Albumin-Bilirubin Grade as a Novel Predictor of the Development and Short-Term Survival of Post-Banding Ulcer Bleeding Following Endoscopic Variceal Ligation in Cirrhotic Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients and Data Collection
2.2. Statistical Analyses
3. Results
3.1. Patient Demographics and Outcomes of the PBUB Group
3.2. Predictive Factors of PBUB
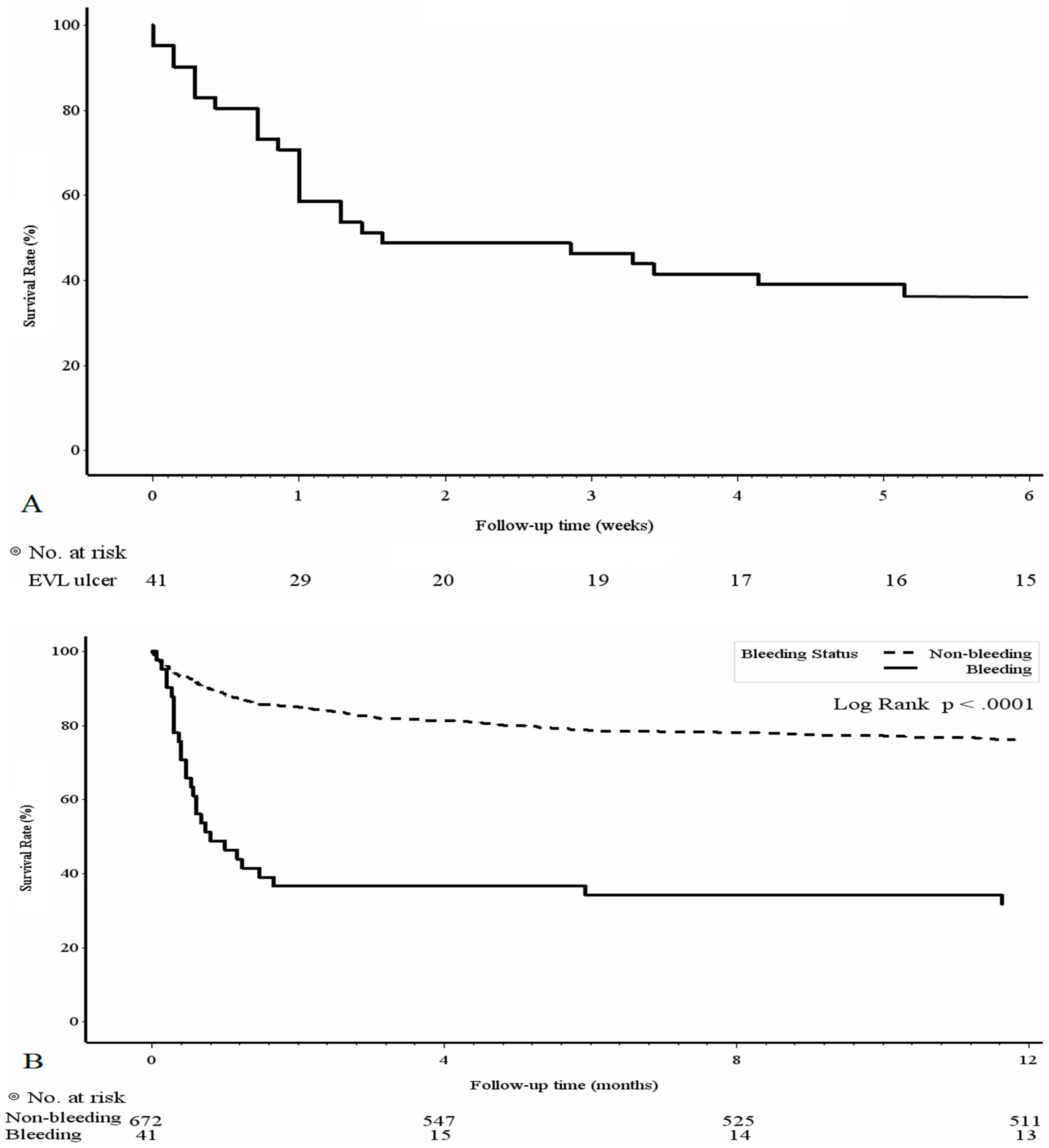
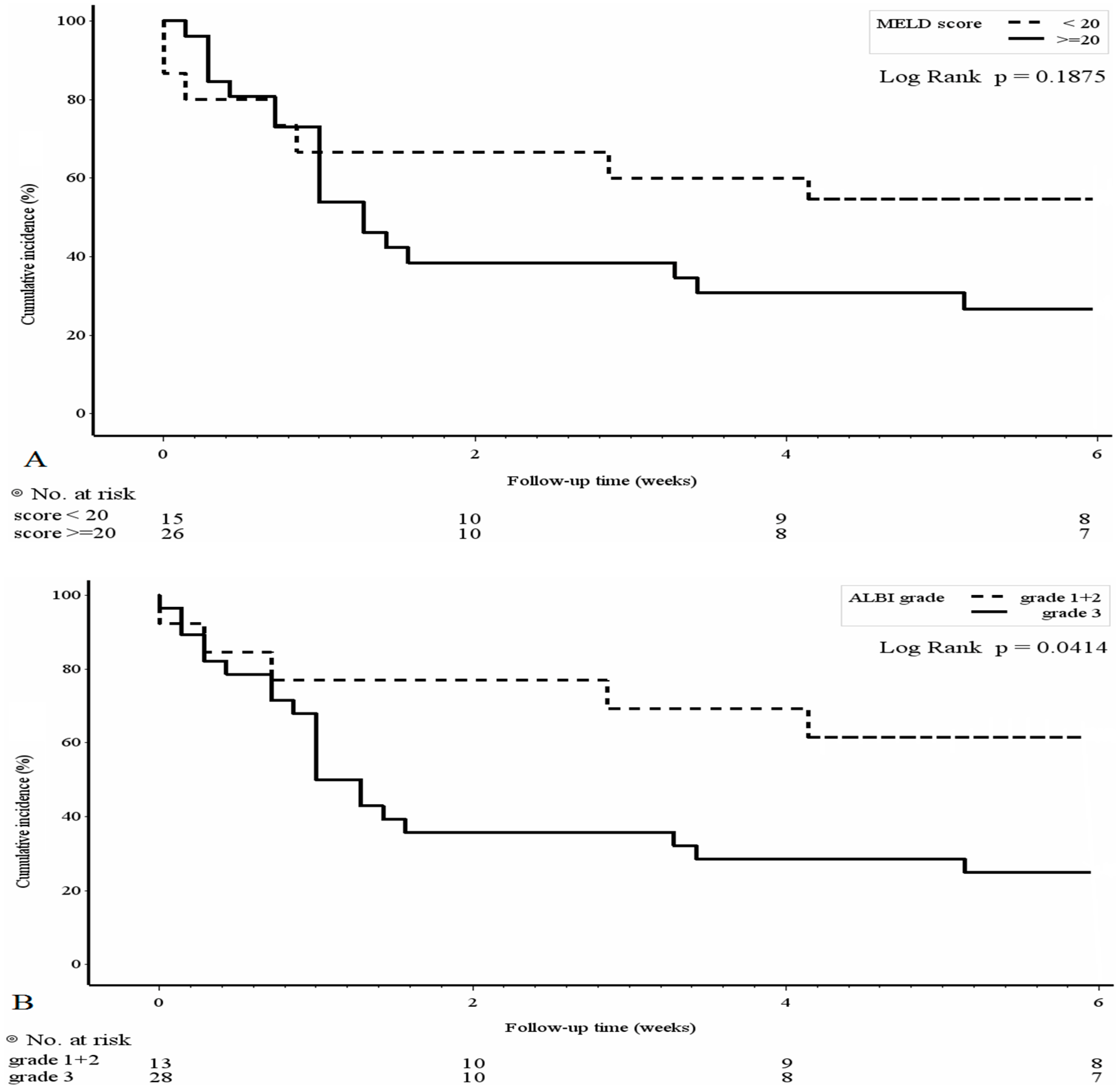
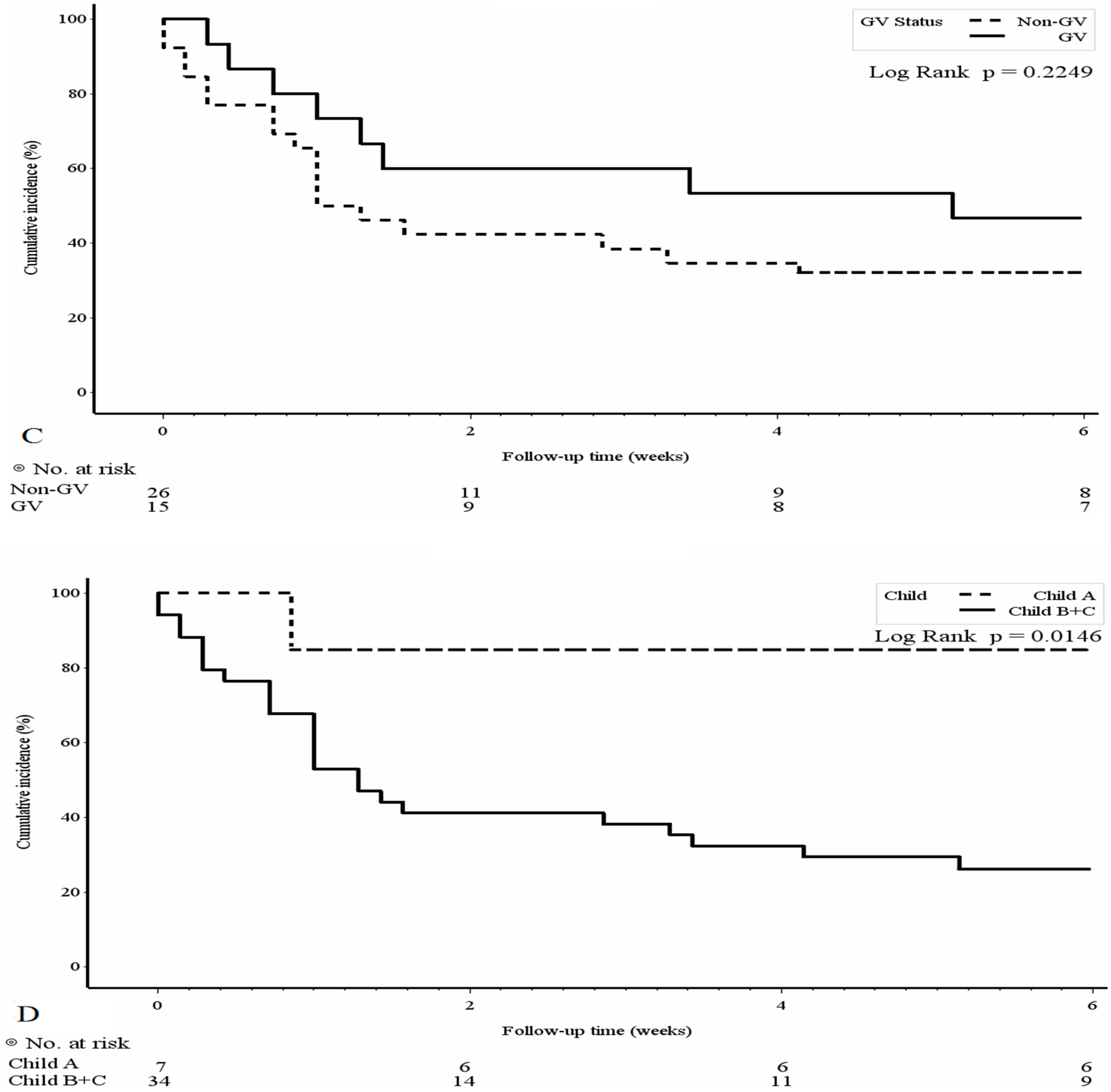
3.3. Mortality Risk Factors and Survival Analyses of the PBUB Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2016, 65, 310–335. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Bosch, J. Management of Varices and Variceal Hemorrhage in Cirrhosis. N. Engl. J. Med. 2010, 362, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.J.; Sharma, P.; Badr, A.S.; Qamar, M.T.; Weston, A.P. Incidence and Management of Esophageal Stricture Formation, Ulcer Bleeding, Perforation, and Massive Hematoma Formation From Sclerotherapy Versus Band Ligation. Am. J. Gastroenterol. 2001, 96, 437–441. [Google Scholar] [CrossRef]
- Stiegmann, G.V.; Sun, J.H.; Hammond, W.S. Results of experimental endoscopic esophageal varix ligation. Am. Surg. 1988, 54, 105–108. [Google Scholar] [PubMed]
- Nijhawan, S.; Rai, R.R.; Nepalia, S.; Pokharana, D.S.; Bharagava, N. Natural history of postligation ulcers. Am. J. Gastroenterol. 1994, 89, 2281–2282. [Google Scholar]
- Dueñas, E.; Cachero, A.; Amador, A.; Rota, R.; Salord, S.; Gornals, J.; Xiol, X.; Castellote, J. Ulcer bleeding after band ligation of esophageal varices: Risk factors and prognosis. Dig. Liver Dis. 2020, 52, 79–83. [Google Scholar] [CrossRef]
