Distally Prophylactic Lymphaticovenular Anastomoses after Axillary or Inguinal Complete Lymph Node Dissection Followed by Radiotherapy: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
Surgical Technique
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shields, J.W. Lymph, lymph glands, and homeostasis. Lymphology 1992, 25, 147–153. [Google Scholar] [PubMed]
- Warren, A.G.; Brorson, H.; Borud, L.J.; Slavin, S.A. Lymphedema: A comprehensive review. Ann. Plast. Surg. 2007, 59, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017, 77, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.; Benchikhi, H.; Roujeau, J.-C.; Bernard, P.; Vaillant, L.; Chosidow, O.; Sassolas, B.; Guillaume, J.-C.; Grob, J.-J.; Bastuji-Garin, S. Risk factors for erysipelas of the leg (cellulitis): Case-control study. BMJ 1999, 318, 1591–1594. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.W.; Masia, J.; Garza, R., 3rd; Skoracki, R.; Neligan, P.C. Lymphedema: Surgical and Medical Therapy. Plast. Reconstr. Surg. 2016, 138, 209S–218S. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoshimatsu, H. Sequential anastomosis for lymphatic supermicrosurgery; multiple lymphaticovenular anastomoses on 1 venule. Ann. Plast. Surg. 2014, 73, 46–49. [Google Scholar] [CrossRef]
- Boccardo, F.M.; Casabona, F.; Friedman, D.; Puglisi, M.; De Cian, F.; Ansaldi, F.; Campisi, C. Surgical Prevention of Arm Lymphedema after Breast Cancer Treatment. Ann. Surg. Oncol. 2011, 18, 2500–2505. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; Wright, M.J.; Morris, K.T. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J. Clin. Oncol. 2008, 26, 5213–5219. [Google Scholar] [CrossRef]
- Sisti, A.; Huayllani, M.T.; Boczar, D.; Restrepo, D.J.; Spaulding, A.C.; Emmanuel, G.; Bagaria, S.P.; McLaughlin, S.A.; Parker, A.S.; Forte, A.J. Breast cancer in women: A descriptive analysis of the national cancer database. Acta. Biomed 2020, 91, 332–341. [Google Scholar] [CrossRef]
- Forte, A.J.; Sisti, A.; Huayllani, M.T.; Boczar, D.; Cinotto, G.; Ciudad, P.; Manrique, O.J.; Lu, X.; McLaughlin, S. Lymphaticovenular anastomosis for breast cancer-related upper extremity lymphedema: A literature review. Gland Surg. 2020, 9, 539–544. [Google Scholar] [CrossRef]
