Effects of Different Drug Therapies and COVID-19 mRNA Vaccination on Semen Quality in a Man with Ankylosing Spondylitis: A Case Report
Abstract
:1. Introduction
The Case
2. Materials and Methods
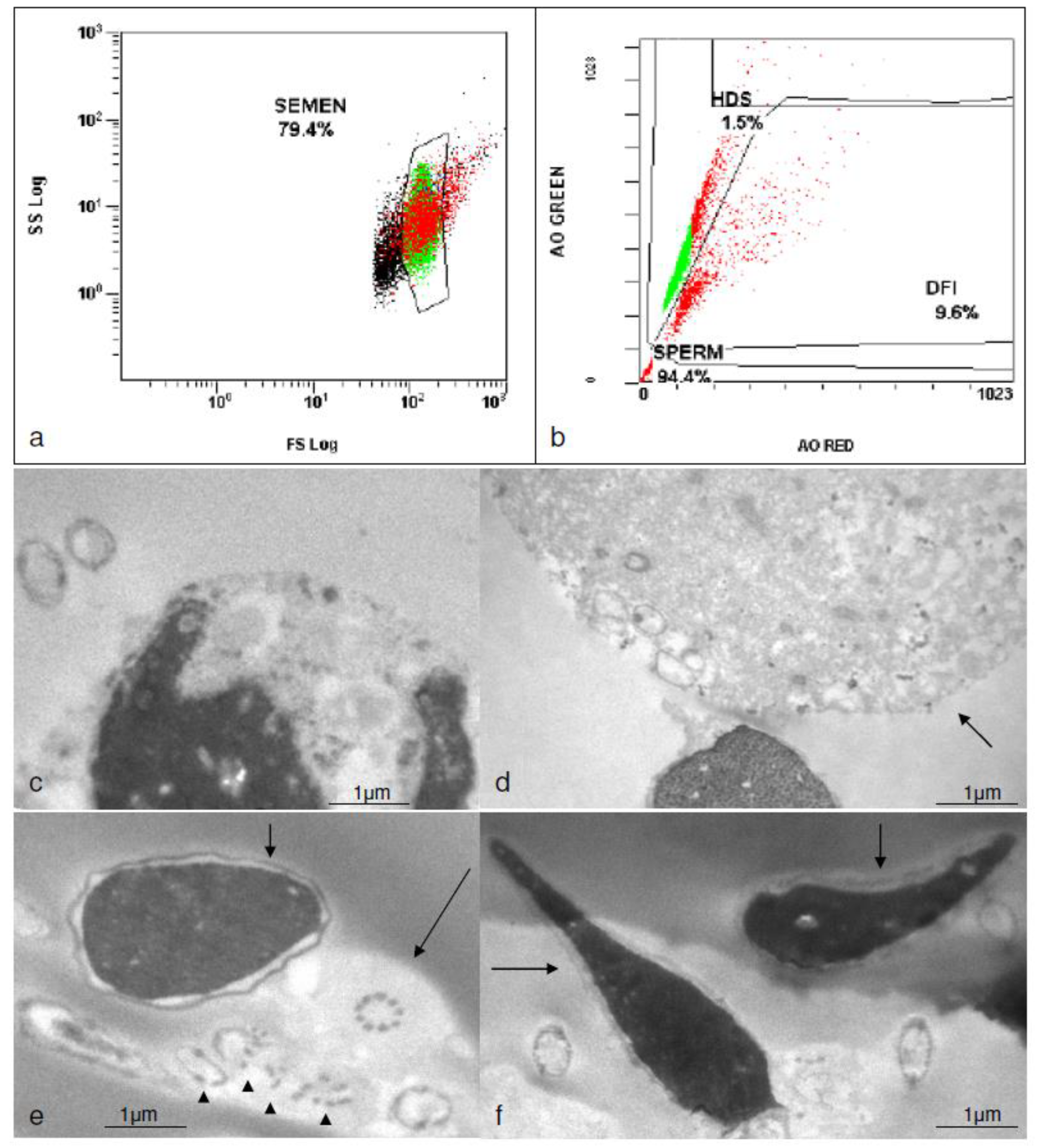
2.1. Standard Semen Analysis
2.2. Transmission Electron Microscopy (TEM)
2.3. Flow Cytometry
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voruganti, A.; Bowness, P. New developments in our understanding of ankylosing spondylitis pathogenesis. Immunology 2020, 161, 94–102. [Google Scholar] [CrossRef]
- Reveille, J.D. Recent studies on the genetic basis of ankylosing spondylitis. Curr. Rheumatol. Rep. 2009, 11, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, B.C.; Cocuzza, M.; Bonfa, E.; Srougi, M.; Silva, C.A. Male fertility potential alteration in rheumatic diseases: A systematic review. Int. Braz. J. Urol. 2016, 42, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, B.P.; Saad, C.G.S.; Souza, F.H.C.; Moraes, J.C.B.; Nukumizu, L.A.; Viana, V.S.T.; Bonfá, E.; Silva, C.A. Testicular Sertoli cell function in ankylosing spondylitis. Clin. Rheumatol. 2013, 32, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Nukumizu, L.; Saad, C.G.; Ostensen, M.; Almeida, B.; Cocuzza, M.; Gonçalves, C.; Saito, O.; Bonfa, E.; Silva, C. Gonadal function in male patients with ankylosing spondylitis. Scand. J. Rheumatol. 2012, 41, 476–481. [Google Scholar] [CrossRef]
- Østensen, M. Sexual and reproductive health in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Bazzani, C.; Andreoli, L.; Agosti, M.; Nalli, C.; Tincani, A. Antirheumatic drugs and reproduction in women and men with chronic arthritis: Table 1. RMD Open 2015, 1, e000048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramonda, R.; Foresta, C.; Ortolan, A.; Bertoldo, A.; Oliviero, F.; Lorenzin, M.; Pizzol, D.; Punzi, L.; Garolla, A. Influence of tumor necrosis factor α inhibitors on testicular function and semen in spondyloarthritis patients. Fertil. Steril. 2014, 101, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Saougou, I.; Markatseli, T.E.; Papagoras, C.; Kaltsonoudis, E.; Voulgari, P.V.; Drosos, A.A. Fertility in male patients with seronegative spondyloarthropathies treated with infliximab. Jt. Bone Spine 2013, 80, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Gordon, D.; Beastall, G.H.; Sturrock, R.D. Androgenic Status and Sexual Function in Males with Rheumatoid Arthritis and Ankylosing Spondylitis. Qjm: Int. J. Med. 1986, 60, 671–679. [Google Scholar] [CrossRef]
