Predictive Factors for the Prognosis of Alcoholic Liver Cirrhosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. First Admission
3.2. Multiple Admissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeverino-Gutiérrez, M.L.; González-González, M.D.R.; González-Santiago, O. Mortality From Alcohol-Related Liver Cirrhosis in Mexico (2000–2017). Front. Public Health 2020, 8, 524356. [Google Scholar] [CrossRef]
- Julien, J.; Ayer, T.; Bethea, E.D.; Tapper, E.B.; Chhatwal, J. Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019-40: A modelling study. Lancet Public Health 2020, 5, e316–e323. [Google Scholar] [CrossRef]
- Termeie, O.; Fiedler, L.; Martinez, L.; Foster, J.; Perumareddi, P.; Levine, R.S.; Hennekens, C.H. Alarming Trends: Mortality from Alcoholic Cirrhosis in the United States. Am. J. Med. 2022, 135, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Cholankeril, G.; Ahmed, A. Alcoholic Liver Disease Replaces Hepatitis C Virus Infection as the Leading Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2018, 16, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Basra, S.; Anand, B.S. Definition, epidemiology and magnitude of alcoholic hepatitis. World J. Hepatol. 2011, 3, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Parikh, N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: Observational study. BMJ 2018, 362, k2817. [Google Scholar] [CrossRef]
- Mellinger, J.L.; Shedden, K.; Winder, G.S.; Tapper, E.; Adams, M.; Fontana, R.J.; Volk, M.L.; Blow, F.C.; Lok, A.S. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology 2018, 68, 872–882. [Google Scholar] [CrossRef]
- Silva, J.M.; Silva, M.J.; Calinas, F.; Nogueira, P.J. Burden of Liver Cirrhosis in Portugal between 2010 and 2017. J. Gastroenterol. 2021, 28, 153–161. [Google Scholar] [CrossRef]
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef]
- World Health Organization-Global Status Report on Alcohol and Health. 2018. Available online: https://www.who.int/substance_abuse/publications/global_alcohol_report/en (accessed on 19 March 2021).
- OECD. State of Health in the EU; România: European Observatory on Health Systems and Policies: Brussels, Belgium, 2019. [Google Scholar]
