vaccination coverage rates among diabetes sufferers, predictors of adherence and time trends from 2003 to 2010 in Spain. Hum. Vaccin. Immunother. 2013, 9, 1326–1332. [Google Scholar] [CrossRef][Green Version]
- Verger, P.; Fressard, L.; Collange, F.; Gautier, A.; Jestin, C.; Launay, O.; Raude, J.; Pulcini, C.; Peretti-Watel, P. Vaccine Hesitancy Among General Practitioners and Its Determinants During Controversies: A National Cross-sectional Survey in France. EBioMedicine 2015, 2, 891–897. [Google Scholar] [CrossRef]
- Verger, P.; Cortaredona, S.; Pulcini, C.; Casanova, L.; Peretti-Watel, P.; Launay, O. Characteristics of patients and physicians correlated with regular influenza vaccination in patients treated for type 2 diabetes: A follow-up study from 2008 to 2011 in southeastern France. Clin. Microbiol. Infect. 2015, 21, 930.e1–930.e9. [Google Scholar] [CrossRef]
- Wilson, R.; Scronias, D.; Zaytseva, A.; Ferry, M.A.; Chamboredon, P.; Dube, E.; Verger, P. Seasonal influenza self-vaccination behaviours and attitudes among nurses in Southeastern France. Hum. Vaccin. Immunother. 2019, 15, 2423–2433. [Google Scholar] [CrossRef]
- Isenor, J.E.; Edwards, N.T.; Alia, T.A.; Slayter, K.L.; MacDougall, D.M.; McNeil, S.A.; Bowles, S.K. Impact of pharmacists as immunizers on vaccination rates: A systematic review and meta-analysis. Vaccine 2016, 34, 5708–5723. [Google Scholar] [CrossRef]
- Isenor, J.E.; O’Reilly, B.A.; Bowles, S.K. Evaluation of the impact of immunization policies, including the addition of pharmacists as immunizers, on influenza vaccination coverage in Nova Scotia, Canada: 2006 to 2016. BMC Public Health 2018, 18, 787. [Google Scholar] [CrossRef]
- Czech, M.; Balcerzak, M.; Antczak, A.; Byliniak, M.; Piotrowska-Rutkowska, E.; Drozd, M.; Juszczyk, G.; Religioni, U.; Vaillancourt, R.; Merks, P. Flu Vaccinations in Pharmacies-A Review of Pharmacists Fighting Pandemics and Infectious Diseases. Int. J. Environ. Res. Public Health 2020, 17, 7945. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Dalton, L.; Dey, A.; Macartney, K.; Beard, F. Letter: Impact of the COVID-19 pandemic on pharmacist-administered vaccination services. Res. Soc. Adm. Pharm. 2021, 17, 2040–2041. [Google Scholar] [CrossRef] [PubMed]
- Breuker, C.; Macioce, V.; Mura, T.; Castet-Nicolas, A.; Audurier, Y.; Boegner, C.; Jalabert, A.; Villiet, M.; Avignon, A.; Sultan, A. Medication Errors at Hospital Admission and Discharge: Risk Factors and Impact of Medication Reconciliation Process to Improve Healthcare. J. Patient Saf. 2021, 17, e645–e652. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S.; Rodney, X.; Sturdivant, R.X. Model-Building Strategies and Methods for Logistic Regression. In Applied Logistic Regression, 3rd ed.; Statistics WSiPa, Ed.; Wiley: Louisville, KY, USA, 2013. [Google Scholar]
- Hosmer, D.W.; Hosmer, T.; Le Cessie, S.; Lemeshow, S. A comparison of goodness-of-fit tests for the logistic regression model. Stat. Med. 1997, 16, 965–980. [Google Scholar] [CrossRef]
- Blanchi, S.; Vaux, J.; Toque, J.M.; Hery, L.; Laforest, S.; Piccoli, G.B.; Crochette, N. Impact of a Catch-Up Strategy of DT-IPV Vaccination during Hospitalization on Vaccination Coverage among People Over 65 Years of Age in France: The HOSPIVAC Study (Vaccination during Hospitalization). Vaccines 2020, 8, 292. [Google Scholar] [CrossRef]
- Guillot, C.; Duputel, B.; Servy, H.; Sultan, A.; Bauduceau, B. Le rapport à la vaccination des personnes diabétiques. Résultats préliminaires d’une étude auprès de 3731 personnes diabétiques. Méd. Mal. Métab. 2020, 14, 6. [Google Scholar] [CrossRef]
- Wellcome. Wellcome Global Monitor 2018 Rapports. Available online: https://wellcome.ac.uk/reports/wellcome-global-monitor/2018 (accessed on 26 March 2021).
