Development and Validation of a Difficulty Scoring System for Laparoscopic Liver Resection to Treat Hepatolithiasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Techniques
2.3. Definitions
2.4. Statistics
3. Results
3.1. Patient Characteristics
3.2. Surgical Outcomes
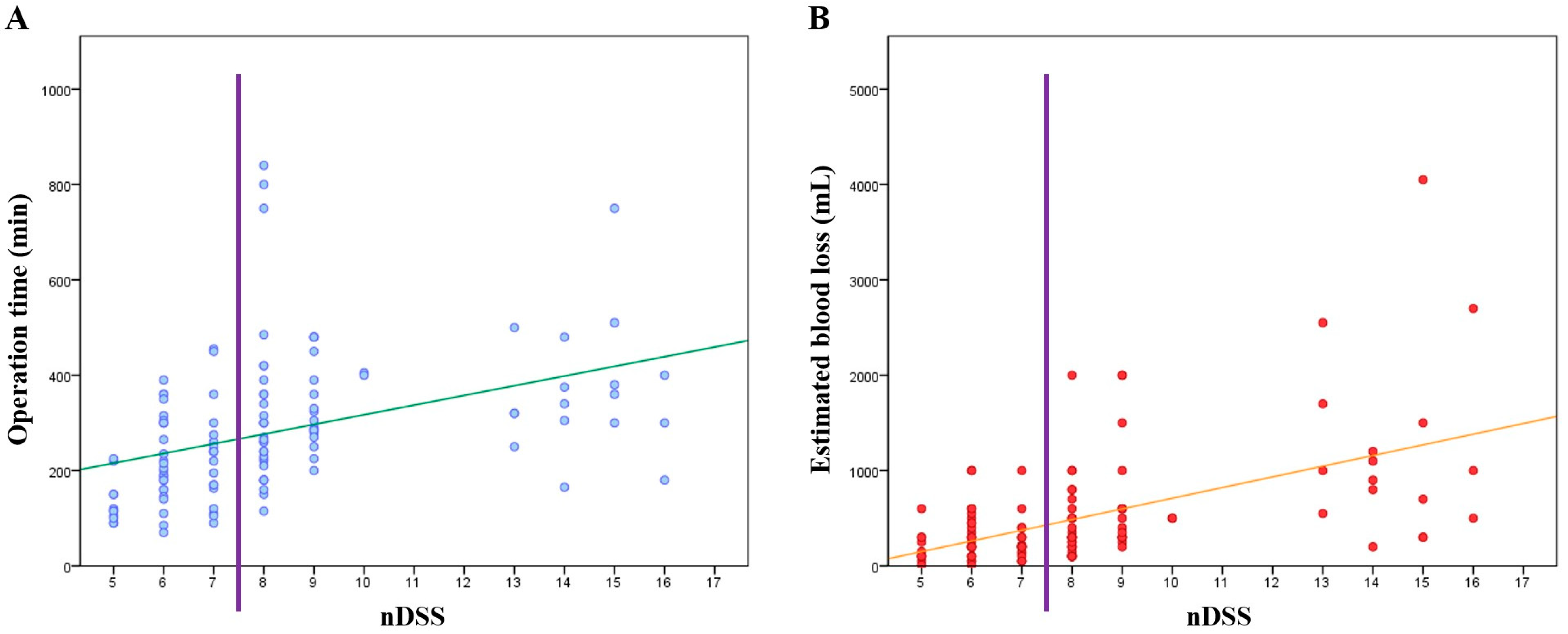
3.3. Development of the nDSS and Associations between nDSS and Short-Term Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, T.H.; Kim, S.Y.; Tang, A.; Lee, J.M. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin. Mol. Hepatol. 2019, 25, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Vivek, M.A.; Augustine, A.J.; Rao, R. A comprehensive predictive scoring method for difcult laparoscopic cholecystectomy. J. Minim. Access Surg. 2014, 10, 62–67. [Google Scholar] [CrossRef]
- Khoshrang, H.; Falahatkar, S.; Heidarzadeh, A.; Abad, M.; Herfeh, N.R.; Nabi, B.N. Predicting difculty score for spinal anesthesia in transurethral lithotripsy surgery. Anesth. Pain Med. 2014, 4, e16244. [Google Scholar] [CrossRef] [PubMed]
- Adnet, F.; Borron, S.W.; Racine, S.X.; Clemessy, J.L.; Fournier, J.L.; Plaisance, P.; Lapandry, C. The intubation difficulty scale (IDS): Proposal and evaluation of a new score characterizing the complexity of endotracheal intubation. Anesthesiology 1997, 87, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Osborne, S.A.; Severn, P.; Bunce, C.V.; Fraser, S.G. The use of a pre-operative scoring system for the prediction of phacoemulsification case difficulty and the selection of appropriate cases to be performed by trainees. BMC Ophthalmol. 2006, 6, 38. [Google Scholar] [CrossRef]
- Lee, M.K.; Gao, F.; Strasberg, S.M. Perceived complexity of various liver resections: Results of a survey of experts with development of a complexity score and classification. J. Am. Coll. Surg. 2015, 220, 64–69. [Google Scholar] [CrossRef]
- Muangkaew, P.; Cho, J.Y.; Han, H.S.; Yoon, Y.S.; Choi, Y.; Jang, J.Y.; Choi, H.; Jang, J.S.; Kwon, S.U. Defining surgical difficulty according to the perceived complexity of liver resection: Validation of a complexity classification in patients with hepatocellular carcinoma. Ann. Surg. Oncol. 2016, 23, 2602–2609. [Google Scholar] [CrossRef]