- GBD 2017 Cirrhosis CollaboratorsThe global, regional, and national burden of cirrhosis by cause in 195 countries and rerritories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [CrossRef]
- Reddy, S.R.; Mouchli, M.; Summey, R.; Walsh, C.; Mir, A.; Bierle, L.; Rubio, M.G. Outcomes of Young Patients With Alcoholic Cirrhosis After First Hospitalization for Cirrhosis: A Carilion Clinic Experience. Cureus 2021, 13, e16695. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hortlik, H.; Erasmus, H.-P.; Schaaf, L.; Zeleke, Y.; Uschner, F.E.; Ferstl, P.; Schulz, M.; Peiffer, K.H.; Queck, A.; et al. Trends and the course of liver cirrhosis and its complications in Germany: Nationwide population based study (2005 to 2018). Lancet Reg. Health-Eur. 2022, 12, 100240. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; Rodrigues, S.; Silva, M.; Costa-Moreira, P.; Morais, R.; Andrade, P.; Cardoso, H.; Albuquerque, A.; Liberal, R.; Macedo, G. Predictive models of mortality and hospital readmission of patients with decompensated liver cirrhosis. Dig. Liver Dis. 2019, 51, 1423–1429. [Google Scholar] [CrossRef]
- European Association for the study of the liver-EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef]
- Bhattarai, S. Complications and Mortality in Hospitalised Patients With Decompensated Cirrhosis of Liver in a Tertiary Care Centre in Nepal. Cureus 2020, 12, e9996. [Google Scholar] [CrossRef]
- Seraj, S.M.; Campbell, E.J.; Argyropoulos, S.K.; Wegermann, K.; Chung, R.T.; Richter, J.M. Hospital readmissions in decompensated cirrhotics: Factors pointing toward a prevention strategy. World J. Gastroenterol. 2017, 23, 6868–6876. [Google Scholar] [CrossRef]
- Scaglione, S.J.; Metcalfe, L.; Kliethermes, S.; Vasilyev, I.; Tsang, R.; Caines, A.; Mumtaz, S.; Goyal, V.; Khalid, A.; Shoham, D.; et al. Early hospital readmissions and mortality in patients with decompensated cirrhosis enrolled in a large national health insurance administrative database. J. Clin. Gastroenterol. 2017, 51, 839–844. [Google Scholar] [CrossRef]
- Choi, C.; Choi, D.H.; Spears, G.M.; Serafim, L.P.; Gajic, O.; Kamath, P.S.; Shah, V.H.; de Moraes, A.G.; Simonetto, D.A. Relationship Between Etiology of Cirrhosis and Survival Among Patients Hospitalized in Intensive Care Units. Mayo Found. Med. Educ. Res. Mayo Clin. Proc. 2021, 97, 274–284. [Google Scholar] [CrossRef]
