Diagnostic Value of Non-invasive Scoring Systems in the Prediction of Esophageal Varices in Patients with Liver Cirrhosis—Single Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Evaluation of EVs
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics
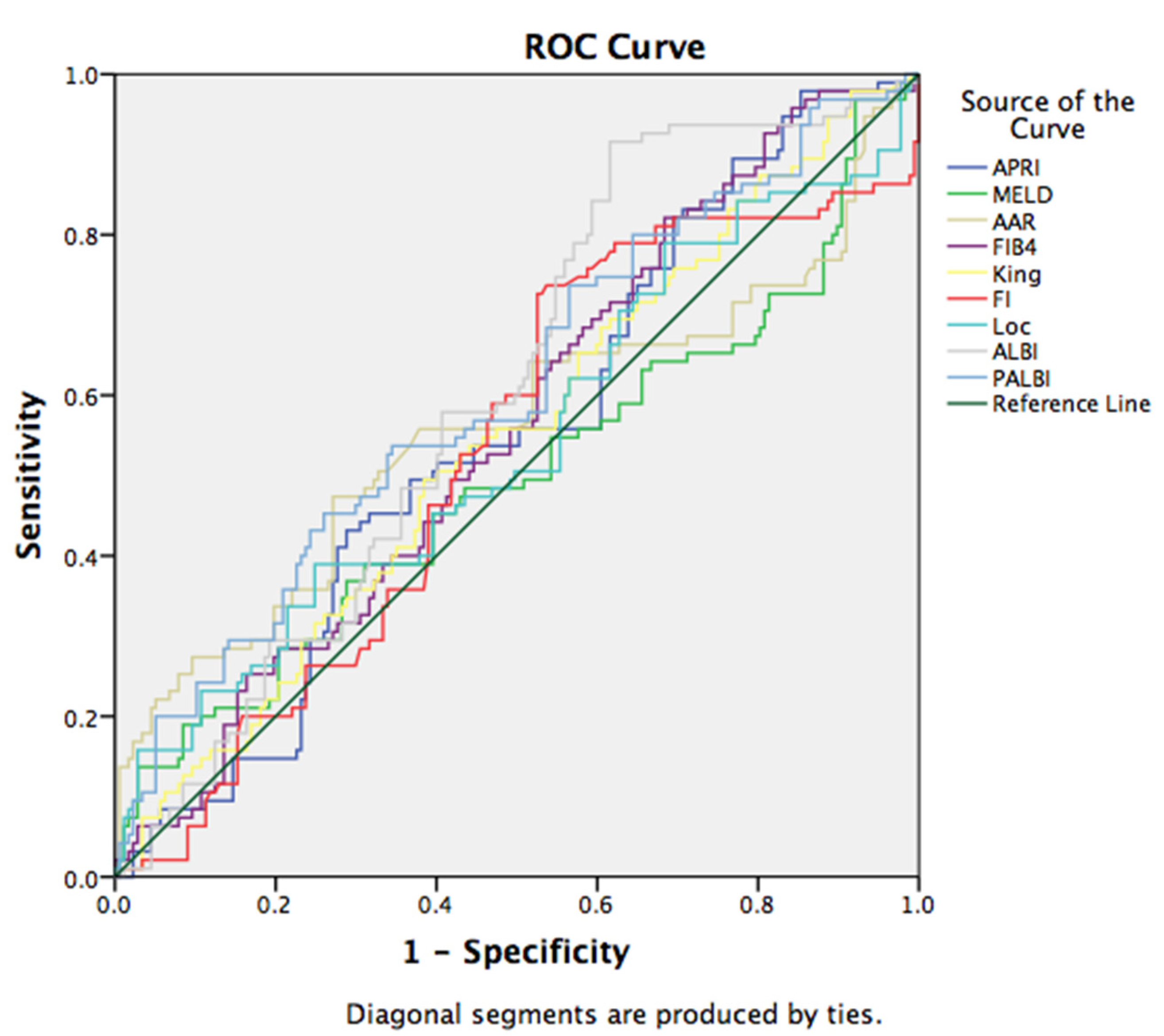
3.2. Non-Invasive Scores in Prediction of EV
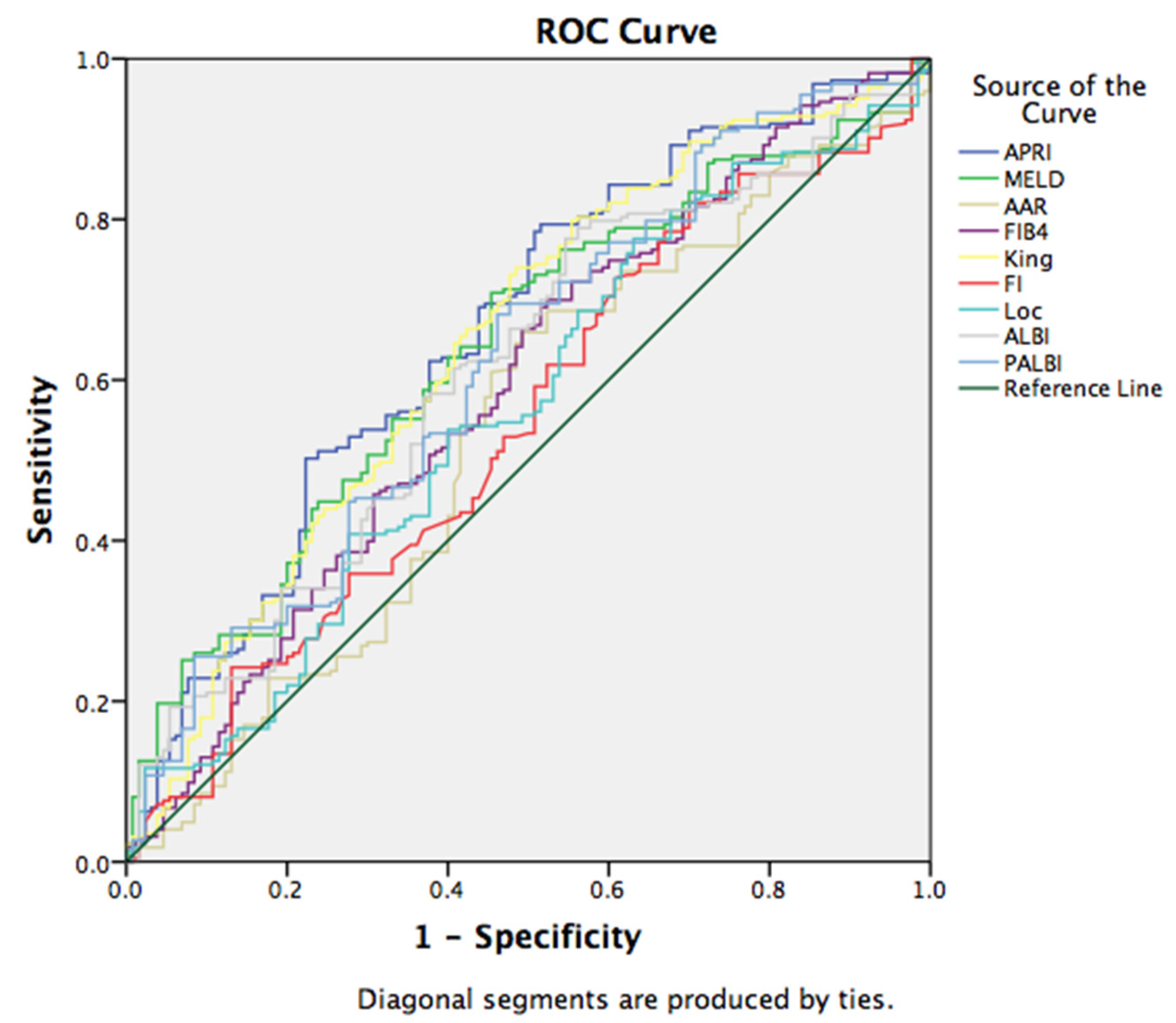
3.3. Non-Invasive Scores in Prediction of EV Bleeding
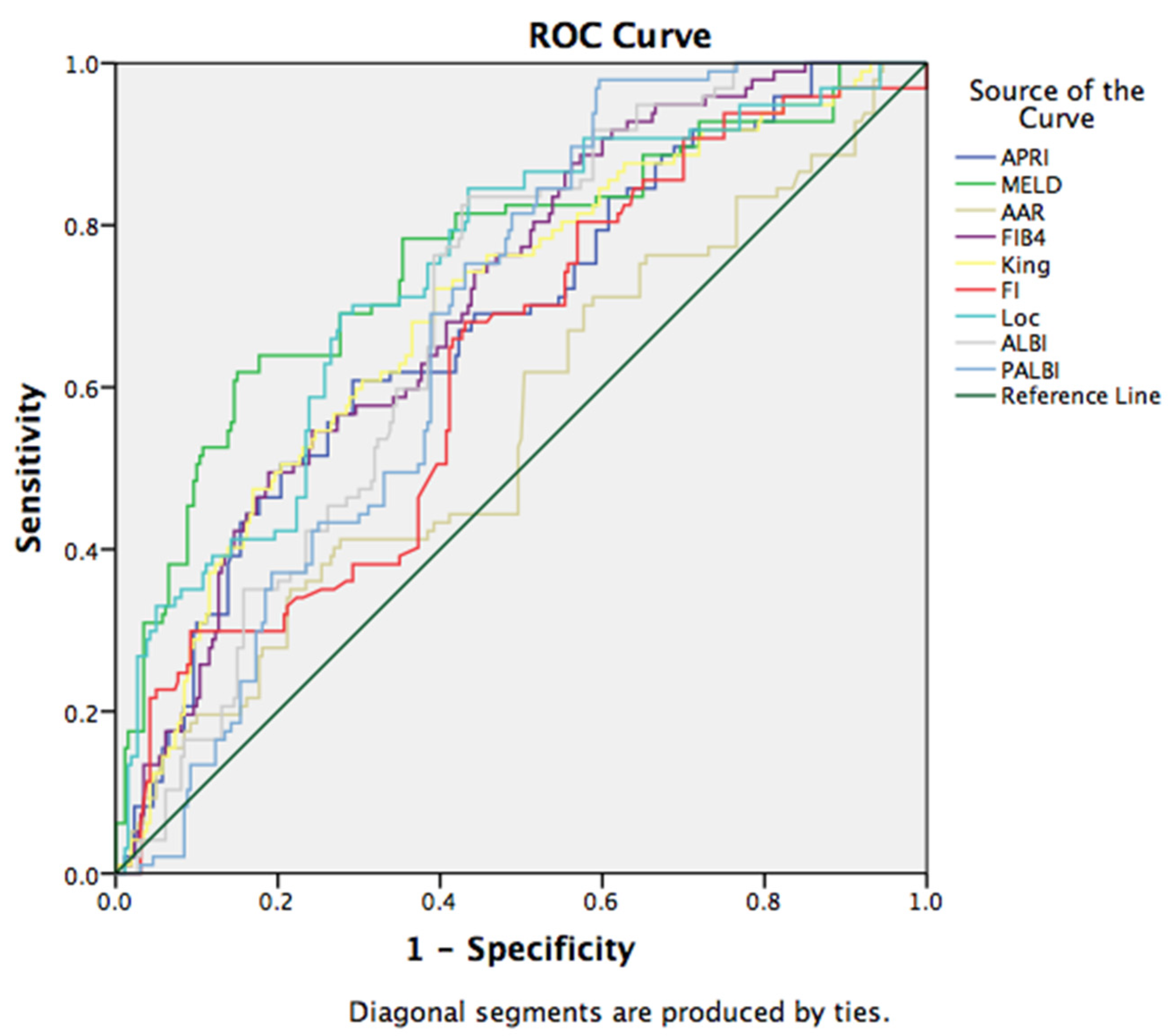
3.4. Non-Invasive Scores in Prediction of Short Term Mortality
4. Discussion
5. Limitation of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berzigotti, A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J. Hepatol. 2017, 67, 399–411. [Google Scholar] [CrossRef]
- Garbuzenko, D.V.; Arefyev, N.O. Primary prevention of bleeding from esophageal varices in patients with liver cirrhosis: An update and review of the literature. J. Evid. Based Med. 2020, 13, 313–324. [Google Scholar] [CrossRef]
- Garcia-Tsao, G. Current management of the complications of cirrhosis and portal hypertension: Variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 2001, 120, 726–748. [Google Scholar] [CrossRef]
- Triger, D.R.; Smart, H.L.; Hosking, S.W.; Johnson, A.G. Prophylactic sclerotherapy for esophageal varices: Long-term results of a singlecenter trial. Hepatology 1991, 13, 117–123. [Google Scholar] [CrossRef]
- Paquet, K.J.; Kalk, J.F.; Klein, C.P.; Gad, H.A. Prophylactic sclerotherapy for esophageal varices in high-risk cirrhotic patients selected by endoscopic and hemodynamic criteria: A randomized, singlecenter controlled trial. Endoscopy 1994, 26, 734–740. [Google Scholar] [CrossRef]
- Kim, D.J.; Choi, M.S. Life-sustaining treatment and palliative care in patients with liver cirrhosis—Legal, ethical, and practical issues. Clin. Mol. Hepatol. 2017, 23, 115–122. [Google Scholar] [CrossRef]
- De Franchis, R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W.D. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am. J. Gastroenterol. 2007, 102, 2086–2102. [Google Scholar] [CrossRef]
