Changes in Body Weight, Body Composition, Phase Angle, and Resting Metabolic Rate in Male Patients with Stage IV Non-Small-Cell Lung Cancer Undergoing Therapy
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patients
2.3. Anthropometric Measurements
2.4. Body Composition Measurements
2.5. Resting Metabolic Rate (RMR) Measurement and Related Parameters
2.6. Lifestlyle Variables
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Changes in Weight, Body Composition, and RMR Parameters in the Whole Sample
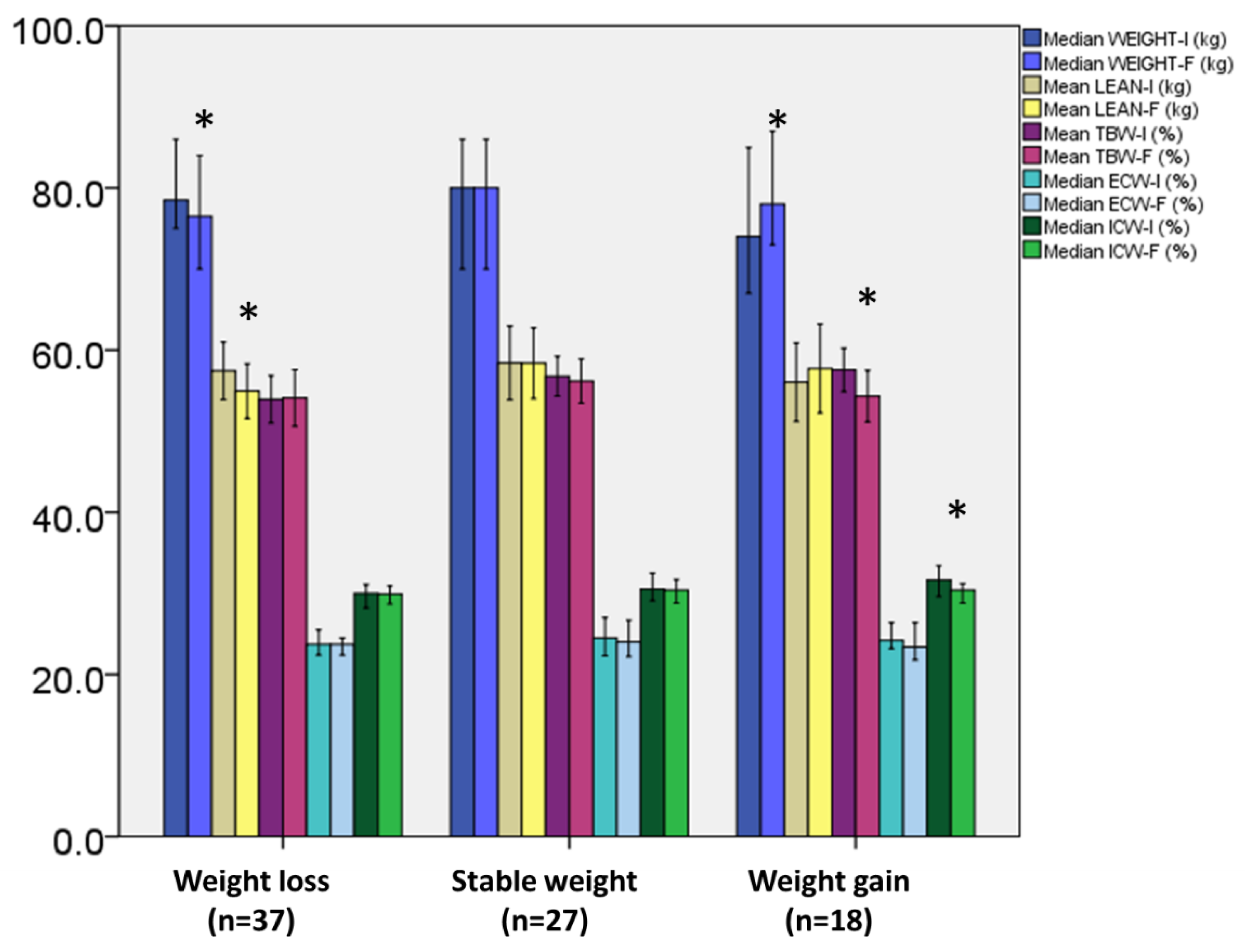
3.3. Changes in Weight, Body Composition, and RMR Parameters according to Weight Change Stratification
3.4. Changes in Weight, Body Composition, and RMR Parameters according Baseline Diet
3.5. Changes in Weight, Body Composition, and RMR Parameters according to Medical Treatment
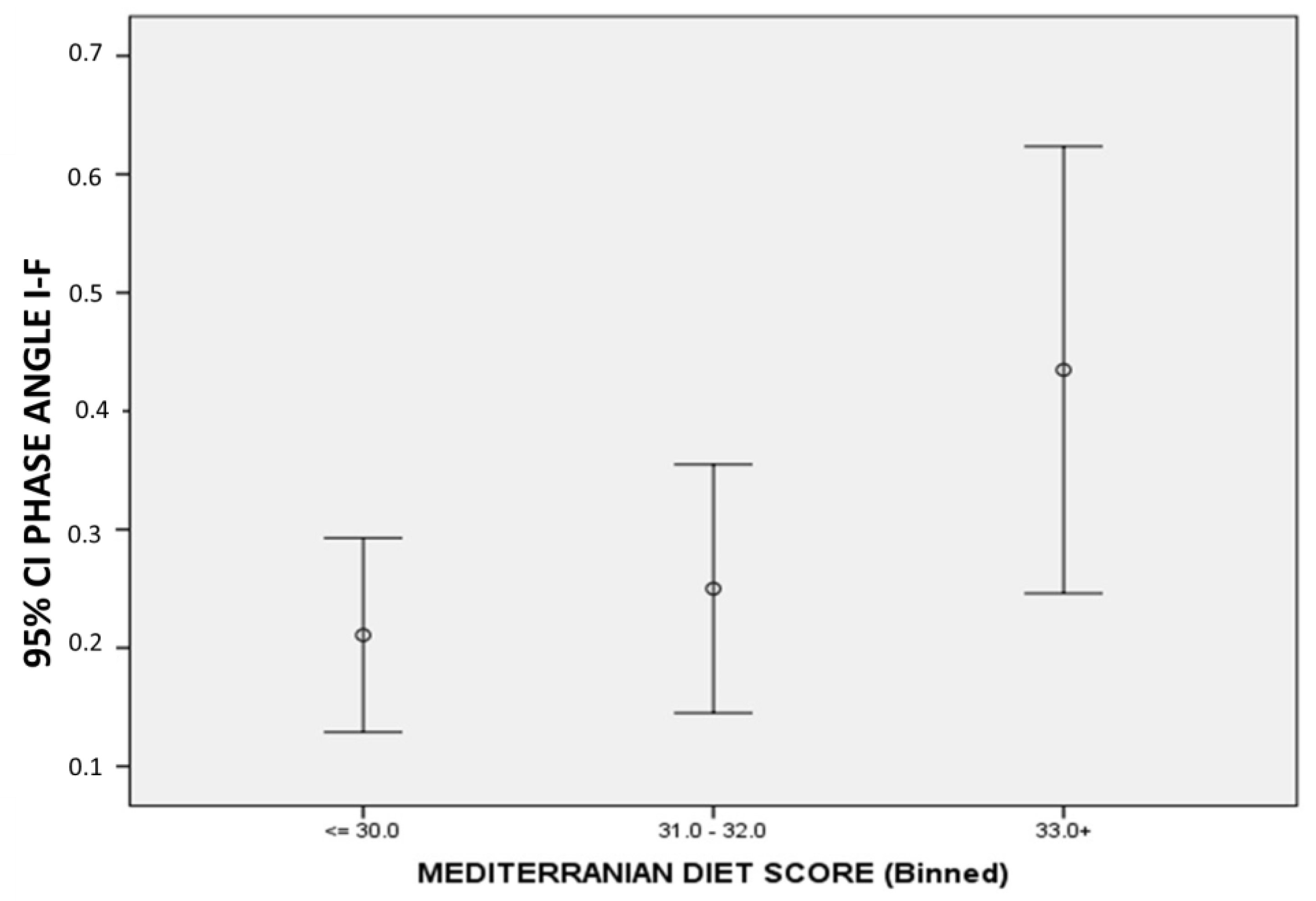
3.6. Correlations between Changes in Body Composition, PhA, and MedDietScore
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BIA | B ioelectrical impedance analysis |
| MedDietScore | Mediterranean diet score |
| NSCLC | Non-small-cell lung cancer |
| PhA | Phase angle |
| R | Resistance |
| Xc | Capacitive reactance |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Poltronieri, T.S.; Pérsico, R.S.; Falcetta, F.S.; Viana, L.V. Changes in Body Adiposity in Women Undergoing Breast Cancer Treatment: A Scoping Review. Nutr. Cancer 2022, 74, 3431–3445. [Google Scholar] [CrossRef]
- Detopoulou, P.; Tsiouda, T.; Pilikidou, M.; Palyvou, F.; Mantzorou, M.; Perzirkianidou, P.; Kyrka, K.; Methenitis, S.; Kondyli, F.S.; Voulgaridou, G.; et al. Dietary Habits Are Related to Phase Angle in Male Patients with Non-Small-Cell Lung Cancer. Curr. Oncol. 2022, 29, 8074–8083. [Google Scholar] [CrossRef]
- Tsiouda, T.; Sardeli, C.; Porpodis, K.; Pilikidou, M.; Apostolidis, G.; Kyrka, K.; Miziou, A.; Kyrka, K.; Tsingerlioti, Z.; Papadopoulou, S.; et al. Sex Differences and Adverse Effects between Chemotherapy and Immunotherapy for Non-Small Cell Lung Cancer. J. Cancer 2020, 11, 3407–3415. [Google Scholar] [CrossRef]