- Osborne, S.A.; Adams, W.E.; Bunce, C.V.; Fraser, S.G. Validation of two scoring systems for the prediction of posterior capsule rupture during phacoemulsification surgery. Br. J. Ophthalmol. 2006, 90, 333–336. [Google Scholar] [CrossRef][Green Version]
- Jia, C.; Li, H.; Wen, N.; Chen, J.; Wei, Y.; Li, B. Laparoscopic liver resection: A review of current indications and surgical techniques. Hepatobiliary Surg. Nutr. 2018, 7, 277–288. [Google Scholar] [CrossRef]
- Wakabayashi, G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg. Nutr. 2016, 5, 281–289. [Google Scholar] [CrossRef]
- Tanaka, S.; Kawaguchi, Y.; Kubo, S.; Kanazawa, A.; Takeda, Y.; Hirokawa, F.; Nitta, H.; Nakajima, T.; Kaizu, T.; Kaibori, M.; et al. Validation of index-based IWATE criteria as an improved difficulty scoring system for laparoscopic liver resection. Surgery 2019, 165, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Ban, D.; Tanabe, M.; Ito, H.; Otsuka, Y.; Nitta, H.; Abe, Y.; Hasegawa, Y.; Katagiri, T.; Takagi, C.; Itano, O.; et al. A novel difficulty scoring system for laparoscopic liver resection. J. Hepatobiliary Pancreat. Sci. 2014, 21, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Im, C.; Cho, J.Y.; Han, H.S.; Yoon, Y.S.; Choi, Y.; Jang, J.Y.; Choi, H.; Jang, J.S.; Kwon, S.U. Validation of difficulty scoring system for laparoscopic liver resection in patients who underwent laparoscopic left lateral sectionectomy. Surg. Endosc. 2017, 31, 430–436. [Google Scholar] [CrossRef]
- Han, H.S.; Cho, J.Y.; Yoon, Y.S. Techniques for performing laparoscopic liver resection in various hepatic locations. J. Hepatobiliary Pancreat. Surg. 2009, 16, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Han, H.S.; Shin, S.H.; Cho, J.Y.; Min, S.K.; Lee, H.K. Laparoscopic treatment for intrahepatic duct stones in the era of laparoscopy: Laparoscopic intrahepatic duct exploration and laparoscopic hepatectomy. Ann. Surg. 2009, 249, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B. Histopathology of a benign bile duct lesion in the liver: Morphologic mimicker or precursor of intrahepatic cholangiocarcinoma. Clin. Mol. Hepatol. 