- Tierney, A.; Toriz, B.E.; Mian, S.; Brown, K.E. Interventions and outcomes of treatment of postbanding ulcer hemorrhage after endoscopic band ligation: A single-center case series. Gastrointest. Endosc. 2013, 77, 136–140.e1. [Google Scholar] [CrossRef]
- Drolz, A.; Schramm, C.; Seiz, O.; Groth, S.; Vettorazzi, E.; Horvatits, T.; Wehmeyer, M.H.; Goeser, T.; Roesch, T.; Lohse, A.W.; et al. Risk factors associated with bleeding after prophylactic endoscopic variceal ligation in cirrhosis. Endoscopy 2020, 53, 226–234. [Google Scholar] [CrossRef]
- Cho, E.; Jun, C.H.; Cho, S.B.; Park, C.H.; Kim, H.S.; Choi, S.K.; Rew, J.S. Endoscopic variceal ligation-induced ulcer bleeding: What are the risk factors and treatment strategies? Medicine 2017, 96, e7157. [Google Scholar] [CrossRef]
- Sinclair, M.; Vaughan, R.; Angus, P.W.; Gow, P.J.; Parker, F.; Hey, P.; Efthymiou, M. Risk factors for band-induced ulcer bleeding after prophylactic and therapeutic endoscopic variceal band ligation. Eur. J. Gastroenterol. Hepatol. 2015, 27, 928–932. [Google Scholar] [CrossRef]
- Da Rocha, E.C.V.; D’Amico, E.A.; Caldwell, S.H.; Da Rocha, T.R.F.; Silva, C.S.S.S.E.; Bomfim, V.D.S.; Felga, G.; Barbosa, W.F.; Kassab, F.; Polli, D.; et al. A Prospective Study of Conventional and Expanded Coagulation Indices in Predicting Ulcer Bleeding After Variceal Band Ligation. Clin. Gastroenterol. Hepatol. 2009, 7, 988–993. [Google Scholar] [CrossRef]