- Micu, M.C.; Micu, R.; Surd, S.; Gîrlovanu, M.; Bolboacă, S.D.; Ostensen, M. TNF-α inhibitors do not impair sperm quality in males with ankylosing spondylitis after short-term or long-term treatment. Rheumatology 2014, 53, 1250–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minozzi, S.; Bonovas, S.; Lytras, T.; Pecoraro, V.; González-Lorenzo, M.; Bastiampillai, A.J.; Gabrielli, E.M.; Lonati, A.C.; Moja, L.; Cinquini, M.; et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: A systematic review and meta-analysis. Expert Opin. Drug Saf. 2016, 15, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-L.; Yang, C.-H.; Chi, C.-C. Drug Survival of Biologics in Treating Ankylosing Spondylitis: A Systematic Review and Meta-analysis of Real-World Evidence. BioDrugs 2020, 34, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.; Kerschbaumer, A.; Dörner, T.; Dougados, M.; Fleischmann, R.M.; Geissler, K.; McInnes, I.; Pope, J.E.; van der Heijde, D.; Stoffer-Marx, M.; et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: A consensus statement. Ann. Rheum. Dis. 2021, 80, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Balderas, E.; Sanchez-Cardenas, C.; Chavez, J.; De La Vega Beltrán, J.L.; Gomez-Lagunas, F.; Trevino, C.L.; Darszon, A. The anti-inflammatory drug celecoxib inhibits t-type Ca2+ currents in spermatogenic cells yet it elicits the acrosome reaction in mature sperm. FEBS Lett. 2013, 587, 2412–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loricera, J.; Galíndez-Aguirregoikoa, E.; Blanco, R. Safety of secukinumab for the treatment of active ankylosing spondylitis. Expert Opin. Drug Saf. 2021, 20, 1–8. [Google Scholar] [CrossRef]
- Abdulla, J.S.; Shi, J.; Roy, B.S.; Zhanwen, Z.; Liu, C. Patients with ankylosing spondylitis treatment by golimumab: A systematic review and meta-analysis. Eur. Spine J. 2020, 29, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Leu, J.H.; Adedokun, O.J.; Gargano, C.; Hsia, E.C.; Xu, Z.; Shankar, G. Immunogenicity of golimumab and its clinical relevance in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Rheumatology 2018, 58, 441–446. [Google Scholar] [CrossRef]
- Winnall, W.R.; Muir, J.A.; Liew, S.; Hirst, J.J.; Meachem, S.J.; Hedger, M.P. Effects of chronic celecoxib on testicular function in normal and lipopolysaccharide-treated rats. Int. J. Androl. 2009, 32, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-C.; Lu, M.-C.; Huang, K.-Y.; Huang, H.-l.; Liu, S.-Q.; Huang, H.-B.; Lai, N.-S. Sulfasalazine Treatment Suppresses the Formation of HLA-B27 Heavy Chain Homodimer in Patients with Ankylosing Spondylitis. Int. J. Mol. Sci. 2016, 17, 46. [Google Scholar] [CrossRef] [Green Version]