- Bhattacharyya, M.; Barman, N.N.; Goswami, B. Clinical profile of cirrhosis of liver in a tertiary care hospital of Assam, North East India. IOSR-JDMS 2016, 15, 21–27. [Google Scholar]
- Wu, S.L.; Zheng, Y.X.; Tian, Z.W.; Chen, M.S.; Tan, H.Z. Prediction of mortality in decompensated liver cirrhosis. World J. Clin. Cases 2018, 6, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Cholongitas, E.; Papatheodorids, G.V.; Vangeli, M.; Terreni, N.; Patch, D.; Burroughs, A.K. Systematic review: The model for endstage liver disease-should it replace Child-Pugh’s classification for assessing prognosisin cirrhosis? Aliment. Pharm. 2005, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.; Chopra, S. Cirrhosis in Adults: Etiologies, Clinical Manifestations, and Diagnosis; Up To Date Inc.: Waltham, MA, USA, 2016. [Google Scholar]
- Papatheodoridis, G.V.; Cholongitas, E.; Dimitriadoue, E.; Touloumi, G.; Sevastianos, V.; Archimandritis, A.J. MELD versus Child-Pugh and creatinine-modified Child-Pugh score for predicting survival in patients with decompensated cirrhosis. World J. Gastroenterol. 2005, 11, 3099–3104. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Shasthry, S.M.; Choudhury, A.K.; Maiwall, R.; Kumar, G.; Bharadwaj, A.; Arora, V.; Vijayaraghavan, R.; Jindal, A.; Sharma, M.K.; et al. Alcohol associated liver cirrhotics have higher mortality after index hospitalization: Long-term data of 5.138 patients. Clin. Mol. Hepatol. 2021, 27, 175. [Google Scholar] [CrossRef] [PubMed]
- Dupont, B.; Delvincourt, M.; Koné, M.; Du Cheyron, D.; Ollivier-Hourmand, I.; Piquet, M.A.; Terzi, N.; Dao, T. Retrospective evaluation of prognostic score performances in cirrhotic patients admitted to an intermediate care unit. Dig. Liver Dis. 2015, 47, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Abbasy, M.; Zaghla, H.; Elhelbawy, M.; Ramadan, M.; Zakareya, T. Predicting in-hospital mortality of cirrhotic patients hospitalized with hepatic encephalopathy. Egypt Liver J. 2022, 12, 13. [Google Scholar]
