Papillary Squamotransitional Cell Carcinoma of the Uterine Cervix with Atypical Presentation: A Case Report with a Literature Review
Abstract
1. Introduction
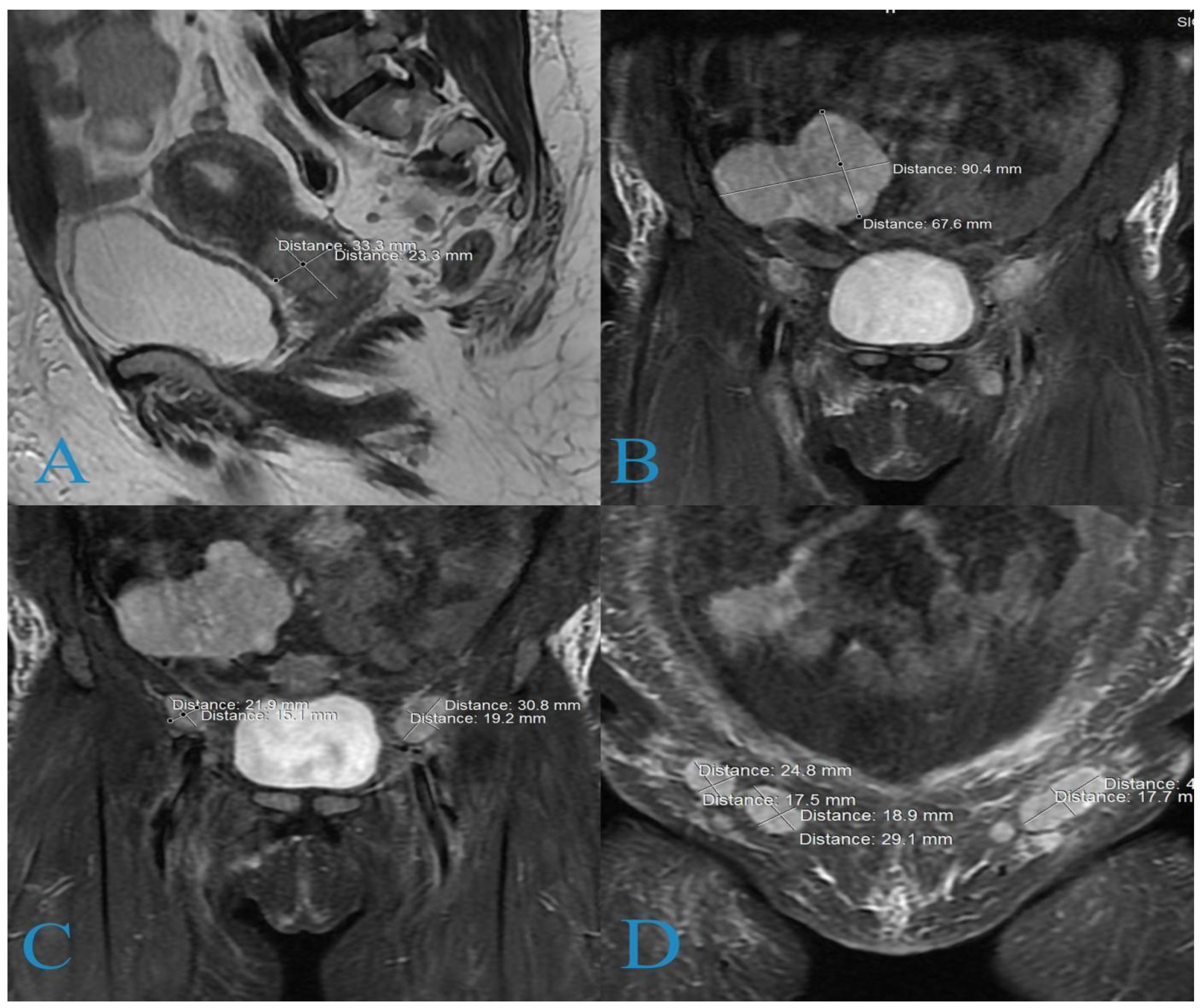