2016, 22, 400–405. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Kim, K.J.; Hong, S.C.; Jeong, S.H.; Ju, Y.T.; Lee, Y.J.; Choi, S.K.; Ha, W.S.; Park, S.T.; Jung, E.J. Laparoscopic left hemihepatectomy for left intrahepatic duct stones. J. Korean Surg. Soc. 2012, 83, 149–154. [Google Scholar] [CrossRef]
- Liu, X.; Miao, X.; Zhong, D.; Yao, H.; Wen, Y.; Dai, W.; Liu, G. Laparoscopic left hemihepatectomy for treatment of left intrahepatic duct stones. Am. Surg. 2014, 80, E350–E351. [Google Scholar] [CrossRef]
- Tan, J.; Tan, Y.; Chen, F.; Zhu, Y.; Leng, J.; Dong, J. Endoscopic or laparoscopic approach for hepatolithiasis in the era of endoscopy in China. Surg. Endosc. 2015, 29, 154–162. [Google Scholar] [CrossRef]
- Yang, T.; Lau, W.Y.; Lai, E.C.; Yang, L.Q.; Zhang, J.; Yang, G.S.; Lu, J.H.; Wu, M.C. Hepatectomy for bilateral primary hepatolithiasis: A cohort study. Ann. Surg. 2010, 251, 84–90. [Google Scholar] [CrossRef]
- Suzuki, Y.; Mori, T.; Yokoyama, M.; Nakazato, T.; Abe, N.; Nakanuma, Y.; Tsubouchi, H.; Sugiyama, M. Hepatolithiasis: Analysis of Japanese nationwide surveys over a period of 40 years. J. Hepatobiliary Pancreat. Sci. 2014, 21, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann. Surg. 2009, 250, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Shehta, A.; Ahn, S.; Yoon, Y.S.; Cho, J.Y.; Choi, Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: Case-matched study with propensity score matching. J. Hepatol. 2015, 63, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’Rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Cherqui, D. Laparoscopic liver resection: A new paradigm in the management of hepatocellular carcinoma? J. Hepatol. 2015, 63, 540–542. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 583–705. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, D.G.; Kim, J.; Lee, K.; Lee, K.W.; Ryu, J.H.; Kim, B.W.; Choi, D.L.; You, Y.K.; Kim, D.S.; et al. Outcomes after liver transplantation in Korea: Incidence and risk factors from Korean transplantation registry. Clin. Mol. Hepatol. 2021, 27, 451–462. [Google Scholar] [CrossRef]