- Madhotra, R.; Mulcahy, H.E.; Willner, I.; Reuben, A. Prediction of esophageal varices in patients with cirrhosis. J. Clin. Gastroenterol. 2002, 34, 81–85. [Google Scholar] [CrossRef]
- Giannini, E.; Botta, F.; Borro, P.; Risso, D.; Romagnoli, P.; Fasoli, A.; Mele, M.R.; Testa, E.; Mansi, C.; Savarino, V.; et al. Platelet count/spleen diameter ratio: Proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut 2003, 52, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Giannini, E.G.; Zaman, A.; Kreil, A.; Floreani, A.; Dulbecco, P.; Testa, E.; Sohaey, R.; Verhey, P.; Peck-Radosavljevic, M.; Mansi, C.; et al. Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: Results of a multicenter, prospective, validation study. Am. J. Gastroenterol. 2006, 101, 2511–2519. [Google Scholar] [CrossRef]
- Sebastiani, G.; Tempesta, D.; Fattovich, G.; Castera, L.; Halfon, P.; Bourliere, M.; Noventa, F.; Angeli, P.; Saggioro, A.; Alberti, A. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: Results of a multicenter, largescale study. J. Hepatol. 2010, 53, 630–638. [Google Scholar] [CrossRef]
- Tafarel, J.R.; Tolentino, L.H.; Correa, L.M.; Bonilha, D.R.; Piauilino, P.; Martins, F.P.; Rodrigues, R.A.; Nakao, F.S.; Libera, E.D.; Ferrari, A.P.; et al. Prediction of esophageal varices in hepatic cirrhosis by noninvasive markers. Eur. J. Gastroenterol. Hepatol. 2011, 23, 754–758. [Google Scholar] [CrossRef]
- Deng, H.; Qi, X.; Peng, Y.; Li, J.; Li, H.; Zhang, Y.; Liu, X.; Sun, X.; Guo, X. Diagnostic Accuracy of APRI, AAR, FIB-4, FI, and King Scores for Diagnosis of Esophageal Varices in Liver Cirrhosis: A Retrospective Study. Med. Sci. Monit. 2015, 21, 3961–3977. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.D.; Zhu, Q.H.; Huang, Z.M.; Chen, X.R.; Jiang, Z.C.; Xu, S.H.; Jin, K. Predictors of esophageal varices in patients with HBV-related cirrhosis: A retrospective study. BMC Gastroenterol. 2009, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- González-Ojeda, A.; Cervantes-Guevara, G.; Chávez-Sánchez, M.; Dávalos-Cobián, C.; Ornelas-Cázares, S.; Macías-Amezcua, M.D.; Chávez-Tostado, M.; Ramírez-Campos, K.M.; Ramírez-Arce Adel, R.; Fuentes-Orozco, C. Platelet count/spleen diameter ratio to predict esophageal varices in Mexican patients with hepatic cirrhosis. World J. Gastroenterol. 2014, 20, 2079–2084. [Google Scholar] [CrossRef]