- Vanbiervliet, G.; Giudicelli-Bornard, S.; Piche, T.; Berthier, F.; Gelsi, E.; Filippi, J.; Anty, R.; Arab, K.; Huet, P.-M.; Hebuterne, X.; et al. Predictive factors of bleeding related to post-banding ulcer following endoscopic variceal ligation in cirrhotic patients: A case-control study. Aliment. Pharmacol. Ther. 2010, 32, 225–232. [Google Scholar] [CrossRef]
- Wai, C.-T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.-F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of Liver Function in Patients With Hepatocellular Carcinoma: A New Evidence-Based Approach—The ALBI Grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Reverter, E.; Tandon, P.; Augustin, S.; Turon, F.; Casu, S.; Bastiampillai, R.; Keough, A.; Llop, E.; González, A.; Seijo, S.; et al. A MELD-Based Model to Determine Risk of Mortality Among Patients With Acute Variceal Bleeding. Gastroenterology 2014, 146, 412–419 e413. [Google Scholar] [CrossRef]
- Conejo, I.; Guardascione, M.A.; Tandon, P.; Cachero, A.; Castellote, J.; Abraldes, J.G.; Amitrano, L.; Genescà, J.; Augustin, S. Multicenter External Validation of Risk Stratification Criteria for Patients With Variceal Bleeding. Clin. Gastroenterol. Hepatol. 2018, 16, 132–139 e138. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Kumada, T.; Izumi, N.; Kadoya, M.; Kokudo, N.; Kubo, S.; Matsuyama, Y.; Nakashima, O.; Sakamoto, M.; et al. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer 2017, 6, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lin, S. Albumin-bilirubin (ALBI) score at admission predicts possible outcomes in patients with acute-on-chronic liver failure. Medicine 2017, 96, e7142. [Google Scholar] [CrossRef]