- Cai, X.; Wang, Y.; Yu, H.; Liang, X.; Peng, S. Laparoscopic hepatectomy for hepatolithiasis: A feasibility and safety study in 29 patients. Surg. Endosc. 2007, 21, 1074–1078. [Google Scholar] [CrossRef]
- Lai, E.C.; Ngai, T.C.; Yang, G.P.; Li, M.K. Laparoscopic approach of surgical treatment for primary hepatolithiasis: A cohort study. Am. J. Surg. 2010, 199, 716–721. [Google Scholar] [CrossRef]
- Kim, Y.K.; Han, H.S.; Yoon, Y.S.; Cho, J.Y.; Lee, W. Laparoscopic approach for right-sided intrahepatic duct stones: A comparative study of laparoscopic versus open treatment. World J. Surg. 2015, 39, 1224–1230. [Google Scholar] [CrossRef]
- Gough, V.; Stephens, N.; Ahmed, Z.; Nassar, A.H. Intrahepatic choledochoscopy during trans-cystic common bile duct exploration; technique, feasibility and value. Surg. Endosc. 2012, 26, 3190–3194. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Fuks, D.; Kokudo, N.; Gayet, B. Difficulty of laparoscopic liver resection: Proposal for a new classification. Ann. Surg. 2018, 267, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Wakabayashi, G.O.; Nitta, H.; Takahara, T.; Katagiri, H.; Umemura, A.; Makabe, K.; Sasaki, A. A novel model for prediction of pure laparoscopic liver resection surgical difficulty. Surg. Endosc. 2017, 31, 5356–5363. [Google Scholar] [CrossRef] [PubMed]
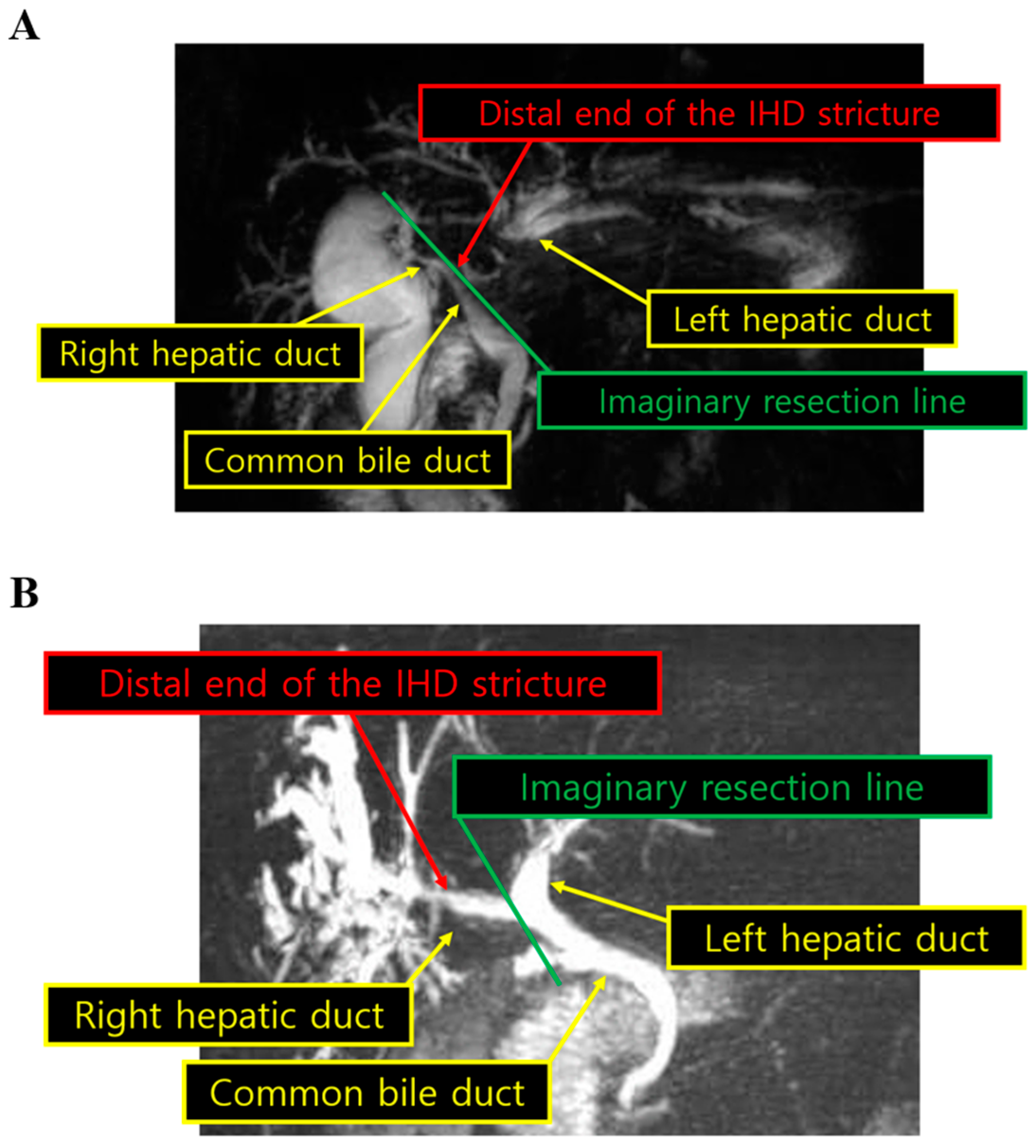
| Characteristics (n = 123) | Value |
|---|---|
| Age, years (mean) | 59.98 ± 9.25 |
| Sex, n (%) | |
| Male | 46 (37.4%) |
| Female | 77 (62.6%) |
| BMI, kg/m2 (mean) | 23.67 ± 3.01 |
| History of upper abdominal surgery, n (%) | 29 (23.6%) |
| Resection type, n (%) | |
| Left lateral sectionectomy | 43 (35.0%) |
| Major hepatectomy | 80 (65.0%) |
| Resection side, n (%) | |
| Left hemiliver | 106 (86.2%) |
| Right hemiliver | 17 (13.8%) |
| Liver parenchyma atrophy, n (%) | 64 (52.0%) |
| Bile duct exploration, n (%) | 51 (41.5%) |
| IHD stricture <1 mm from the bifurcation, n (%) | 63 (51.2%) |
| Variable | Value |