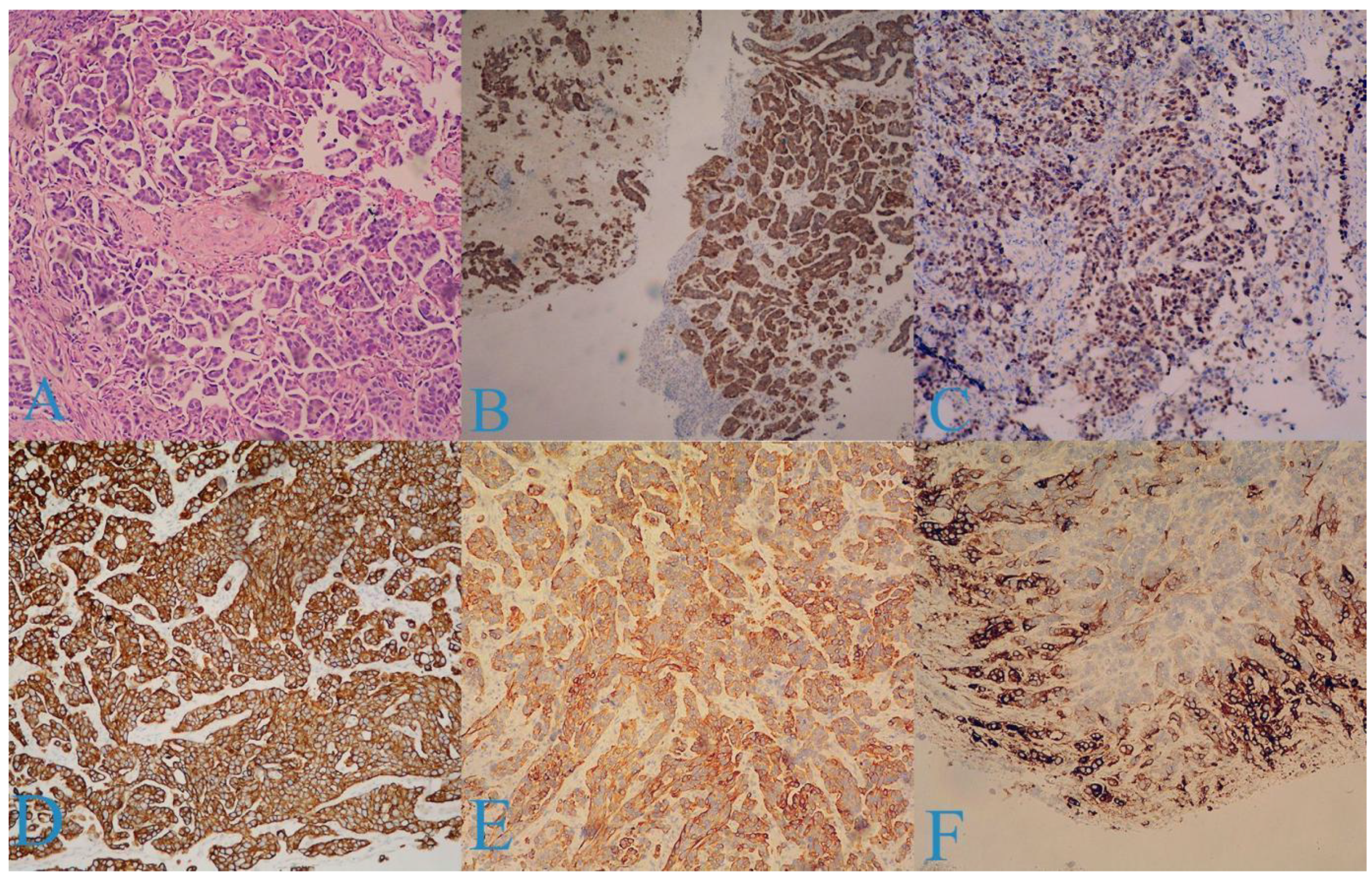
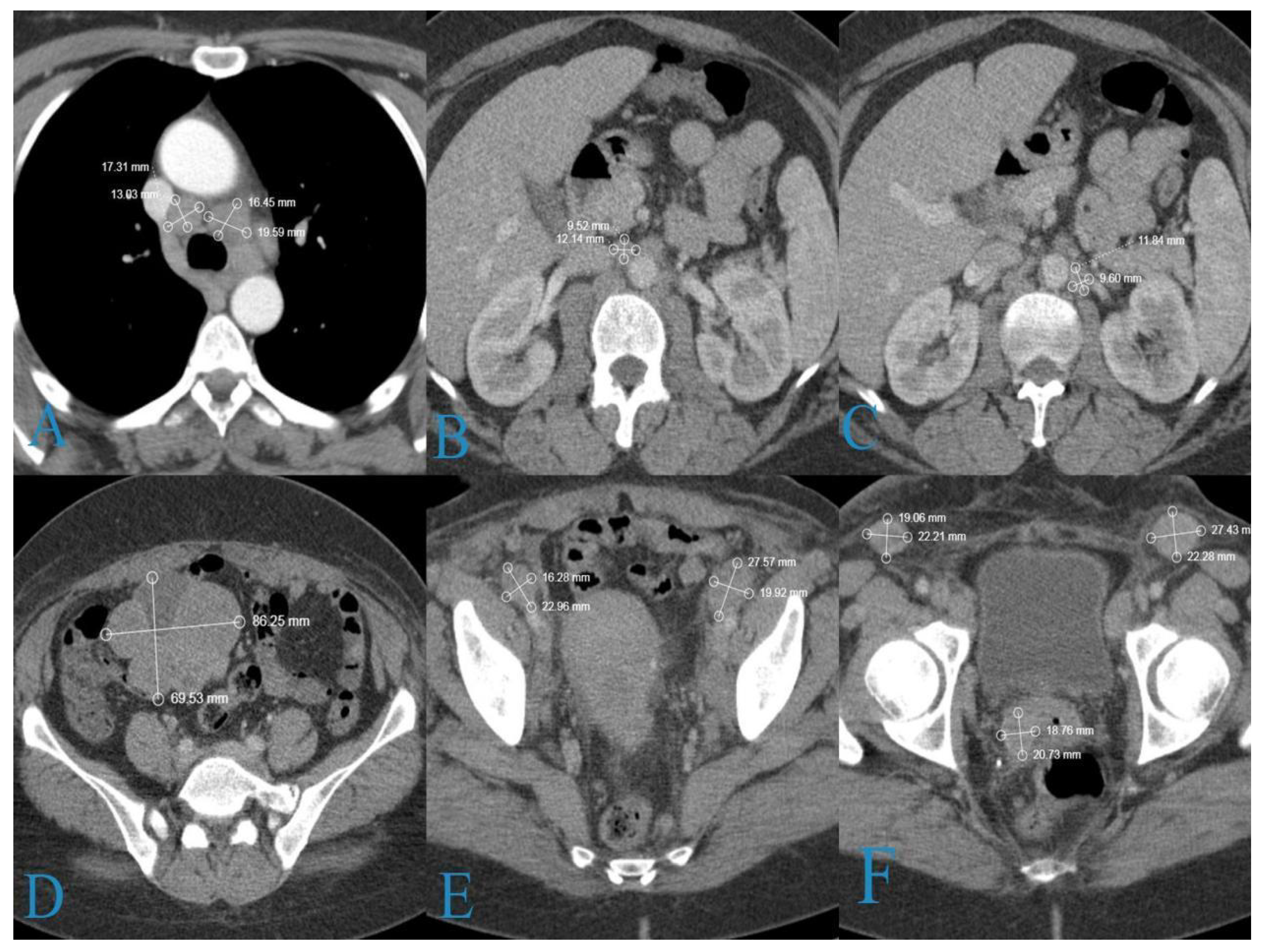
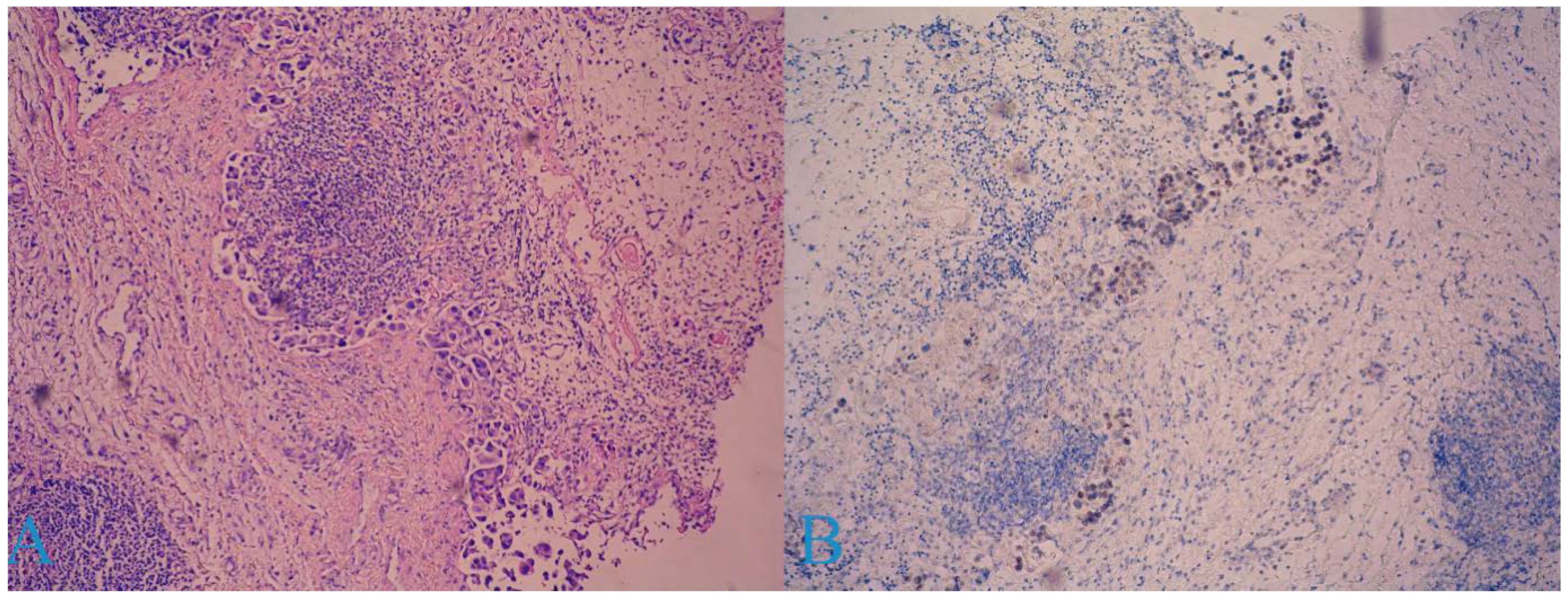