- Lee, P.-C.; Chen, Y.-T.; Chao, Y.; Huo, T.-I.; Li, C.-P.; Su, C.-W.; Lee, M.-H.; Hou, M.-C.; Lee, F.-Y.; Lin, H.-C.; et al. Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma. Liver Int. 2017, 38, 321–330. [Google Scholar] [CrossRef]
- Deng, M.; Ng, S.W.Y.; Cheung, S.T.; Chong, C.C.N. Clinical application of Albumin-Bilirubin (ALBI) score: The current status. Surg. J. R. Coll. Surg. Edinb. Irel. 2020, 18, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, A.; Takamura, M.; Sakai, N.; Watanabe, Y.; Arao, Y.; Kimura, N.; Setsu, T.; Abe, H.; Yokoo, T.; Kamimura, H.; et al. Longitudinal increase in albumin–bilirubin score is associated with non-malignancy-related mortality and quality of life in patients with liver cirrhosis. PLoS ONE 2022, 17, e0263464. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.B.; Wiesner, R.H.; Harper, A.; McDiarmid, S.V.; Lake, J.; Edwards, E.; Merion, R.; Wolfe, R.; Turcotte, J.; Teperman, L. The new liver allocation system: Moving toward evidence-based transplantation policy. Liver Transplant. 2002, 8, 851–858. [Google Scholar] [CrossRef]
- De Franchis, R.; Baveno, V.I.F. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
| PBUB, N = 41 (5.8%) | Non-PBUB, N = 672 (94.2%) | p Value | |
|---|---|---|---|
| Sex, male | 29 (70.7%) | 480 (71.4%) | 0.924 |
| Age, years (Mean ± S.D.) | 53.95 ± 11.53 | 58.35 ± 12.48 | 0.028 |
| Cirrhosis etiology | 0.348 | ||
| HBV | 11 (26.8%) | 160 (23.8%) | |
| HCV | 13 (31.7%) | 208 (31.0%) | |
| Alcoholism | 1 (2.4%) | 110 (16.4%) | |
| HBV + alcoholism | 9 (22.0%) | 91 (13.5%) | |
| HCV + alcoholism | 2 (4.9%) | 29 (4.3%) | |
| HBV + HCV + alcoholism | 0 | 8 (1.2%) | |
| Others | 3 (7.3%) | 45 (6.7%) | |