- Scaglioni, M.F.; Fontein, D.B.Y.; Arvanitakis, M.; Giovanoli, P. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery 2017, 37, 947–953. [Google Scholar] [CrossRef]
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treat-ment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.; Sørensen, J.A. The effect of prophylactic lymphovenous anastomosis and shunts for pre-venting cancer related lymphedema: A systematic review and meta-analysis. Microsurgery. 2017, 38, 576–585. [Google Scholar] [CrossRef]
- Hidding, J.T.; Viehoff, P.B.; Beurskens, C.H. Measurement properties of instruments for measuring of lymphedema: Systematic review. Phys Ther. 2016, 96, 1965–1981. [Google Scholar] [CrossRef]
- Ogata, F.; Azuma, R.; Kikuchi, M.; Koshima, I.; Morimoto, Y. Novel Lymphography Using Indocyanine Green Dye for Near-Infrared Fluorescence Labeling. Ann. Plast. Surg. 2007, 58, 652–655. [Google Scholar] [CrossRef]
- Chen, W.F. How to Get Started Performing Supermicrosurgical Lymphaticovenular Anastomosis to Treat Lymphedema. Ann. Plast. Surg. 2018, 81, S15–S20. [Google Scholar] [CrossRef]
- Shaitelman, S.F.; Cromwell, K.D.; Rasmussen, J.C.; Stout, N.L.; Armer, J.M.; Lasinski, B.B.; Cormier, J.N. Recent progress in the treatment and prevention of cancer-related lymphedema. CA A Cancer J. Clin. 2015, 65, 55–81. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Coroneos, C.J. Surgical Treatment of Lymphedema. Plast. Reconstr. Surg. 2019, 144, 738–758. [Google Scholar] [CrossRef]
- Kaciulyte, J.; Garutti, L.; Spadoni, D.; Velazquez-Mujica, J.; Losco, L.; Ciudad, P.; Marcasciano, M.; Lo Torto, F.; Casella, D.; Ribuffo, D.; et al. Genital Lymphedema and How to Deal with It: Pearls and Pitfalls from over 38 Years of Experience with Unusual Lymphatic System Impairment. Medicina 2021, 57, 1175. [Google Scholar] [CrossRef]
- Campisi, C.; Bellini, C.; Accogli, S.; Bonioli, E.; Boccardo, F.M.; Campisi, C. Microsurgery for lymphedema: Clinical research and long-term results. Microsurgery 2010, 30, 256–260. [Google Scholar] [CrossRef]
- Di Taranto, G.; Bolletta, A.; Chen, S.H.; Losco, L.; Elia, R.; Cigna, E.; Rubino, C.; Ribuffo, D.; Chen, H.C. A prospective study on combined lymphedema surgery: Gastroepiploic vascularized lymph nodes transfer and lymphaticovenous anastomosis followed by suction lipectomy. Microsurgery 2021, 41, 34–43. [Google Scholar] [CrossRef]