- O’Morain, C.; Smethurst, P.; Dore, C.J.; Levi, A.J. Reversible male infertility due to sulphasalazine: Studies in man and rat. Gut 1984, 25, 1078–1084. [Google Scholar] [CrossRef] [Green Version]
- Freeman, J.; Reece, V.; Venables, C. Sulphasalazine and Spermatogenesis. Digestion. 1982, 23, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Chatzimeletiou, K.; Galanis, N.; Karagiannidis, A.; Sioga, A.; Pados, G.; Goulis, D.; Kalpatsanidis, A.; Tarlatzis, B.C. Fertility potential in a man with ankylosing spondylitis as revealed by semen analysis by light, electron and fluorescence microscopy. SAGE Open Med. Case Rep. 2018, 6, 2050313X18759898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, S.A.; Lecarpentier, J.; Mani, V.; Goodman, M.J.; Mandal, B.K.; Turnberg, L.A. Sulphasalazine induced seminal abnormalities in ulcerative colitis: Results of mesalazine substitution. Gut 1987, 28, 1008–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelissen, P.M.J.; Van Hattum, J.; Poen, H.; Scholten, P.; Gerritse, R.; Velde, E.R.T. Influence of Salazosulphapyridine and 5-Aminosalicylic Acid on Seminal Qualities and Male Sex Hormones. Scand. J. Gastroenterol. 1988, 23, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G.; Noonan, E.; Von Eckardstein, S.; Auger, J.; Gordon Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Chatzimeletiou, K.; Sioga, A.; Oikonomou, L.; Charalampidou, S.; Kantartzi, P.; Zournatzi, V.; Panidis, D.; Goulis, D.G.; Papadimas, I.; Tarlatzis, B.C. Semen analysis by electron and fluorescence microscopy in a case of partial hydatidiform mole reveals a high incidence of abnormal morphology, diploidy, and tetraploidy. Fertil. Steril. 2011, 95, 2430.e1–2430.e5. [Google Scholar] [CrossRef]
- Lazaros, L.A.; Xita, N.V.; Chatzikyriakidou, A.L.; Kaponis, A.I.; Grigoriadis, N.G.; Hatzi, E.G.; Grigoriadis, I.G.; Sofikitis, N.V.; Zikopoulos, K.A.; Georgiou, I.A. Association of TNF, TNFR1, and TNFR2 Polymorphisms with Sperm Concentration and Motility. J. Androl. 2012, 33, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Chatzikyriakidou, A.; Georgiou, I.; Voulgari, P.V.; Drosos, A.A. The role of tumor necrosis factor (TNF)-alpha and TNF receptor polymorphisms in susceptibility to ankylosing spondylitis. Clin. Exp. Rheumatol. 2009, 27, 645–648. [Google Scholar]
- Eliasson, R. Standards for investigation of human semen Untersuchungsstandards für das menschliche Sperma La standardisation de l’analyse du sperme humain. Andrologia 2009, 3, 49–64. [Google Scholar] [CrossRef]
- Menkveld, R.; Holleboom, C.A.G.; Rhemrev, J.P.T. Measurement and significance of sperm morphology. Asian J. Androl. 2011, 13, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouy, S.; Sentein, P. Ultrastructural characteristics of human spermatozoa with elongated head. Pathol. Biol. 1977, 25, 691–697. [Google Scholar]