- Fayad, L.; Narciso-Schiavon, J.L.; Lazzarotto, C.; Ronsoni, M.F.; Wildner, L.M.; Bazzo, M.L.; de Lucca Schiavon, L.; Dantas-Corrêa, E.B. The performance of prognostic models as predictors of mortality in patients with acute decompensation of cirrhosis. Ann. Hepatol. 2015, 14, 83–92. [Google Scholar]
- Radisavljevic, M.M.; Bjelakovic, G.B.; Nagorni, A.V.; Stojanovic, M.P.; Radojkovicn, M.D.; Jovic, J.Z.; Ignjatovic, A.M.; Radisavljevic, M.M.; Simonovic, M.M. Predictors of Mortality in Long-Term Follow-Up of Patients with Terminal Alcoholic Cirrhosis: Is It Time to Accept Remodeled Scores. Med. Princ. Pract. 2017, 26, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, S.; Oh, S.Y.; Shin, J.; Kim, J.; Jung, C.W.; Ryu, H.G. Comparison of APACHE IV with APACHE II, SAPS 3, MELD, MELD-Na, and CTP scores in predicting mortality after liver transplantation. Sci. Rep. 2017, 7, 10884. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.; Jan, I. Analysis of mortality prognostic factors using model for end-stage liver disease with incorporation of serum-sodium classification for liver cirrhosis complications A retrospective cohort study. Medicine 2019, 98, 45. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, C.W.; Choi, J.Y.; Lee, C.D.; Lee, S.H.; Kim, M.Y.; Jang, B.K.; Woo, H.Y. Complications Requiring Hospital Admission and Causes of In-Hospital Death over Time in Alcoholic and Nonalcoholic Cirrhosis Patients. Gut Liver 2016, 10, 95–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dos Santos, S.G.R.; Mattos, A.A.; Guimarães, M.M.; Boger, B.D.S.; Coral, G.P. Alcohol Consumption Influences Clinical Outcome in Patients Admitted to a Referral Center for Liver Disease. Ann. Hepatol. 2018, 17, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Morales, B.P.; Planas, R.; Bartoli, R.; Morillas, R.M.; Sala, M.; Casas, I.; Armengol, C.; Masnou, H. HEPACONTROL. A program that reduces early readmissions, mortality at 60 days, and healthcare costs in decompensated cirrhosis. Dig. Liver Dis. 2018, 50, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Morales, B.P.; Planas, R.; Bartoli, R.; Morillas, R.M.; Sala, M.; Cabré, E.; Casas, I.; Masnou, H. 2017. Early hospital readmission in decompensated cirrhosis: Incidence, impact on mortality, and predictive factors. Dig Liver Dis. 2017, 49, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Reddy, S.; Mouchli, M.; Summey, R.; Walsh, C.; Mir, A.; Bierle, L.; Rubio, M. Gender-Specific Risk Factors Contributing to Mortality in Patients Hospitalized With Alcoholic Cirrhosis. Cureus 2021, 13, e16271. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, E.; Garcovich, M.; Borzio, M.; Leandro, G.; Majumdar, A.; Tsami, A.; Arvaniti, V.; Roccarina, D.; Pinzani, M.; Burroughs, A.K.; et al. Bacterial infections change natural history of cirrhosis irrespective of liver disease severity. Am. J. Gastroenterol. 2017, 112, 588–596. [Google Scholar] [CrossRef]
- Fernández, J.; Acevedo, J.; Wiest, R.; Gustot, T.; Amoros, A.; Deulofeu, C.; Reverter, E.; Martínez, J.; Saliba, F.; Jalan, R.; et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2018, 67, 1870–1880. [Google Scholar] [CrossRef]
- Prieto-Ortiz, J.E.; Garzón-Orjuela, N.; Sánchez-Pardo, S.; Prieto-Ortiz, R.G.; Eslava-Schmalbach, J. Survival in Patients with Cirrhosis According to Etiology. Retrospective Cohort. Rev. Colomb. Gastroenterol. 2022, 37, 24–32. [Google Scholar] [CrossRef]
- Piano, S.; Brocca, A.; Mareso, S.; Angeli, P. Infections complicating cirrhosis. Liver Int. 2018, 38, 126–133. [Google Scholar] [CrossRef]
- Grgurevic, I.; Trkulja, V.; Bozin, T.; Madir, A.; Miletic, M.; Marusic, S.; Skrlin, J.; Sestan Crnek, S.; Dobrovic, K. Infection as a predictor of mortality in decompensated liver cirrhosis: Exploring the relationship to severity of liver failure. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1458–1465. [Google Scholar] [CrossRef]
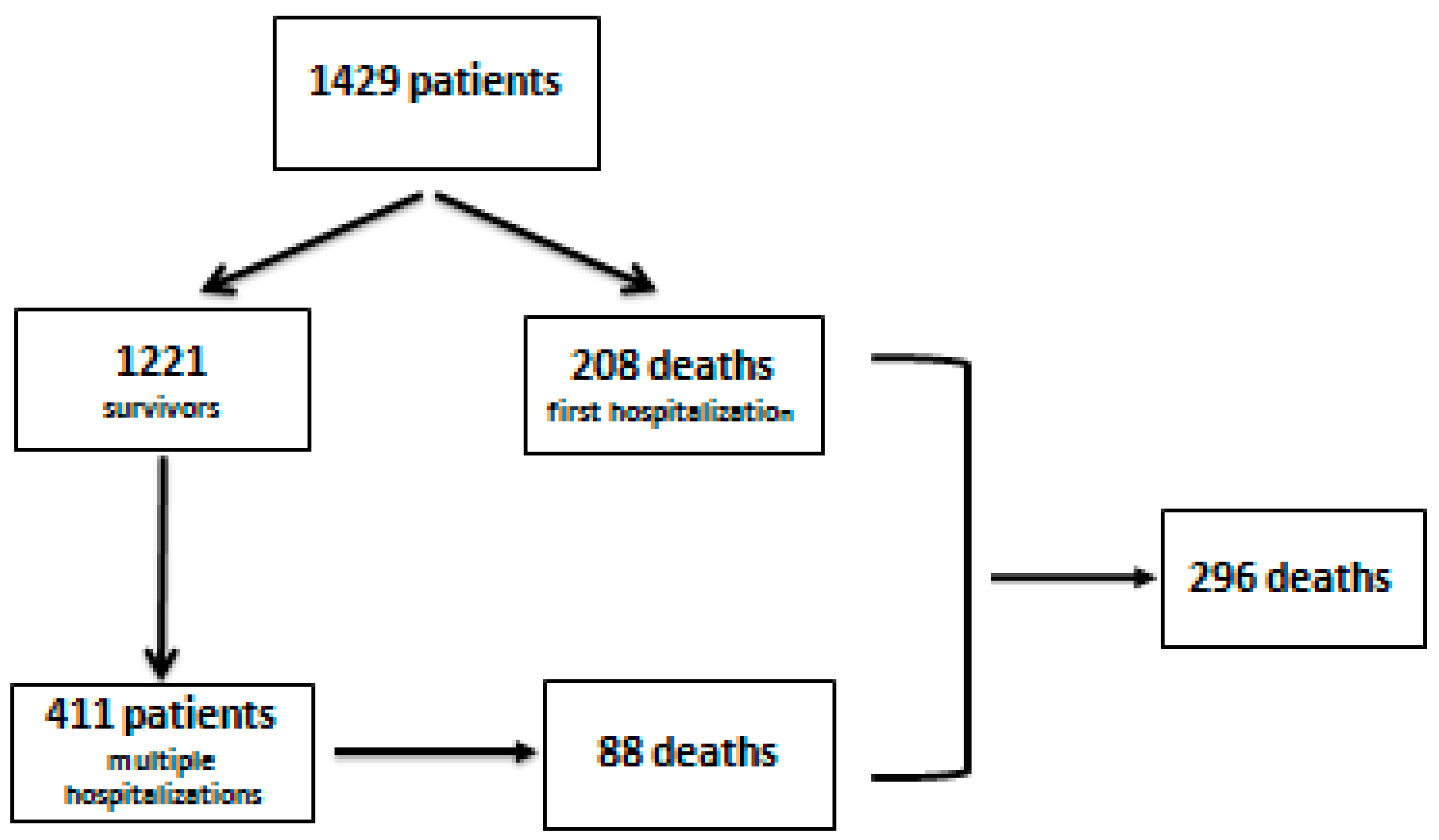

| Variables | Patients | Survivors | Deceased | p |
|---|---|---|---|---|
| Patients n (%) | 1429 (100%) | 1133 (79.3%) | 296 (20.7%) | - |
| Age (average ± SD) | 56.15 ± 11.49 | 56.05 ± 11.61 | 55.18 ± 10.77 | 0.079 |
| (limits/median) | (25–92/57) | (25–92/57) | (27–83/58) | |
| Gender | 0.625 | |||
| Male, n (%) | 968 (67.7%) | 771 (68.0%) | 197 (66.6%) | |
| Female, n (%) | 461 (32.3%) | 362 (32.0%) | 99 (33.4%) | |
| Residence | 0.506 | |||
| Urban, n (%) | 623 (43.6%) | 499 (44.0%) | 124 (41.9%) | |
| Rural, n (%) | 806 (56.4%) | 634 (55.0%) | 172 (58.1%) | |
| Etiology | ||||
| Alcohol, n (%) | 1159 (81.1%) | 903 (79.7%) | 256 (86.5%) | |