- Larson, H.J.; de Figueiredo, A.; Xiahong, Z.; Schulz, W.S.; Verger, P.; Johnston, I.G.; Cook, A.R.; Jones, N.S. The State of Vaccine Confidence 2016: Global Insights Through a 67-Country Survey. EBioMedicine 2016, 12, 295–301. [Google Scholar] [CrossRef]
- Le Monde with AFP. WHO Justifies Its Management of Influenza A. Available online: https://www.lemonde.fr/epidemie-grippe-a/article/2010/01/26/l-oms-justifie-sa-gestion-de-la-grippe-a_1296718_1225408.html (accessed on 26 March 2021).
- Paterson, P.; Meurice, F.; Stanberry, L.R.; Glismann, S.; Rosenthal, S.L.; Larson, H.J. Vaccine hesitancy and healthcare providers. Vaccine 2016, 34, 6700–6706. [Google Scholar] [CrossRef]
- Breuker, C.; Guedj, A.M.; Allan, M.; Coinus, L.; Molinari, N.; Chapet, N.; Roubille, F.; Le Quintrec, M.; Duhalde, V.; Jouglen, J.; et al. The COVID-19 Pandemic Led to a Small Increase in Changed Mentality Regarding Infection Risk without Any Change in Willingness to Be Vaccinated in Chronic Diseases Patients. J. Clin. Med. 2021, 10, 3967. [Google Scholar] [CrossRef]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef]
- Breuker, C.; Abraham, O.; di Trapanie, L.; Mura, T.; Macioce, V.; Boegner, C.; Jalabert, A.; Villiet, M.; Castet-Nicolas, A.; Avignon, A.; et al. Patients with diabetes are at high risk of serious medication errors at hospital: Interest of clinical pharmacist intervention to improve healthcare. Eur. J. Intern. Med. 2017, 38, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lohan, L.; Clement, F.; Duflos, C.; Villiet, M.; Castet-Nicolas, A.; Boegner, C.; Avignon, A.; Sultan, A.; Breuker, C. Hypoglycemia While Driving in Insulin-Treated Patients: Incidence and Risk Factors. J. Patient Saf. 2021, 17, e1034–e1039. [Google Scholar] [CrossRef] [PubMed]
- Lohan, L.; Galtier, F.; Manson, T.; Mura, T.; Castet-Nicolas, A.; Faure, D.; Chapet, N.; Leclercq, F.; Pasquie, J.L.; Roubille, F.; et al. Compliance with Prescription Guidelines for Glucose-Lowering Therapies According to Renal Function: Real-Life Study in Inpatients of Internal Medicine, Endocrinology and Cardiology Units. Medicina 2021, 57, 1376. [Google Scholar] [CrossRef] [PubMed]
- Breuker, C.; Clement, F.; Mura, T.; Macioce, V.; Castet-Nicolas, A.; Audurier, Y.; Boegner, C.; Morcrette, E.; Jalabert, A.; Villiet, M.; et al. Non-achievement of LDL-cholesterol targets in patients with diabetes at very-high cardiovascular risk receiving statin treatment: Incidence and risk factors. Int. J. Cardiol. 2018, 268, 195–199. [Google Scholar] [CrossRef]
| Incomplete Immunization Coverage at Admission | Vaccination during Hospital Stay | |||
|---|---|---|---|---|