- Torto, F.L.; Kaciulyte, J.; Mori, F.L.; Frattaroli, J.M.; Marcasciano, M.; Casella, D.; Cigna, E.; Losco, L.; Manrique, O.J.; Nicoli, F.; et al. Microsurgical lymphedema treatment: An objective evaluation of the quality of online information. J. Plast. Reconstr. Aesthetic Surg. 2020, 74, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Verhey, E.M.; Torres-Guzman, R.A.; Avila, F.R.; Forte, A.J.; Rebecca, A.M.; Teven, C.M. Outcomes of Lymphovenous Anastomosis for Upper Extremity Lymphedema: A Systematic Review. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3770. [Google Scholar] [CrossRef] [PubMed]
- Cigna, E.; Pierazzi, D.M.; Sereni, S.; Marcasciano, M.; Losco, L.; Bolletta, A. Lymphatico-venous anastomosis in chronic ulcer with venous insufficiency: A case report. Microsurgery 2021, 41, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Huang, J.J.; Wu, C.W. The mechanism of vascularized lymph node transfer for lymphedema: Natural lymphaticovenous drainage. Plast. Reconstr. Surg. 2014, 133, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Taranto, G.; Chen, S.H.; Elia, R.; Bolletta, A.; Amorosi, V.; Sitpahul, N.; Chan, J.C.; Ribuffo, D.; Chen, H.C. Free gas-troepiploic lymph nodes and omentum flap for treatment of lower limb ulcers in severe lymphedema: Killing two birds with one stone. J. Surg. Oncol. 2020, 121, 168–174. [Google Scholar]
- Gasteratos, K.; Morsi-Yeroyannis, A.; Vlachopoulos, N.C.; Spyropoulou, G.A.; Del Corral, G.; Chaiyasate, K. Microsurgical techniques in the treatment of breast cancer-related lymphedema: A systematic review of efficacy and patient out-comes. Breast Cancer 2021, 28, 1002–1015. [Google Scholar] [CrossRef]
- Morrell, R.M.; Halyard, M.Y.; Schild, S.E.; Ali, M.S.; Gunderson, L.L.; Pockaj, B.A. Breast cancer-related lymphedema. Mayo Clin. Proc. 2005, 80, 1480–1484. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer- related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef]