- Drinane, M.C.; Shah, V.H. Alcoholic Hepatitis: Diagnosis and Prognosis. Clin. Liver Dis. 2013, 2, 80. [Google Scholar] [CrossRef]
- Dominguez, M.; Rincon, D.; Gonzalez-Abraldes, J.; Miquel, R.; Colmenero, J.; Bellot, P.; Garcia-Pagan, J.C.; Fernández, R.; Moreno, M.; Bañares, R.; et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am. J. Gastroenterol. 2008, 103, 2747–2756. [Google Scholar] [CrossRef]
- Louvet, A.; Naveau, S.; Abdelnour, M.; Ramond, M.-J.; Diaz, E.; Fartoux, L.; Dhareancy, S.; Texier, F.; Hollebecque, A.; Serfaty, L.; et al. The Lille model: A new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007, 45, 1348–1354. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Roayaie, S.; Jibara, G.; Berhane, S.; Tabriz-ian, P.; Park, J.W.; Yang, J.; Yan, L.; Han, G.; Izzo, F.; Chen, M.; et al. 851 PALBI-An Objective Score Based on Platelets, Albumin Bilirubin Stratifies HCC Patients Undergoing Resection & Ablation Better than Child’s Classification. Hepatology 2015, 62, 624A–690A. [Google Scholar]
- Elshaarawy, O.; Samea, E.; Gomaa, A.; Allam, N.; Saad, M.; Waked, I. PALBI-the platelet-albumin-bilirubin score—A better predictor of outcome of acute variceal bleeding. J. Hepatol. 2017, 66, S564. [Google Scholar] [CrossRef]
- Kamath, P.S.; Kim, W.R. The model for end-stage liver disease (MELD). Hepatology 2007, 45, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.-F. A simple noninvasive index can pre dict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Giannini, E.; Risso, D.; Botta, F.; Chiarbonello, B.; Fasoli, A.; Malfatti, F.; Romagnoli, P.; Testa, E.; Ceppa, P.; Testa, R. Validity and Clinical Utility of the Aspartate Aminotransferase–Alanine Aminotransferase Ratio in Assessing Disease Severity and Prognosis in Patients With Hepatitis C Virus–Related Chronic Liver Disease. Arch. Intern. Med. 2003, 163, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accu rate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Ohta, T.; Sakaguchi, K.; Fujiwara, A.; Fujioka, S.