| NO | YES | p-Value | ||
| n | 208 | 151 (72.6) | 57 (27.4) | |
| Sex, male | 119 (57.2) | 91 (60.3) | 28 (49.1) | 0.16 |
| Age (years), mean (sd) | 59.5 ± 15.0 | 59.1 ± 15.8 | 60.3 ± 12.9 | 0.57 |
| Type 2 diabetes | 145 (69.7) | 102 (67.6) | 43 (75.4) | 0.31 |
| Length of stay, days | 8.6 ± 8.8 | 8.9 ± 9.6 | 7.9 ± 5.9 | 0.38 |
| Diabetes duration ≥10 years | 155 (75.2) | 112 (74.7) | 43 (76.8) | 0.75 |
| HbA1c, % | 8.8 ± 2.2 | 8.8 ± 2.3 | 8.6 ± 1.8 | 0.45 |
| Body mass index ≥30 kg/m2 | 81 (38.9) | 53 (35.1) | 28 (49.1) | 0.08 |
| Number of medications | 7.8 ± 4.1 | 7.8 ± 4.3 | 8.0 ± 3.8 | 0.75 |
| Insulin treatment (yes) | 142 (68.3) | 103 (68.2) | 39 (68.4) | 0.98 |
| Diabetes care units | <0.001 | |||
| Full-time inpatient unit 1 | 53 (25.5) | 34 (22.5) | 19 (33.3) | |
| Weekday hospitalization unit | 76 (36.5) | 42 (27.8) | 34 (59.6) | |
| Full-time inpatient unit 2 | 79 (38.0) | 75 (49.7) | 4 (7.0) | |
| Admission reasons | 0.24 | |||
| Imbalanced diabetes | 135 (66.8) | 97 (66.9) | 38 (66.7) | |
| Diabetic foot | 55 (27.2) | 37 (25.5) | 18 (31.6) | |
| Insulin pump installation | 12 (5.9) | 11 (7.6) | 1 (1.7) | |
| Knowledge of mandatory vaccine (yes) | 163 (78.4) | 121 (80.1) | 42 (73.7) | 0.35 |
| Knowledge of recommended vaccines | 0.04 | |||
| No | 150 (72.1) | 111 (73.5) | 39 (68.4) | |
| Yes | 5 (2.4) | 1 (2.0) | 4 (7.0) | |
| Incomplete | 53 (25.5) | 39 (25.8) | 14 (24.6) | |
| Feelings about vaccination | 0.01 | |||
| For | 100 (48.1) | 64 (42.4) | 36 (63.2) | |
| Against | 19 (9.1) | 17 (11.2) | 2 (3.5) | |
| Mixed | 53 (25.5) | 45 (29.8) | 8 (14.0) | |
| Without opinion | 36 (17.3) | 25 (16.6) | 11 (19.3) | |
| Documentation in the medical record of the pharmacist’s assessment of the vaccination coverage on hospital admission (yes) | 171 (82.2) | 116 (76.8) | 55 (96.5) | 0.0009 |
| Immunization coverage of Tdap (tetanus, diphtheria, and pertussis) vaccines at hospital admission | 0.15 | |||
| No | 73 (35.1) | 48 (31.8) | 25 (43.9) | |
| Yes | 91 (43.7) | 72 (47.7) | 19 (33.3) | |
| Unknown | 44 (21.2) | 31 (20.5) | 13 (22.8) | |
| Immunization coverage of pneumococcal vaccines | 0.17 | |||
| No | 174 (83.7) | 122 (80.8) | 52 (91.2) | |
| Yes | 10 (4.8) | 8 (5.3) | 2 (3.5) | |
| Unknown | 24 (11.5) | 21 (13.9) | 3 (5.3) | |
| Immunization coverage of influenza vaccines | 0.22 | |||
| No | 118 (56.7) | 90 (59.6) | 28 (49.1) | |
| Yes | 88 (42.3) | 60 (39.7) | 28 (49.1) | |
| Unknown | 2 (1.0) | 1 (0.7) | 1 (1.8) | |
| Data are the mean ± SD, or n (%); HbA1c: hemoglobin A1c. | ||||
| Immunization Coverage at Admission | Immunization Coverage at Discharge | |||||