| Child-Pugh score | 0.16 | ||
| A | 7 (17.1%) | 181 (26.9%) | |
| B + C | 34 (82.9%) | 491 (73.1%) | |
| First EVL session | 34 (82.9%) | 466 (69.3%) | 0.065 |
| Gastric varices | 15 (36.6%) | 162 (24.1%) | 0.073 |
| Varices in endoscopy | 0.535 | ||
| F1 | 4 (9.8%) | 47 (7.0%) | |
| F2 | 21 (51.2%) | 401 (59.7%) | |
| F3 | 16 (39.0%) | 224 (33.3%) | |
| Prophylactic | 3 (7.3%) | 94 (14.0%) | 0.226 |
| emergency | 38 (92.7%) | 578 (82.0%) | |
| Hepatocellular carcinoma | 20 (48.8%) | 284 (42.3%) | 0.413 |
| Portal vein thrombosis | 5 (12.2%) | 59 (8.8%) | 0.458 |
| Ascites | 26 (63.4%) | 400 (59.5%) | 0.622 |
| Hepatic encephalopathy | 9 (22.0%) | 138 (20.5%) | 0.828 |
| WBC (1000 u/L) | 9.26 ± 6.20 | 7.98 ± 5.82 | 0.176 |
| Hemoglobin (g/dL) | 9.13 ± 1.90 | 9.26 ± 2.02 | 0.692 |
| Platelet (1000 u/L) | 105.93 ± 75.36 | 98.31 ± 65.85 | 0.476 |
| P.T/INR | 1.81 ± 0.56 | 1.56 ± 0.56 | 0.007 |
| Creatinine (mg/dL) | 1.60 ± 1.89 | 1.36 ± 1050 | 0.346 |
| Total. Bilirubin (mg/dL) | 9.57 ± 10.4 | 4.1 ± 6.47 | <0.001 |
| AST (U/L) | 268.3 ± 732 | 150.4 ± 415.2 | 0.096 |
| ALT (U/L) | 117.9 ± 354.1 | 68.5 ± 126.6 | 0.04 |
| Na (mEq/L) | 138.1 ± 8.1 | 137.0 ± 5.8 | 0.249 |
| K (mEq/L) | 4.16 ± 0.85 | 3.98 ± 0.81 | 0.169 |
| Albumin (g/dL) | 2.67 ± 0.62 | 2.89 ± 0.57 | 0.018 |
| APRI (mean ± S.D.) | 7.68 ± 19.98 | 5.14 ± 14.58 | 0.29 |
| FIB-4 | 15.39 ± 18.66 | 12.37 ± 17.16 | 0.277 |
| ALBI | −1.00 ± 0.74 | −1.42 ± 0.65 | <0.001 |
| MELD | 22.9 ± 9.37 | 17.5 ± 8.25 | <0.001 |
| Univariate OR | 95% CI | p Value | Multivariate OR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| Male | 0.967 | 0.48–1.93 | 0.92 | |||