-i.; Iwasaki, Y.; Makino, Y.; Araki, Y.; Shiratori, Y. Simple surrogate index of the fibro sis stage in chronic hepatitis C patients using platelet count and serum al bumin level. Acta Med. Okayama 2006, 60, 77–84. [Google Scholar]
- Cross, T.J.S.; Rizzi, P.; Berry, P.A.; Bruce, M.; Portmann, B.; Harrison, P.M. King’s Score: An accurate marker of cirrhosis in chronic hepatitis C. Eur. J. Gastroenterol. Hepatol. 2009, 21, 730–738. [Google Scholar] [CrossRef]
- Lok, A.S.; Ghany, M.G.; Goodman, Z.D.; Wright, E.C.; Everson, G.T.; Sterling, R.K.; Everhart, J.E.; Lindsay, K.L.; Bonkovsky, H.L.; Di Bisceglie, A.M.; et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: Results of the HALT-C cohort. Hepatology 2005, 42, 282–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal Hypertensive Bleeding in Cirrhosis: Risk Stratification, Diagnosis, and Management: 2016 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraja, B.; Mone, I.; Akshija, I.; Koçollari, A.; Prifti, S.; Burazeri, G. Predictors of esophageal varices and first variceal bleeding in liver cirrhosis patients. World J. Gastroenterol. 2017, 23, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Elshaarawy, O.; Allam, N.; Abdelsameea, E.; Gomaa, A.; Waked, I. Platelet-albumin-bilirubin score—A predictor of outcome of acute variceal bleeding in patients with cirrhosis. World J. Hepatol. 2020, 12, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Lee, K.C.; Wang, Y.W.; Yang, Y.Y.; Hou, M.C.; Huo, T.I.; Lin, H.C. Correlation and prognostic accuracy between noninvasive liver fibrosismarkers and portal pressure in cirrhosis: Role of ALBI score. PLoS ONE 2018, 13, e0208903. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, T.; Goulis, I.; Doumtsis, P.; Tzoumari, T.; Akriviadis, E.; Cholongitas, E. ALBI and PALBI Grades Are Associated with the Outcome of Patients with Stable Decompensated Cirrhosis. Ann. Hepatol. 