|---|---|---|---|---|---|---|
| Total Population | Unit 2 | Unit 1 and Weekday Unit | Total Population | Unit 2 | Unit 1 and Weekday Unit | |
| n | 222 | 86 (38.7) | 136 (61.3) | 222 | 86 (38.7) | 136 (61.3) |
| Tdap vaccines | ||||||
| No | 73 (32.9) | 23 (26.7) | 50 (36.8) | 52 (23.4) | 22 (25.6) | 30 (22.0) |
| Yes | 105 (47.3) | 44 (51.2) | 61 (44.8) | 133 (59.9) | 46 (53.5) | 87 (64.0) |
| Unknown | 44 (19.8) | 19 (22.1) | 25 (18.4) | 37 (16.7) | 18 (2039) | 19 (14.0) |
| Pneumococcal vaccines | ||||||
| No | 174 (78.4) | 65 (75.6) | 109 (80.1) | 132 (59.4) | 64 (74.4) | 68 (50.0) |
| Yes | 24 (10.8) | 10 (11.6) | 14 (10.3) | 69 (31.1) | 11 (12.8) | 58 (42.6) |
| Unknown | 24 (10.8) | 11 (12.8) | 13 (9.6) | 21 (9.5) | 11 (12.8) | 10 (7.4) |
| Influenza vaccines | ||||||
| No | 118 (53.2) | 50 (58.1) | 68 (50.0) | 109 (49.1) | 49 (57.0) | 60 (44.1) |
| Yes | 102 (45.9) | 35 (40.7) | 67 (49.3) | 111 (50.0) | 36 (41.9) | 75 (55.2) |
| Unknown | 2 (0.9) | 1 (1.2) | 1 (0.7) | 2 (0.9) | 1 (1.1) | 1 (0.7) |
| Data are n (%). Tdap, tetanus, diphtheria, pertussis | ||||||
| Total | |
|---|---|
| n | 222 |
| Knowledge of mandatory vaccine (yes) | 171 (77.0) |
| Knowledge of recommended vaccines | |
| No | 155 (69.8) |
| Yes | 8 (3.6) |
| Incomplete | 59 (26.6) |
| Feelings about vaccination | |
| For | 113 (50.9) |
| Against | 19 (8.6) |
| Mixed | 54 (24.3) |
| Without opinion | 36 (16.2) |
| Reasons for patients against and with mixed feelings about vaccines (n = 63/73) | |
| Vaccines are not very efficient | 17 (27.0) |
| Side effects of vaccines | 19 (30.2) |
| Vaccination only if mandatory | 18 (28.5) |
| Fear of vaccines | 7 (11.1) |
| Others | 2 (3.2) |
| Possession of a vaccination record booklet | 59 (26.6%) |
| Data are n (%) | |
| Characteristics | Univariate Analysis Odds Ratio 95% CI | p-Value | Multivariate Analysis Odds Ratio 95% CI | p-Value |
|---|---|---|---|---|
| Sex, female (vs. male) | 1.57 (0.85–2.90) | 0.15 | 2.64 (1.05–6.64) | 0.04 |
| Diabetes care units | <0.0001 | <0.0001 | ||
| Full-time inpatient unit 1 (vs. full-time inpatient unit 2) | 10.48 (3.31–33.14) | 9.15 (2.33–35.97) | ||
| Weekday hospitalization unit (vs. full-time inpatient unit 2) | 15.18 (5.04–45.71) | 22.62 (6.26–81.74) | ||
| Admission reasons | 0.24 | 0.13 | ||
| Diabetic foot (vs. imbalanced diabetes) | 1.24 (0.63–2.44) | 3.42 (0.93–12.59) | ||
| Insulin pump installation (vs. imbalanced diabetes) | 0.23 (0.03–1.86) | 0.30 (0.02–4.54) | ||
| Type 2 diabetes (vs. type 1 diabetes) | 1.48 (0.74–2.95) | 0.31 | 0.59 (0.21–1.66) | 0.13 |
| Feelings about vaccination | 0.02 | 0.0001 | ||
| Against (vs. for) | 0.21 (0.05–0.96) | 0.08 (0.01–0.42) | ||