- Prisant, N.; Escalier, D.; Soufir, J.-C.; Morillon, M.; Schoevaert, D.; Misrahi, M.; Tachdjian, G. Ultrastructural nuclear defects and increased chromosome aneuploidies in spermatozoa with elongated heads. Hum. Reprod. 2007, 22, 1052–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehti, M.S.; Sironen, A. Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 2016, 151, R43–R54. Available online: https://rep.bioscientifica.com/view/journals/rep/151/4/R43.xml (accessed on 1 November 2021). [CrossRef] [Green Version]
- Liu, R.; Wan, Q.; Zhao, R.; Xiao, H.; Cen, Y.; Xu, X. Risk of non-melanoma skin cancer with biological therapy in common inflammatory diseases: A systemic review and meta-analysis. Cancer Cell Int. 2021, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, M.; Tavalaee, M.; Abbasi, H.; Nomikos, M.; Nasr-Esfahani, M.H. Increased de novo DNA Methylation Enzymes in Sperm of Individuals with Varicocele. Cell J. 2021, 23, 389–396. [Google Scholar] [PubMed]
- Eisermann, J.; Register, K.B.; Strickler, R.C.; Collins, J.L. The Effect of Tumor Necrosis Factor on Human Sperm MotilityIn Vitro. J. Androl. 1989, 10, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Villiger, P.M.; Caliezi, G.; Cottin, V.; Förger, F.; Senn, A.; Østensen, M. Effects of TNF antagonists on sperm characteristics in patients with spondyloarthritis. Ann. Rheum. Dis. 2010, 69, 1842–1844. [Google Scholar] [CrossRef] [PubMed]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, D.C.; Nassau, D.E.; Khodamoradi, K.; Ibrahim, E.; Blachman-Braun, R.; Ory, J.; Ramasamy, R. Sperm Parameters Before and After COVID-19 mRNA Vaccination. JAMA: J. Am. Med. Assoc. 2021, 326, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Taylor, T.H.; Messini, C.I.; Chatzimeletiou, K.; Daponte, A.; Ioannou, D.; Tempest, H.G. The Impact of SARS-CoV-2 on Sperm Cryostorage, Theoretical or Real Risk? Medicina 2021, 57, 946. [Google Scholar] [CrossRef] [PubMed]
- Moshrefi, M.; Ghasemi-Esmailabad, S.; Ali, J.; Findikli, N.; Mangoli, E.; Khalili, M.A. The probable destructive mechanisms behind COVID-19 on male reproduction system and fertility. J. Assist. Reprod. Genet. 2021, 38, 1691–1708. [Google Scholar] [CrossRef] [PubMed]

| Cryopreserved Sperm Sample during Therapy with Celecoxib Sulphasalazine Thawed 6 Years Post-Cryopreservation | Fresh Sperm Sample during Therapy with Golimumab before Vaccination | Fresh Sperm Sample during Current Therapy with Golimumab after Vaccination | |
|---|---|---|---|
| Volume | 4.3 mL | 3.2 mL | 3.5 mL |