2019, 18, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Qi, X.; Zhu, C.; Ning, Z.; Hou, F.; Zhao, J.; Peng, Y.; Li, J.; Deng, H.; Guo, X. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Turk. J. Gastroenterol. 2016, 27, 180–186. [Google Scholar] [CrossRef]
| MELD score = 9.57 × ln(Cr) + 3.78 × ln(TBIL) + 11.2 × ln (INR) + 6.43 |
| APRI = ((AST/UNL) × 100)/PLT |
| AAR = AST/ALT |
| FIB-4 = (age × AST)/PLT × ALT1/2 |
| FI = 8 − 0.01 × PLT-ALB |
| King = age × AST × INR/PLT LogOddsLok = (1.26 × AST/ALT) + (5.27 × INR) − (0.0089 × PLT) − 5.56 |
| Lok index = e(LogOddsLok)/(1 + e(LogOddsLok)) |
| ALBI = ((log10 bilirubin × 0.66) + (albumin × (−0.085))) |
| PALBI = 2.02 × log10 bilirubin − 0.37 × (log10 bilirubin)2 − 0.04 × albumin − 3.48 × log10 platelets + 1.01 × (log10 platelets)2 |
| ABIC = (age × 0.1) + (serum bilirubin × 0.08) + (serum creatinine × 0.3) + (INR × 0.8) |
| Variables | Total Patients (n = 386) |
|---|---|
| Sex (m/f) | 309/77 |
| Age (years) | 62.4 ± 13.14 |
| Etiology, n (%) | |
| Alcohol | 273 (70.7) |
| Hepatitis B virus | 17 (4.4) |
| Hepatitis C virus | 18 (4.7) |
| Autoimmune disease | 26 (6.7) |
| Wilson disease | 3 (0.8) |
| Toxic | 3 (0.8) |
| Cryptogenic | 52 (13.5) |
| Laboratory test | |
| Hb (g/L) a | 111.10 ± 81.62 |
| WBC (109/L) a | 9.35 ± 5.23 |
| PLT (109/L) a | 129.887 ± 85.65 |
| TBIL (mmol/L) b | 81 (100) |
| Alb (g/L) b | 27 (5) |
| AST (U/L) b | 82 (97) |
| ALT (U/L) b | 31 (27) |
| ALP (U/L) b | 156 (213) |
| GGT (U/L) b | 68 (1590) |
| BUN (mmol/ L) b | 13.2 (25) |
| Cr (µmol/L) b | 115 (268) |
| INR b | 1.7 (1.78) |
| D-dimer (mg/L) b | 6.18 (28.1) |
| CRP (mg/L) b | 43.5 (127) |
| Pct (ng/L) b | 3.0 (12.7) |
| Na (mmol/L) b | 136.20 ± 6.14 |
| K (mmol/L) b | 4.28 ± 0.92 |
| LDH (U/L) b | 675 (1168) |
| NH4 (µmol/L) b | 89 (59) |
| Cholesterol (mmol/L) | 3.30 ± 1.97 |
| Triglycerides (mmol/L) | 1.27 ± 0.82 |
| MELD score b | 16.4 (13.5) |
| APRI score b | 1.48 (2.64) |
| AAR score b | 1.9 (1.12) |
| FIB-4 score b | 6.15 (7.66) |