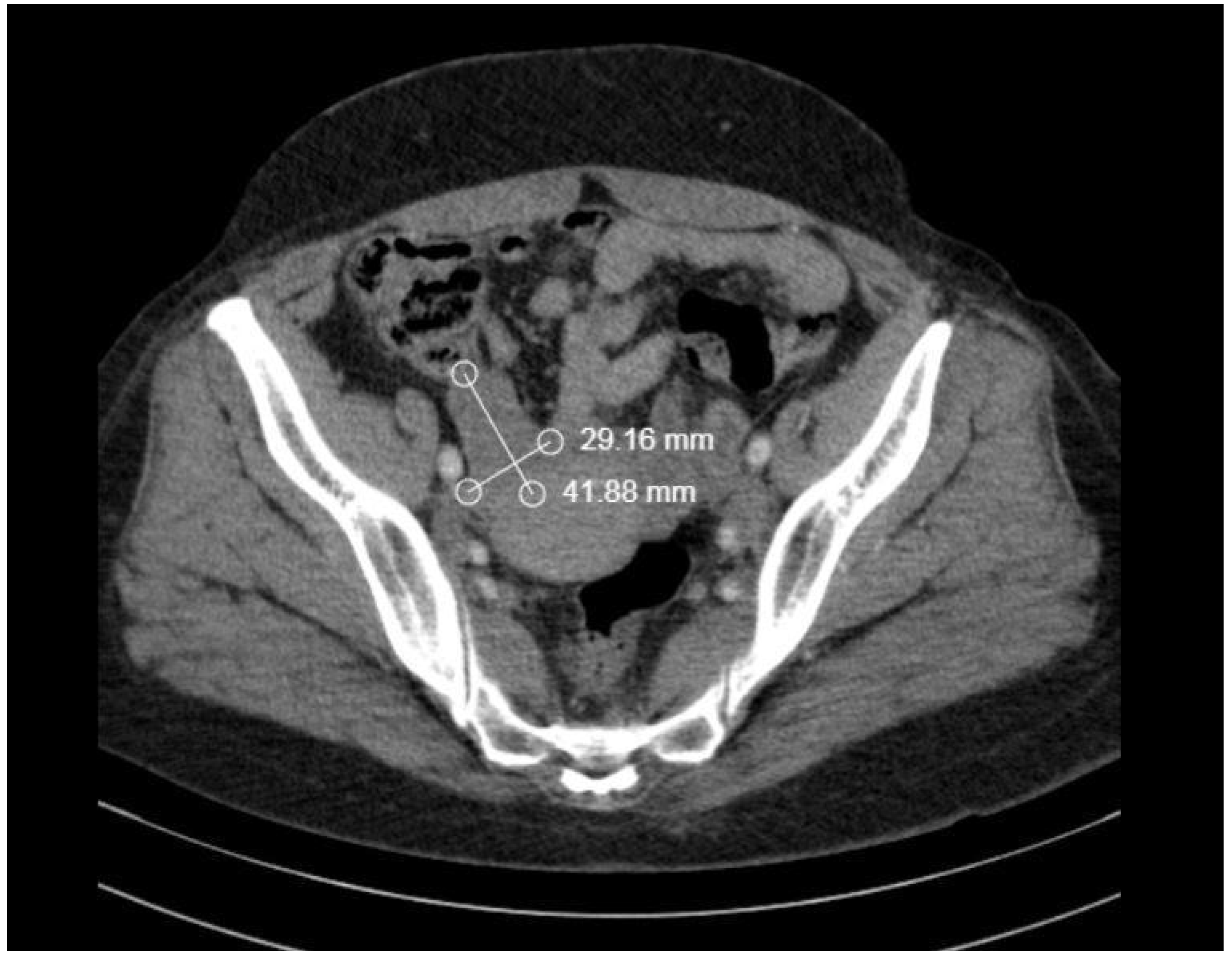
2. Case Report
3. Discussion
- Predominantly squamous (28.1%);
- Mixed squamous and transitional (50%);
- Predominantly transitional (21.9%).