| Number/mL | 47 × 106/ mL | 108 × 106/ mL | 142 × 106/ mL |
| Total Number/ejaculation | 202,100,000 | 345,600,000 | 497,000,000 |
| Motility | |||
| Linear progression | 53% | 82% | 85% |
| No progression—tail moving | 26% | 7% | 8% |
| Immotile | 21% | 11% | 7% |
| Morphology | |||
| Normal | 7% | 1% | 1% |
| Abnormal | 93% | 99% | 99% |
| Big head | 5 | 1 | 2 |
| Small head | 4 | 0 | 0 |
| Long head | 4 | 118 | 132 |
| Pear shaped head | 28 | 6 | 5 |
| Round head | 1 | 0 | 0 |
| Amorphous head | 18 | 11 | 8 |
| Vacuoles | 54 | 20 | 15 |
| Small acrosome | 2 | 1 | 1 |
| Short tail | 1 | 1 | 1 |
| Double tail | 3 | 1 | 2 |
| Fourchette | 2 | 1 | 1 |
| Broken tail | 1 | 1 | 1 |
| Spiral tail | 5 | 1 | 1 |
| Asymmetric tail extrusion | 1 | 1 | 1 |
| Broken neck | 14 | 5 | 4 |
| Cytoplasmic droplet | 32 | 17 | 14 |
| Thick mid piece | 25 | 15 | 12 |
| Round spermatids | 7 × 106/ mL | 1 × 106/ mL | 1 × 106/ mL |
| White cells | 2 × 106/ mL | - | - |
| DNA fragmentation | 9.6% | 7.6% | 6.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzimeletiou, K.; Fleva, A.; Sioga, A.; Georgiou, I.; Nikolopoulos, T.-T.; Markopoulou, M.; Petrogiannis, N.; Anifandis, G.; Patrikiou, A.; Kolibianakis, E.; et al. Effects of Different Drug Therapies and COVID-19 mRNA Vaccination on Semen Quality in a Man with Ankylosing Spondylitis: A Case Report. Medicina 2022, 58, 173. https://doi.org/10.3390/medicina58020173
Chatzimeletiou K, Fleva A, Sioga A, Georgiou I, Nikolopoulos T-T, Markopoulou M, Petrogiannis N, Anifandis G, Patrikiou A, Kolibianakis E, et al. Effects of Different Drug Therapies and COVID-19 mRNA Vaccination on Semen Quality in a Man with Ankylosing Spondylitis: A Case Report. Medicina. 2022; 58(2):173. https://doi.org/10.3390/medicina58020173
Chicago/Turabian StyleChatzimeletiou, Katerina, Alexandra Fleva, Antonia Sioga, Ioannis Georgiou, Theodoros-Thomas Nikolopoulos, Maria Markopoulou, Nikos Petrogiannis, George Anifandis, Antonios Patrikiou, Efstratios Kolibianakis, and et al. 2022. "Effects of Different Drug Therapies and COVID-19 mRNA Vaccination on Semen Quality in a Man with Ankylosing Spondylitis: A Case Report" Medicina 58, no. 2: 173. https://doi.org/10.3390/medicina58020173
APA StyleChatzimeletiou, K., Fleva, A., Sioga, A., Georgiou, I., Nikolopoulos, T.-T., Markopoulou, M., Petrogiannis, N., Anifandis, G., Patrikiou, A., Kolibianakis, E., Giannakou, A., & Grimbizis, G. (2022). Effects of Different Drug Therapies and COVID-19 mRNA Vaccination on Semen Quality in a Man with Ankylosing Spondylitis: A Case Report. Medicina, 58(2), 173. https://doi.org/10.3390/medicina58020173