| Alcohol + B virus, n (%) | 129 (9.0%) | 112 (9.9%) | 17 (5.7%) | 0.024 |
| Alcohol + C virus, n (%) | 141 (9.9%) | 118 (10.4%) | 23 (7.8%) | |
| Complication | ||||
| Ascites,n (%) | 589 (41.2%) | 445 (39.3%) | 144 (48.6%) | 0.004 |
| Jaundice,n (%) | 191 (13.4%) | 142 (12.5%) | 49 (16.6%) | 0.070 |
| Digestive bleeding | ||||
| Variceal, n (%) | 292 (20.5%) | 197 (17.4%) | 95 (32.1%) | <0.001 |
| Non-variceal, n (%) | 109 (7.6%) | 88 (7.8%) | 21 (7.1%) | 0.698 |
| Infections,n (%) | 209 (14.6%) | 97 (8.6%) | 112 (37.8%) | <0.001 |
| SBP, n (%) | 87 (6.1%) | 32 (2.8%) | 55 (18.6%) | <0.001 |
| Sepsis, n (%) | 63 (4.4%) | 16 (1.4%) | 47 (15.9%) | <0.001 |
| Other infections,n (%) | 90 (6.3%) | 56 (4.9%) | 34 (11.5%) | <0.001 |
| Encephalopathy,n (%) | 713 (49.9%) | 504 (44.5%) | 209 (70.6%) | <0.001 |
| Hepatic carcinoma,n (%) | 55 (3.8%) | 34 (3.0%) | 21 (7.1%) | 0.001 |
| Hepatorenal syndrome,n (%) | 59 (4.1%) | 25 (2.2%) | 34 (11.5%) | <0.001 |
| Acute alcoholic hepatitis,n (%) | 112 (7.8%) | 89 (7.9%) | 23 (7.8%) | 0.961 |
| Prognostic score | ||||
| CTP Class | ||||
| An (%) | 207 (14.5%) | 205 (18.1%) | 2 (0.7%) | <0.001 |
| Bn (%) | 487 (34.1%) | 451 (39.8%) | 36 (12.2%) | <0.001 |
| Cn (%) | 735 (51.4%) | 477 (42.1%) | 258 (87.2%) | <0.001 |
| CTP (average ± SD) | 9.37 ± 2.50 | 8.91 ± 2.37 | 12.10 ± 1.33 | <0.001 |
| (limits/median) | (5–15/10) | (5–15/9) | (6–15/12) | |
| MELD-Na (average ± SD) | 17.41 ± 7.72 | 15.52 ± 7.08 | 24.94 ± 6.28 | <0.001 |
| (limits/median) | (5–43/17) | (5–40/15) | (8–43/25) |
| Variables | Deceased (n = 208) | Survivors (n = 1221) | Odds Ratio (CI 95%) | p |
|---|---|---|---|---|
| Variceal bleedingn (%) | 73 (35%) | 219 (18.0%) | 2.47 (1.80–3.41) | <0.001 |
| Non-variceal bleedingn (%) | 14 (6.8%) | 95 (7.8%) | 0.86 (0.48–1.53) | 0.527 |
| Infectionsn (%) | 100 (49.0%) | 109 (8.9%) | 9.45 (6.75–13.21) | <0.001 |
| SBPn (%) | 53 (25.5%) | 34 (2.8%) | 11.94 (7.52–18.95) | <0.001 |
| Sepsisn (%) | 47 (22.6%) | 16 (1.3%) | 21.98 (12.18–39.69) | <0.001 |
| Other infectionsn (%) | 25 (12.0%) | 65 (5.3%) | 2.43 (1.41–2.91) | <0.001 |
| Hepatic encephalopathyn (%) | 155 (74.5%) | 558 (45.7%) | 3.47 (2.49–4.84) | <0.001 |