| King score b | 1036 (1841.28) |
| FI score b | 4.1 (1.33) |
| Loc score b | 4.08 (3.97) |
| ALBI score b | −1.11 (0.89) |
| PALBI score b | −1.54 (0.66) |
| ABIC score b | 8.43 (2.15) |
| Variables | With Varices Pts | Without Varices Pts | p Value |
|---|---|---|---|
| Hb (g/L) a | 114.61 ± 113.93 | 102.67 ± 23.45 | 0.041 |
| WBC (109/L) a | 9.715 ±5.52 | 9.612 ± 5.16 | 0.785 |
| Plt (109/L) a | 124.59± 65.95 | 142.51 ± 120.02 | 0.000 |
| TBil (mmol/L) b | 37.6 | 48.6 | 0.009 |
| Alb (g/L) b | 29.0 | 27.0 | 0.028 |
| AST (U/L) b | 47.0 | 66.0 | 0.006 |
| ALT (U/L) b | 27.0 | 28.5 | 0.238 |
| ALP (U/L) b | 88.0 | 110.5 | 0.024 |
| GGT (U/L) b | 77.0 | 82.5 | 0.010 |
| BUN (mmol/L) b | 10.3 | 8.4 | 0.184 |
| Cr (µmol/L) b | 81.0 | 73.0 | 0.009 |
| INR b | 1.49 | 1.48 | 0.965 |
| D-dimer (mg/L) b | 3.45 | 5.57 | 0.007 |
| CRP (mg/L) b | 14.85 | 30.9 | 0.056 |
| Pct (ng/L) b | 0.57 | 1.32 | 0.282 |
| Na (mmol/L) | 136.76 ± 5.92 | 136.02 ± 6.75 | 0.005 |
| LDH (U/L) b | 451.0 | 591.5 | 0.000 |
| NH4 (µmol/L) b | 59.0 | 81.0 | 0.091 |
| Cholesterol (mmol/L) | 3.238 ± 1.30 | 2.717 ± 1.31 | 0.699 |
| Triglycerides (mmol/L) | 1.145 ± 0.52 | 1.382 ± 1.17 | 0.002 |
| MELD score b | 14.79 | 16.53 | 0.049 |
| APRI score b | 1.30 | 1.51 | 0.132 |
| AAR score b | 1.78 | 2.08 | 0.037 |
| FIB-4 score b | 5.55 | 6.91 | 0.047 |
| King score b | 887.9 | 1085.7 | 0.171 |
| ALBI score b | −1.29 | −1.12 | 0.002 |
| PALBI score b | −1.67 | −1.52 | 0.005 |
| ABIC score b | 7.93 | 8.59 | 0.815 |
| Etiology | Pts with Varices (%) | Pts without Varices (%) | p |
|---|---|---|---|
| Alcohol | 118 (59.0%) | 82 (41.0) | 0.010 |
| HBV cirrhosis | 10 (76.9) | 3 (23.1) | 0.242 |
| HCV cirrhosis | 14 (87.5) | 2 (12.5) | 0.033 |
| Autoimmune disease | 16 (69.6) | 7 (30.4) | 0.359 |
| Wilson disease | 2 (100) | 0 (0) | 0.406 |
| Toxic disease | 0 (0) | 1 (100) | 0.362 |
| Cryptogenic disease | 35 (76.1) | 11 (23.9) | 0.040 |
| Scores | Pts with Variceal Bleeding | Pts without Variceal Bleeding | p |
|---|---|---|---|
| APRI | 3.35 | 1.87 | 0.000 |
| MELD | 18.95 | 17.15 | 0.000 |
| AAR | 3.12 | 1.92 | 0.887 |