| Age (>50 y/o) | 0.59 | 0.31–1.13 | 0.11 | 0.75 | 0.38–1.49 | 0.41 |
| Alcoholism | 1.54 | 0.82–2.9 | 0.17 | |||
| Child-Pugh score (B + C) | 1.79 | 0.78–4.11 | 0.17 | |||
| Gastric varices | 1.81 | 0.93–3.51 | 0.07 | 2.1 | 1.06–4.16 | 0.03 |
| EV (F2 + F3) | 0.69 | 0.23–2.03 | 0.5 | |||
| HCC | 1.31 | 0.69–2.44 | 0.41 | |||
| PVT | 1.44 | 0.54–3.81 | 0.46 | |||
| HE | 1.08 | 0.5–2.33 | 0.82 | |||
| Ascites | 1.18 | 0.61–2.26 | 0.62 | |||
| Emergent EVL | 2.06 | 0.62–6.8 | 0.23 | |||
| First EVL | 2.14 | 0.93–4.92 | 0.07 | 1.84 | 0.79–4.31 | 0.16 |
| Hemoglobin (>8.0 g/dL) | 0.91 | 0.45–1.82 | 0.79 | |||
| Thrombocytopenia (<150 K/uL) | 0.76 | 0.34–1.7 | 0.5 | |||
| P.T (INR > 2.3) | 2.78 | 1.10–7.01 | 0.03 | 1.2 | 0.44–3.35 | 0.69 |
| Creatinine (>2.0 mg/dL) | 1.73 | 0.80–3.75 | 0.16 | |||
| Total bilirubin (>3.0 mg/dL) | 4.2 | 2.18–8.12 | <0.001 | 3.57 | 1.72–7.41 | 0.001 |
| AST (>200 U/L) | 1.21 | 0.49–2.98 | 0.66 | |||
| ALT(>200 U/L) | 0.76 | 0.18–3.29 | 0.72 | |||
| Na (>150 mEq/L) | 3.71 | 1.02–13.46 | 0.04 | 2.56 | 0.67–9.78 | 0.17 |
| K (>5.0 mEq/L) | 0.93 | 0.322–2.68 | 0.89 | |||
| Albumin (>2.8 g/dL) | 0.56 | 0.29–1.06 | 0.07 | 0.85 | 0.42–1.71 | 0.66 |
| FIB-4 | 1.00 | 0.99–1.02 | 0.28 | |||
| MELD (≥20) | 3.49 | 1.81–6.72 | <0.001 | 3.77 | 1.94–7.33 | <0.001 * |
| ALBI(grade3) | 2.40 | 1.22–4.71 | 0.011 | 2.67 | 1.34–5.30 | 0.005 ** |
| Univariate OR | 95% CI | p Value | |
|---|---|---|---|
| Child-Pugh A | 1 | ||
| Child-Pugh B | 33.0 | 2.45–443.6 | 0.008 |
| Child-Pugh C | 12.0 | 1.20–120 | 0.034 |
| Child-Pugh score | 16.67 | 1.75–158.1 | 0.014 |
| (B + C) | |||