| Ascitesn (%) | 103 (49.5%) | 486 (39.8%) | 1.48 (1.10–1.99) | 0.009 |
| Jaundicen (%) | 33 (15.9%) | 159 (13.0%) | 1.26 (0.84–1.89) | 0.267 |
| Hepatocellular carcinoman (%) | 21 (10.1%) | 34 (2.8%) | 3.92 (2.23–6.90) | <0.001 |
| Acute alcoholic hepatitisn (%) | 13 (6.3%) | 99 (8.1%) | 0.76 (0.42–1.37) | 0.919 |
| Hepatorenal syndromen (%) | 34 (16.3%) | 25 (2.0%) | 9.35 (5.45–16.07) | <0.001 |
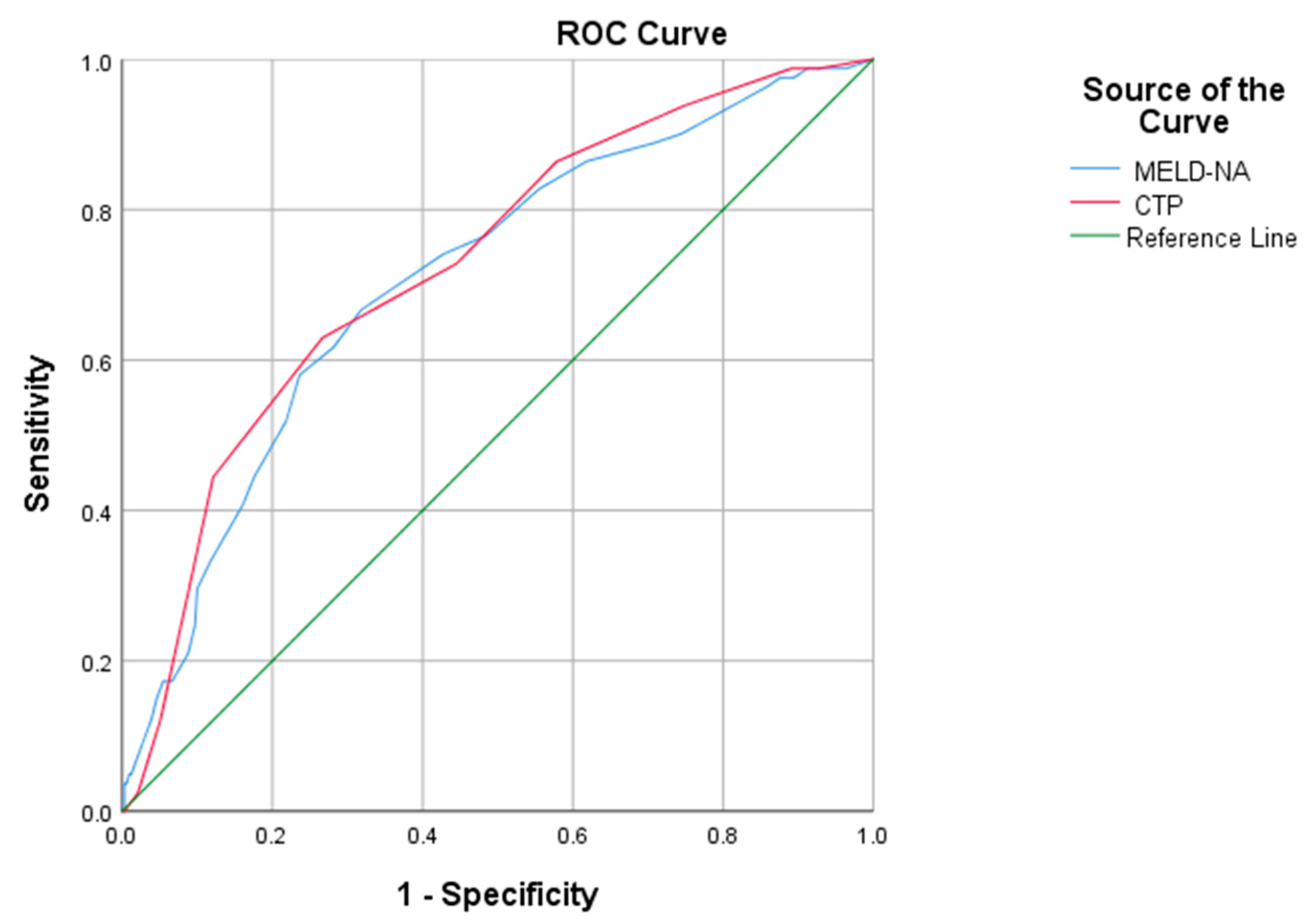
| Scor | AUROC | p | CI 95% | Cutoff | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| CTP | 0.876 | 0.001 | 0.854–0.898 | 10.5 | 89% | 64% | 31% | 63% |
| MELD-Na | 0.822 | 0.001 | 0.794–0.850 | 19 | 88% | 83% | 58% | 64% |
| Variabile | Deceased (n = 88) | Survivors (n = 323) | Odds Ratio | CI 95% | p |
|---|---|---|---|---|---|
| Non-variceal bleedingn (%) | 6 (6.8%) | 21 (6.5%) | 1.05 | 0.41–2.69 | 0.915 |
| Variceal bleedingn (%) | 39 (44.3%) | 74 (22.9%) | 2.68 | 1.63–4.39 | 0.001 |
| Infectionsn (%) | 56 (63.6%) | 55 (17.0%) | 8.53 | 5.06–14.38 | <0.001 |
| SBPn (%) | 34 (38.6%) | 17 (5.3%) | 11.33 | 5.92–21.71 | <0.001 |
| Sepsisn (%) | 20 (22.7%) | 5 (1.5%) | 18.71 | 6.78–51.58 | <0.001 |
| Other infectionsn (%) | 13 (14.8%) | 38 (11.8%) | 1.30 | 0.70–2.56 | 0.449 |
| Hepatic encephalopathyn (%) | 72 (81.8%) | 198 (61.3%) | 2.84 | 1.58–5.11 | <0.001 |
| Ascitesn (%) | 54 (61.4%) | 182 (56.3%) | 1.23 | 0.78–1.99 | 0.843 |
| Jaundicen (%) | 17 (19.3%) | 38 (11.8%) | 1.79 | 0.96–3.37 | 0.068 |
| Hepatocellular carcinoman (%) | 6 (6.8%) | 30 (9.3%) | 0.71 | 0.29–1.78 | 0.450 |
| Hepatorenal syndromen (%) | 23 (26.1%) | 16 (5.0%) | 6.79 | 3.40–13.56 | <0.001 |
| Acute alcoholic hepatitisn (%) | 3 (3.4%) | 14 (4.3%) | 0.78 | 0.22–2.77 | 0.385 |
| Score | AUROC | p | CI 95% | Cutoff | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| CTP | 0.726 | 0.001 | 0.666–0.787 | 10.5 | 86% | 57.9% | 19.7% | 80.29% |
| MELD-Na | 0.709 | 0.001 | 0.647–0.772 | 19 | 82.7% | 55.5% | 19.7% | 80.29% |
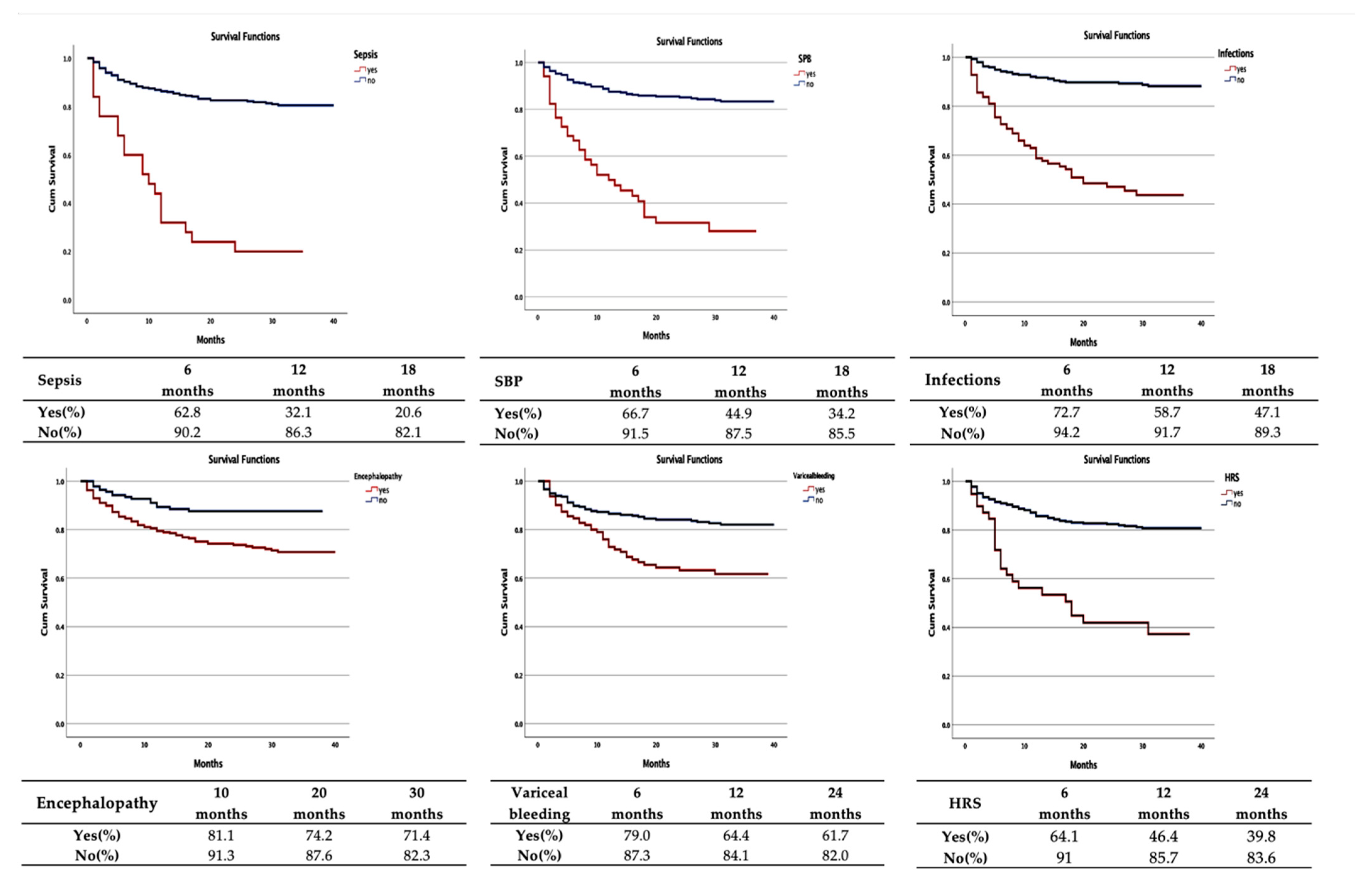
| Variables | Deaths | Average Survival Period/ CI 95% (Months) | Log-Rank Test |
|---|---|---|---|
| Patients | 88 | - | - |
| Variceal bleeding | 39 | 27.8 (24.9–30.7) | <0.001 |
| Infections | 56 | 21.4 (18.5–24.3) | <0.001 |