| FIB-4 | 13.75 | 6.83 | 0.109 |
| King | 1289.39 | 1179.46 | 0.000 |
| ALBI | −1.00 | −1.09 | 0.027 |
| PALBI | −1.48 | −1.52 | 0.000 |
| ABIC | 8.39 | 8.49 | 0.627 |
| B | S.E. | Wald | df | Sig. | Exp (B) | 95% CI for EXP (B) | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Step 1 | Na Constant | 0.111 −15.756 | 0.022 3.031 | 25.269 27.032 | 1 1 | 0.000 0.000 | 1.117 0.000 | 1.070 | 1.167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glisic, T.; Stojkovic Lalosevic, M.; Milovanovic, T.; Rankovic, I.; Stojanovic, M.; Toplicanin, A.; Aleksic, M.; Milivojevic, V.; Martinov Nestorov, J.; Lolic, I.; et al. Diagnostic Value of Non-invasive Scoring Systems in the Prediction of Esophageal Varices in Patients with Liver Cirrhosis—Single Center Experience. Medicina 2022, 58, 158. https://doi.org/10.3390/medicina58020158
Glisic T, Stojkovic Lalosevic M, Milovanovic T, Rankovic I, Stojanovic M, Toplicanin A, Aleksic M, Milivojevic V, Martinov Nestorov J, Lolic I, et al. Diagnostic Value of Non-invasive Scoring Systems in the Prediction of Esophageal Varices in Patients with Liver Cirrhosis—Single Center Experience. Medicina. 2022; 58(2):158. https://doi.org/10.3390/medicina58020158
Chicago/Turabian StyleGlisic, Tijana, Milica Stojkovic Lalosevic, Tamara Milovanovic, Ivan Rankovic, Marija Stojanovic, Aleksandar Toplicanin, Marko Aleksic, Vladimir Milivojevic, Jelena Martinov Nestorov, Iva Lolic, and et al. 2022. "Diagnostic Value of Non-invasive Scoring Systems in the Prediction of Esophageal Varices in Patients with Liver Cirrhosis—Single Center Experience" Medicina 58, no. 2: 158. https://doi.org/10.3390/medicina58020158
APA StyleGlisic, T., Stojkovic Lalosevic, M., Milovanovic, T., Rankovic, I., Stojanovic, M., Toplicanin, A., Aleksic, M., Milivojevic, V., Martinov Nestorov, J., Lolic, I., & Popovic, D. D. (2022). Diagnostic Value of Non-invasive Scoring Systems in the Prediction of Esophageal Varices in Patients with Liver Cirrhosis—Single Center Experience. Medicina, 58(2), 158. https://doi.org/10.3390/medicina58020158