|---|---|
| Operation time (min) | Mean: 280.46 ± 141.63; median: 260 |
| EBL (mL) | Mean: 507.64 ± 590.43; median: 300 |
| RBC transfusion, n (%) | 28 (22.8%) |
| RBC transfusion (mL) | Mean: 302.44 ± 784.01 |
| POHS (days) | Mean: 10.85 ± 9.70, median: 8 |
| Remnant stone, n (%) | 11 (8.9%) |
| Recurrent stone, n (%) | 6 (4.9%) |
| CDC grade ≥IIIa, n (%) | 22 (17.9%) |
| Fluid collection, n (%) | 10 (8.1%) |
| Biliary fistula, n (%) | 6 (4.9%) |
| Pleural effusion, n (%) | 2 (1.6%) |
| Biliary stricture, n (%) | 1 (0.8%) |
| Septic shock, n (%) | 1 (0.8%) |
| Pseudoaneurysm rupture, n (%) | 1 (0.8%) |
| Wound complication, n (%) | 1 (0.8%) |
| Operation Time ≥ 260 min | Estimated Blood Loss ≥ 300 mL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||||||
| Variables | OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value |
| Age 1 | 0.979 | 0.394–2.435 | 0.964 | - | - | - | 1.123 | 0.461–2.735 | 0.798 | - | - | - |
| Sex 2 | 0.760 | 0.313–1.848 | 0.545 | - | - | - | 0.628 | 20.259–1.520 | 0.302 | - | - | - |
| BMI 3 | 1.771 | 0.701–4.474 | 0.227 | - | - | - | 1.582 | 0.628–3.985 | 0.331 | - | - | - |
| Resection type 4 | 4.479 | 1.702–11.785 | 0.002 | 3.984 | 1.596–9.947 | 0.003 | 1.759 | 0.740–4.183 | 0.201 | - | - | - |
| Resection side 5 | 4.267 | 1.018–17.886 | 0.047 | 4.173 | 1.018–17.104 | 0.047 | 13.172 | 1.562–111.047 | 0.018 | 16.209 | 2.007–130.901 | 0.009 |
| Liver parenchyma atrophy 6 | 0.744 | 0.312–1.770 | 0.504 | - | - | - | 0.622 | 0.267–1.452 | 0.272 | - | - | - |
| Bile duct exploration 7 | 4.172 | 1.761–9.883 | 0.001 | 3.891 | 1.678–9.021 | 0.002 | 2.712 | 1.164–6.318 | 0.021 | 2.812 | 1.225–6.455 | 0.015 |
| Proximity to the bifurcation 8 | 2.744 | 1.136–6.624 | 0.025 | 2.683 | 1.148–6.269 | 0.023 | 1.425 | 0.624–3.255 | 0.400 | - | - | - |
| History of UAS 9 | 1.708 | 0.728–4.004 | 0.218 | - | - | - | 3.096 | 1.155–8.301 | 0.025 | 3.976 | 1.408–11.231 | 0.009 |
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| RBC transfusion | 1.383 | 1.186–1.614 | <0.001 |
| POHS ≥ 8 days | 1.307 | 1.107–1.544 | 0.002 |
| CDC grade ≥ IIIa | 1.267 | 1.092–1.470 | 0.002 |