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Giannini, A.; Bogani, G.; Vizza, E.; Chiantera, V.; Laganà, A.S.; Muzii, L.; Salerno, M.G.; Caserta, D.; D’Oria, O. Advances on Prevention and Screening of Gynecologic Tumors: Are We Stepping Forward? Healthcare 2022, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- CONCORD Working Group; Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, O.; Corrado, G.; Laganà, A.S.; Chiantera, V.; Vizza, E.; Giannini, A. New Advances in Cervical Cancer: From Bench to Bedside. Int. J. Environ. Res. Public Health 2022, 19, 7094. [Google Scholar] [CrossRef]
- Cuzick, J.; Du, R.; Adcock, R.; Kinney, W.; Joste, N.; McDonald, R.M.; English, K.; Torres, S.M.; Saslow, D.; Wheeler, C.M.; et al. Uptake of co-testing with HPV and cytology for cervical screening: A population-based evaluation in the United States. Gynecol. Oncol. 2021, 162, 555–559. [Google Scholar] [CrossRef]
- Bogani, G.; Lalli, L.; Sopracordevole, F.; Ciavattini, A.; Ghelardi, A.; Simoncini, T.; Plotti, F.; Casarin, J.; Serati, M.; Pinelli, C.; et al. Development of a Nomogram Predicting the Risk of Persistence/Recurrence of Cervical Dysplasia. Vaccines 2022, 10, 579. [Google Scholar] [CrossRef]
- Intaraphet, S.; Kasatpibal, N.; Siriaunkgul, S.; Sogaard, M.; Patumanond, J.; Khunamornpong, S.; Chandacham, A.; Suprasert, P. Prognostic impact of histology in patients with cervical squamous cell carcinoma, adenocarcinoma and small cell neuroendocrine carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 5355–5360. [Google Scholar] [CrossRef]
- Available online: https://screening.iarc.fr/atlasclassifwho.php (accessed on 1 May 2022).
- Randall, M.E.; Andersen, W.A.; Mills, S.E.; Kim, J.A. Papillary squamous cell carcinoma of the uterine cervix: A clinicopathologic study of nine cases. Int. J. Gynecol. Pathol. 1986, 5, 1–10. [Google Scholar] [CrossRef]
- Al-Nafussi, A.I.; Al-Yusif, R. Papillary squamotransitional cell carcinoma of the uterine cervix: An advanced stage disease despite superficial location: Report of two cases and review of the literature. Eur. J. Gynaecol. Oncol. 1998, 19, 455–457. [Google Scholar]
- Austin, R.M.; Norris, H.J. Malignant Brenner tumor and transitional cell carcinoma of the ovary: A comparison. Int. J. Gynecol. Pathol. 1987, 6, 29–39. [Google Scholar] [CrossRef]
- Bass, P.S.; Birch, B.; Smart, C.; Theaker, J.M.; Wells, M. Low-grade transitional cell carcinoma of the vagina—An unusual cause of vaginal bleeding. Histopathology 1994, 24, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.R. Papilloma of the cervix. Am. J. Obstet. Gynecol. 1952, 64, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.; Östör, A.G.; Crum, C.P. Tumours of the uterine cervix. In World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs, 3rd ed.; Tavassoli, F.A., Devilee, P., Eds.; IARC: Lyon, France, 2003; pp. 259–289. [Google Scholar]
- Woo, H.Y.; Kim, H.S. Local and Metastatic Relapses in a Young Woman with Papillary Squamous Cell Carcinoma of the Uterine Cervix. Diagnostics 2022, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Koenig, C.; Turnicky, R.P.; Kankam, C.F.; Tavassoli, F.A. Papillary squamotransitional cell carcinoma of the cervix: A report of 32 cases. Am. J. Surg Pathol. 1997, 21, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Gitas, G.; Ertan, K.; Rody, A.; Baum, S.; Tsolakidis, D.; Alkatout, I. Papillary squamotransitional cell carcinoma of the uterine cervix: A case report and review of the literature. J. Med. Case Rep. 2019, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Goyal, N.; Dass, C.; Bodal, V.K.; Mittal, A. Papillary Squamotransitional Cell Carcinoma Cervix- A Rare Case Report. Ann. Int. Med. Den. Res. 2018, 4, 15–17. [Google Scholar] [CrossRef]