| SBP | 34 | 16.9 (12.9–20.9) | <0.001 |
| Sepsis | 20 | 13.4 (8.7–18.2) | <0.001 |
| Hepatic encephalopathy | 72 | 31.2 (29.4–32.9) | <0.001 |
| Ascites | 54 | 32.3 (30.4–34.1) | 0.464 |
| Other infections | 13 | 28.2 (24.3–32.2) | 0.296 |
| Jaundice | 17 | 32.3 (27.9–36.6) | 0.055 |
| Non-variceal bleeding | 6 | 28.2 (24.9–32.7) | 0.990 |
| Hepatorenal syndrome | 23 | 20.2 (15.3–25.0) | <0.001 |
| Hepatocelular carcinoma | 6 | 32.6 (29.3–35.9) | 0.366 |
| Acute alcoholic hepatitis | 3 | 305 (25.6–35.4) | 0.670 |
| Authors | Score | AUROC | Cutoff | Sensitivity | Specificity | PPV | NVP |
|---|---|---|---|---|---|---|---|
| Radisavljevic et al., 2017 [31] | CTP | 0.861 | 11.50 | 85% | 66.7% | - | - |
| MELD-Na | 0.814 | 22.1 | 83.3% | 70% | - | - | |
| Abbasy et al., 2022 [29] | CTP | 0.709 | 9 | 90.7% | 29.13% | 54.7% | 76.9% |
| MELD-Na | 0.717 | 21.93 | 94.5% | 40.7% | 57.5% | 87.5% | |
| Fayad et al., 2015 [30] | MELD-Na | 0.781 | 20 | 70% | 79% | 49% | 90% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifan, A.; Minea, H.; Rotaru, A.; Stanciu, C.; Stafie, R.; Stratina, E.; Zenovia, S.; Nastasa, R.; Singeap, A.-M.; Girleanu, I.; et al. Predictive Factors for the Prognosis of Alcoholic Liver Cirrhosis. Medicina 2022, 58, 1859. https://doi.org/10.3390/medicina58121859
Trifan A, Minea H, Rotaru A, Stanciu C, Stafie R, Stratina E, Zenovia S, Nastasa R, Singeap A-M, Girleanu I, et al. Predictive Factors for the Prognosis of Alcoholic Liver Cirrhosis. Medicina. 2022; 58(12):1859. https://doi.org/10.3390/medicina58121859
Chicago/Turabian StyleTrifan, Anca, Horia Minea, Adrian Rotaru, Carol Stanciu, Remus Stafie, Ermina Stratina, Sebastian Zenovia, Robert Nastasa, Ana-Maria Singeap, Irina Girleanu, and et al. 2022. "Predictive Factors for the Prognosis of Alcoholic Liver Cirrhosis" Medicina 58, no. 12: 1859. https://doi.org/10.3390/medicina58121859
APA StyleTrifan, A., Minea, H., Rotaru, A., Stanciu, C., Stafie, R., Stratina, E., Zenovia, S., Nastasa, R., Singeap, A.-M., Girleanu, I., Muzica, C., Huiban, L., Cuciureanu, T., Chiriac, S., Sfarti, C., & Cojocariu, C. (2022). Predictive Factors for the Prognosis of Alcoholic Liver Cirrhosis. Medicina, 58(12), 1859. https://doi.org/10.3390/medicina58121859