| Mixed (vs. for) | 0.32 (0.13–0.74) | 0.18 (0.06–0.51) | ||
| Without opinion (vs. for) | 0.78 (0.35–1.77) | 0.54 (0.18–1.58) | ||
| Immunization coverage of Tdap (Tetanus, diphtheria, and pertussis) vaccines at hospital admission | 0.16 | 0.21 | ||
| Yes (vs. no) | 0.51 (0.25–1.02) | 0.48 (0.19–1.21) | ||
| Unknown (vs. no) | 0.81 (0.36–1.81) | 1.12 (0.36–3.46) | ||
| Immunization coverage of pneumococcal vaccines | 0.20 | 0.03 | ||
| Yes (vs. no) | 0.59 (0.12–2.86) | 0.27 (0.03–2.16) | ||
| Unknown (vs. no) | 0.34 (0.10–1.17) | 0.1 (0.03–0.65) | ||
| Immunization coverage of influenza vaccines yes (vs. no) | 1.46 (0.79–2.70) | 0.22 | ||
| Documentation in the medical record of the pharmacist’s assessment of the vaccination coverage on hospital admission (vs. no) | 8.30 (1.93–35.74) | <0.001 | 5.14 (1.02–25.95) | 0.04 |
| Knowledge of recommended vaccines | 0.10 | |||
| Yes (vs. no) | 11.38 (1.24–104.94) | |||
| Incomplete (vs. no) | 1.02 (0.50–2.08) | |||
| Body mass index ≥30 kg/m2 (vs. <30 kg/m2) | 1.78 (0.96–3.31) | 0.07 | ||
| all independent variables with a p-value < 0.25 in the bivariate analysis and one variable of interest (type of diabetes) were simultaneously introduced in the models | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohan, L.; Cool, C.; Viault, L.; Cestac, P.; Renard, E.; Galtier, F.; Villiet, M.; Avignon, A.; Sultan, A.; Breuker, C. Impact of Hospitalization in an Endocrinology Department on Vaccination Coverage in People Living with Diabetes: A Real-Life Study. Medicina 2022, 58, 219. https://doi.org/10.3390/medicina58020219
Lohan L, Cool C, Viault L, Cestac P, Renard E, Galtier F, Villiet M, Avignon A, Sultan A, Breuker C. Impact of Hospitalization in an Endocrinology Department on Vaccination Coverage in People Living with Diabetes: A Real-Life Study. Medicina. 2022; 58(2):219. https://doi.org/10.3390/medicina58020219
Chicago/Turabian StyleLohan, Laura, Charlène Cool, Loriane Viault, Philippe Cestac, Eric Renard, Florence Galtier, Maxime Villiet, Antoine Avignon, Ariane Sultan, and Cyril Breuker. 2022. "Impact of Hospitalization in an Endocrinology Department on Vaccination Coverage in People Living with Diabetes: A Real-Life Study" Medicina 58, no. 2: 219. https://doi.org/10.3390/medicina58020219
APA StyleLohan, L., Cool, C., Viault, L., Cestac, P., Renard, E., Galtier, F., Villiet, M., Avignon, A., Sultan, A., & Breuker, C. (2022). Impact of Hospitalization in an Endocrinology Department on Vaccination Coverage in People Living with Diabetes: A Real-Life Study. Medicina, 58(2), 219. https://doi.org/10.3390/medicina58020219