- Koh, W.J.; Greer, B.E.; Abu-Rustum, N.R.; Apte, S.M.; Campos, S.M.; Cho, K.R. Cervical cancer, version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 395–404. [Google Scholar] [CrossRef]
- Turker, L.B.; Gressel, G.M.; Abadi, M.; Frimer, M. Papillary squamous cell carcinoma of the cervix: Two cases and a review of the literature. Gynecol. Oncol. Rep. 2016, 18, 18–21. [Google Scholar] [CrossRef][Green Version]
- Anand, M.; Deshmukh, S.D.; Gulati, H.K. Papillary squamotransitional cell carcinoma of the uterine cervix: A histomorphological and immunohistochemical study of nine cases. Indian J. Med. Paediatr. Oncol. 2013, 34, 66–71. [Google Scholar] [CrossRef][Green Version]
- Nagura, M.; Koshiyama, M.; Matsumura, N.; Kido, A.; Baba, T.; Abiko, K.; Hamanishi, J.; Yamaguchi, K.; Mikami, Y.; Konishi, I. Clinical approaches to treating papillary squamous cell carcinoma of the uterine cervix. BMC Cancer 2014, 14, 784. [Google Scholar] [CrossRef][Green Version]
- Akbar, S.A.; Tunio, M.A.; Al-Dandan, S.; Salamah, K.M.; AlAsiri, M. Papillary Squamotransitional Cell Carcinoma of the Uterine Cervix: A Case Report and Review of the Literature. Case Rep. Obstet. Gynecol. 2016, 2016, 7107910. [Google Scholar] [CrossRef] [PubMed]
- Anusha, M.; Anuradha, P.; Praveen Kumar, M.; Lohith, G. Papillary Squamo-Transitional Cell Carcinoma. Cancer Ther. Oncol. Int. J. 2018, 10, 555792. [Google Scholar] [CrossRef]

| Benign | Malignant |
|---|---|
| Condyloma acuminatum | Warty squamous cell carcinoma |
| Squamous papilloma | Verrucous carcinoma |
| Cervical intraepithelial neoplasia with papillary configuration | Transitional cell carcinoma of the endometrium and endometrial adenocarcinoma |
| Papillary adenofibroma | Villoglandular papillary adenocarcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yordanov, A.; Karaivanov, M.; Kostov, S.; Kornovski, Y.; Ivanova, Y.; Slavchev, S.; Todorova, V.; Vasileva-Slaveva, M. Papillary Squamotransitional Cell Carcinoma of the Uterine Cervix with Atypical Presentation: A Case Report with a Literature Review. Medicina 2022, 58, 1838. https://doi.org/10.3390/medicina58121838
Yordanov A, Karaivanov M, Kostov S, Kornovski Y, Ivanova Y, Slavchev S, Todorova V, Vasileva-Slaveva M. Papillary Squamotransitional Cell Carcinoma of the Uterine Cervix with Atypical Presentation: A Case Report with a Literature Review. Medicina. 2022; 58(12):1838. https://doi.org/10.3390/medicina58121838
Chicago/Turabian StyleYordanov, Angel, Milen Karaivanov, Stoyan Kostov, Yavor Kornovski, Yonka Ivanova, Stanislav Slavchev, Venelina Todorova, and Mariela Vasileva-Slaveva. 2022. "Papillary Squamotransitional Cell Carcinoma of the Uterine Cervix with Atypical Presentation: A Case Report with a Literature Review" Medicina 58, no. 12: 1838. https://doi.org/10.3390/medicina58121838
APA StyleYordanov, A., Karaivanov, M., Kostov, S., Kornovski, Y., Ivanova, Y., Slavchev, S., Todorova, V., & Vasileva-Slaveva, M. (2022). Papillary Squamotransitional Cell Carcinoma of the Uterine Cervix with Atypical Presentation: A Case Report with a Literature Review. Medicina, 58(12), 1838. https://doi.org/10.3390/medicina58121838