- Boccardo, F.; Casabona, F.; De Cian, F.; Friedman, D.; Villa, G.; Bogliolo, S.; Ferrero, S.; Murelli, F.; Campisi, C. Lymphedema Microsurgical Preventive Healing Approach: A New Technique for Primary Prevention of Arm Lymphedema After Mastectomy. Ann. Surg. Oncol. 2009, 16, 703–708. [Google Scholar] [CrossRef]
- Herremans, K.M.; Cribbin, M.P.; Andrea, N.; Riner, A.N.; Neal, D.W.; Hollen, T.L.; Clevenger, P.; Munoz, D.; Blewett, S.; Giap, F.; et al. Five-Year Breast Surgeon Experience in LYMPHA at Time of ALND for Treatment of Clinical T1-4N1-3M0 Breast Cancer. Ann. Surg. Oncol. 2021, 28, 5775–5787. [Google Scholar] [CrossRef]
- Cook, J.A.; Sinha, M.; Lester, M.; Fisher, C.S.; Sen, C.K.; Hassanein, A.H. Immediate Lymphatic Reconstruction to Prevent Breast Cancer Related Lymphedema: A Systematic Review. Adv. Wound Care 2021. [Google Scholar] [CrossRef]
- Orefice, S.; Conti, A.R.; Grassi, M.; Salvadori, B. The use of lympho-venous anastomoses to prevent complications from ilio-inguinal dissection. Tumori. J. 1988, 30, 347–351. [Google Scholar] [CrossRef]
- Onoda, S.; Todokoro, T.; Hara, H.; Azuma, S.; Goto, A. Minimally invasive multiple lymphaticovenular anastomosis at the ankle for the prevention of lower leg lymphedema. Microsurgery 2013, 34, 372–376. [Google Scholar] [CrossRef]
- Nacchiero, E.; Maruccia, M.; Vestita, M.; Elia, R.; Marannino, P.; Giudice, G. Multiple lymphatic-venous anastomoses in reducing the risk of lymphedema in melanoma patients undergoing complete lymph node dissection. A retrospective case-control study. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 642–648. [Google Scholar] [CrossRef]
- Chen, W.F.; Knackstedt, R. Delayed Distally Based Prophylactic Lymphaticovenular Anastomosis: Improved Functionali-ty, Feasibility, and Oncologic Safety? J. Reconstr. Microsurg. 2020, 36, e1–e2. [Google Scholar] [CrossRef]
- Yoshida, S.; Koshima, I.; Imai, H.; Sasaki, A.; Fujioka, Y.; Nagamatsu, S.; Yokota, K.; Harima, M.; Yamashita, S.; Tashiro, K. Indocyanine green lymphography findings in older patients with lower limb lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 8, 251–258. [Google Scholar] [CrossRef]
- Yoshida, S.; Koshima, I.; Imai, H.; Uchiki, T.; Sasaki, A.; Fujioka, Y.; Nagamatsu, S.; Yokota, K.; Yamashita, S. Lymphovenous Anastomosis for Morbidly Obese Patients with Lymphedema. Plast. Reconstr. Surg. Glob. Open 2020, 8, 2860. [Google Scholar] [CrossRef]