| Remnant stone | 0.928 | 0.722–1.194 | 0.564 |
| Recurrent stone | 0.948 | 0.686–1.310 | 0.746 |
| Variable | nDSS 5–7 | nDSS ≥ 8 | OR | p Value |
|---|---|---|---|---|
| Operation time, min (mean) | 210.67 | 240.74 | - | <0.001 |
| Operation time ≥ 260 min, n (%) | 16 (28.1%) | 48 (72.7%) | 6.833 | <0.001 |
| EBL, mL (mean) | 281.93 | 702.58 | - | <0.001 |
| EBL ≥ 300 mL, n (%) | 24 (42.1%) | 51 (77.3%) | 4.675 | <0.001 |
| RBC transfusion, n (%) | 3 (5.3%) | 25 (37.9%) | 10.976 | <0.001 |
| POHS, days (mean) | 8.00 | 13.32 | - | 0.001 |
| POHS ≥ 8 days, n (%) | 20 (35.1%) | 44 (66.7%) | 3.700 | 0.001 |
| CDC grade ≥ IIIa, n (%) | 5 (8.8%) | 17 (25.8%) | 3.608 | 0.014 |
| Remnant stone, n (%) | 4 (7.0%) | 7 (10.6%) | 1.572 | 0.543 |
| Recurrent stone, n (%) | 2 (3.5%) | 4 (6.1%) | 1.774 | 0.685 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, Y.; Cho, J.Y.; Han, H.-S.; Yoon, Y.-S.; Lee, H.W.; Lee, J.S.; Lee, B.; Lee, E.; Park, Y.; Kang, M.; et al. Development and Validation of a Difficulty Scoring System for Laparoscopic Liver Resection to Treat Hepatolithiasis. Medicina 2022, 58, 1847. https://doi.org/10.3390/medicina58121847
Jo Y, Cho JY, Han H-S, Yoon Y-S, Lee HW, Lee JS, Lee B, Lee E, Park Y, Kang M, et al. Development and Validation of a Difficulty Scoring System for Laparoscopic Liver Resection to Treat Hepatolithiasis. Medicina. 2022; 58(12):1847. https://doi.org/10.3390/medicina58121847
Chicago/Turabian StyleJo, Yeongsoo, Jai Young Cho, Ho-Seong Han, Yoo-Seok Yoon, Hae Won Lee, Jun Suh Lee, Boram Lee, Eunhye Lee, Yeshong Park, MeeYoung Kang, and et al. 2022. "Development and Validation of a Difficulty Scoring System for Laparoscopic Liver Resection to Treat Hepatolithiasis" Medicina 58, no. 12: 1847. https://doi.org/10.3390/medicina58121847
APA StyleJo, Y., Cho, J. Y., Han, H.-S., Yoon, Y.-S., Lee, H. W., Lee, J. S., Lee, B., Lee, E., Park, Y., Kang, M., & Lee, J. (2022). Development and Validation of a Difficulty Scoring System for Laparoscopic Liver Resection to Treat Hepatolithiasis. Medicina, 58(12), 1847. https://doi.org/10.3390/medicina58121847