| Gastric varices | 1.97 | 0.53–7.31 | 0.31 |
| MELD (≥20) | 3.10 | 0.82–11.78 | 0.096 |
| ALBI (grade3) | 4.8 | 1.18–19.6 | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-W.; Kuo, C.-J.; Lee, C.-W.; Kuo, T.; Chiu, C.-T.; Lin, C.-J.; Lim, S.-N.; Yeh, C.-T.; Lin, W.-R. Albumin-Bilirubin Grade as a Novel Predictor of the Development and Short-Term Survival of Post-Banding Ulcer Bleeding Following Endoscopic Variceal Ligation in Cirrhotic Patients. Medicina 2022, 58, 1836. https://doi.org/10.3390/medicina58121836
Chen C-W, Kuo C-J, Lee C-W, Kuo T, Chiu C-T, Lin C-J, Lim S-N, Yeh C-T, Lin W-R. Albumin-Bilirubin Grade as a Novel Predictor of the Development and Short-Term Survival of Post-Banding Ulcer Bleeding Following Endoscopic Variceal Ligation in Cirrhotic Patients. Medicina. 2022; 58(12):1836. https://doi.org/10.3390/medicina58121836
Chicago/Turabian StyleChen, Chun-Wei, Chia-Jung Kuo, Chao-Wei Lee, Tony Kuo, Cheng-Tang Chiu, Chun-Jung Lin, Siew-Na Lim, Chau-Ting Yeh, and Wey-Ran Lin. 2022. "Albumin-Bilirubin Grade as a Novel Predictor of the Development and Short-Term Survival of Post-Banding Ulcer Bleeding Following Endoscopic Variceal Ligation in Cirrhotic Patients" Medicina 58, no. 12: 1836. https://doi.org/10.3390/medicina58121836
APA StyleChen, C.-W., Kuo, C.-J., Lee, C.-W., Kuo, T., Chiu, C.-T., Lin, C.-J., Lim, S.-N., Yeh, C.-T., & Lin, W.-R. (2022). Albumin-Bilirubin Grade as a Novel Predictor of the Development and Short-Term Survival of Post-Banding Ulcer Bleeding Following Endoscopic Variceal Ligation in Cirrhotic Patients. Medicina, 58(12), 1836. https://doi.org/10.3390/medicina58121836