- Yoshida, S.; Koshima, I.; Imai, H.; Roh, S.; Mese, T.; Uchiki, T.; Sasaki, A.; Nagamatsu, S. Lymphaticovenous Anastomosis for Age-Related Lymphedema. J. Clin. Med. 2021, 10, 5129. [Google Scholar] [CrossRef]
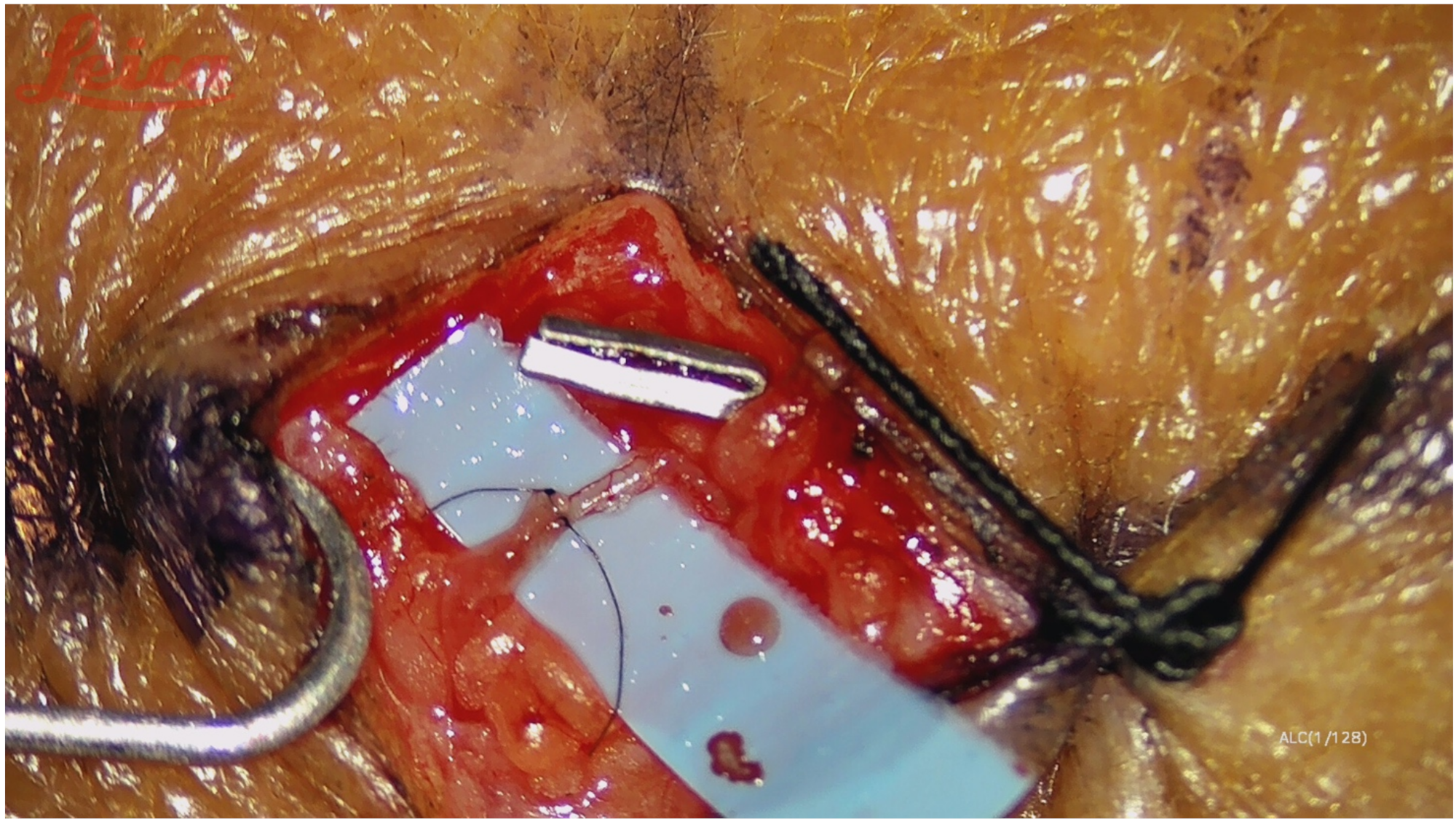
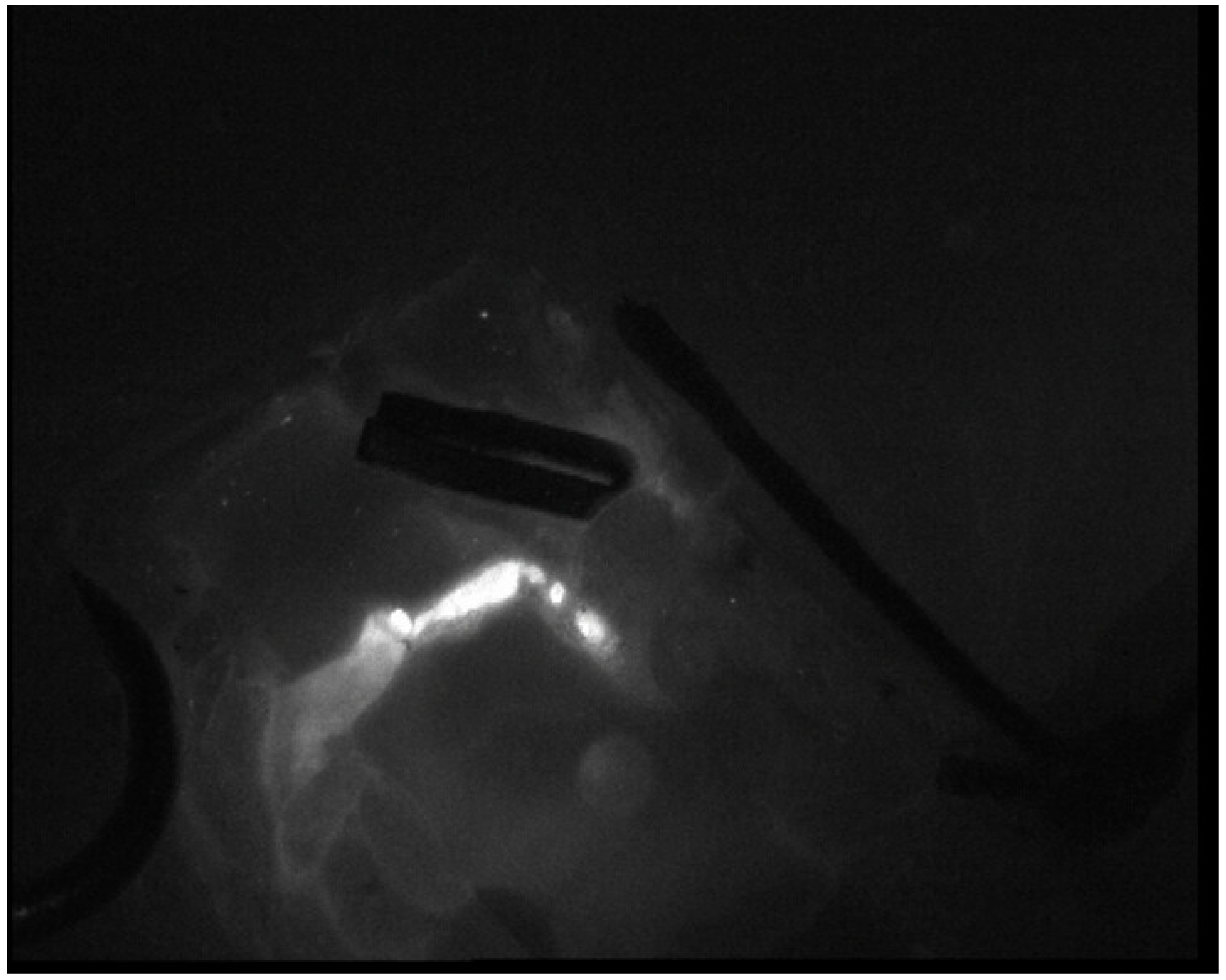
| Case Number | Age (Years) | Sex | BMI | Pathology | Comorbidities |
|---|---|---|---|---|---|
| 1 | 42 | F | 23 | Breast cancer | None |
| 2 | 51 | F | 30 | Breast cancer | None |
| 3 | 55 | F | 28 | Breast cancer | None |
| 4 | 48 | F | 24 | Breast cancer | None |
| 5 | 53 | F | 25 | Breast cancer | None |
| 6 | 60 | F | 21 | Merkel Cell carcinoma | None |
| Case Number | Lymph Node Dissection | Time between Lymph Node Dissection and LVA (Days) | Number of Anastomosis | Length of Stay (Days) | Complications |
|---|---|---|---|---|---|
| 1 | Axillary | 90 | 4 | 2 | None |
| 2 | Axillary | 110 | 4 | 2 | None |
| 3 | Axillary | 85 | 4 | 2 | None |
| 4 | Axillary | 120 | 4 | 2 | None |
| 5 | Axillary | 130 | 4 | 3 | None |
| 6 | Inguinal | 107 | 3 | 3 | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierazzi, D.M.; Arleo, S.; Faini, G. Distally Prophylactic Lymphaticovenular Anastomoses after Axillary or Inguinal Complete Lymph Node Dissection Followed by Radiotherapy: A Case Series. Medicina 2022, 58, 207. https://doi.org/10.3390/medicina58020207
Pierazzi DM, Arleo S, Faini G. Distally Prophylactic Lymphaticovenular Anastomoses after Axillary or Inguinal Complete Lymph Node Dissection Followed by Radiotherapy: A Case Series. Medicina. 2022; 58(2):207. https://doi.org/10.3390/medicina58020207
Chicago/Turabian StylePierazzi, Diletta Maria, Sergio Arleo, and Gianpaolo Faini. 2022. "Distally Prophylactic Lymphaticovenular Anastomoses after Axillary or Inguinal Complete Lymph Node Dissection Followed by Radiotherapy: A Case Series" Medicina 58, no. 2: 207. https://doi.org/10.3390/medicina58020207
APA StylePierazzi, D. M., Arleo, S., & Faini, G. (2022). Distally Prophylactic Lymphaticovenular Anastomoses after Axillary or Inguinal Complete Lymph Node Dissection Followed by Radiotherapy: A Case Series. Medicina, 58(2), 207. https://doi.org/10.3390/medicina58020207