- Willemsen, A.C.H.; Degens, J.H.R.J.; Baijens, L.W.J.; Dingemans, A.-M.C.; Hoeben, A.; Hoebers, F.J.P.; De Ruysscher, D.K.M.; Schols, A.M.W.J. Early Loss of Fat Mass During Chemoradiotherapy Predicts Overall Survival in Locally Advanced Squamous Cell Carcinoma of the Lung, but Not in Locally Advanced Squamous Cell Carcinoma of the Head and Neck. Front. Nutr. 2020, 7, 600612. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Voulgaridou, G.; Papadopoulou, S. Cancer, Phase Angle and Sarcopenia: The Role of Diet in Connection with Lung Cancer Prognosis. Lung 2022, 200, 347–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, H.; Zhou, S.; Wang, D.; Zhu, L.; Hou, J.; Tang, J.; Zhao, J.; Zhong, S. Body Mass Index and Mortality in Lung Cancer Patients: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2018, 72, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.D.; Pereira, J.R.; Chen, J.; Liu, J.; Guba, S.C.; John, W.J.; Orlando, M.; Scagliotti, G.; Bonomi, P.D. Relationship between Efficacy Outcomes and Weight Gain during Treatment of Advanced, Non-Squamous, Non-Small-Cell Lung Cancer Patients. Ann. Oncol. 2016, 27, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Sher, D.J.; Gielda, B.T.; Liptay, M.J.; Warren, W.H.; Batus, M.; Fidler, M.J.; Garg, S.; Bonomi, P. Prognostic Significance of Weight Gain During Definitive Chemoradiotherapy for Locally Advanced Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2013, 14, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Topkan, E.; Parlak, C.; Selek, U. Impact of Weight Change During the Course of Concurrent Chemoradiation Therapy on Outcomes in Stage IIIB Non-Small Cell Lung Cancer Patients: Retrospective Analysis of 425 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Gielda, B.T.; Mehta, P.; Khan, A.; Marsh, J.C.; Zusag, T.W.; Warren, W.H.; Fidler, M.J.; Abrams, R.A.; Bonomi, P.; Liptay, M.; et al. Weight Gain in Advanced Non–Small-Cell Lung Cancer Patients During Treatment With Split-Course Concurrent Chemoradiotherapy Is Associated With Superior Survival. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 985–991. [Google Scholar] [CrossRef]
- Khaddour, K.; Gomez-Perez, S.L.; Jain, N.; Patel, J.D.; Boumber, Y. Obesity, Sarcopenia, and Outcomes in Non-Small Cell Lung Cancer Patients Treated With Immune Checkpoint Inhibitors and Tyrosine Kinase Inhibitors. Front. Oncol. 2020, 10, 576314. [Google Scholar] [CrossRef] [PubMed]
- de Jong, C.; Chargi, N.; Herder, G.J.M.; van Haarlem, S.W.A.; van der Meer, F.; van Lindert, A.S.R.; ten Heuvel, A.; Brouwer, J.; de Jong, P.A.; Devriese, L.A.; et al. The Association between Skeletal Muscle Measures and Chemotherapy-induced Toxicity in Non-small Cell Lung Cancer Patients. J. Cachexia Sarcopenia Muscle 2022, 13, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Chargi, N.; Molenaar-Kuijsten, L.; Huiskamp, L.F.J.; Devriese, L.A.; de Bree, R.; Huitema, A.D.R. The Association of Cisplatin Pharmacokinetics and Skeletal Muscle Mass in Patients with Head and Neck Cancer: The Prospective PLATISMA Study. Eur. J. Cancer 2022, 160, 92–99. [Google Scholar] [CrossRef]
- Cooley, M.E. Symptoms in Adults with Lung Cancer. J. Pain Symptom Manag. 2000, 19, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, V.; Alesi, E.; Myers, J.; Hackney, M.H.; Cuttino, L.; Gewirtz, D.A. An Overview of Chemotoxicity and Radiation Toxicity in Cancer Therapy. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2022; Volume 155, pp. 1–27. ISBN 978-0-323-90087-4. [Google Scholar]
- Tüccar, T.; Tek, N. Determining the Factors Affecting Energy Metabolism and Energy Requirement in Cancer Patients. J. Res. Med. Sci. 2021, 26, 124. [Google Scholar] [CrossRef]
- Pilikidou, M.; Palyvou, F.; Papadopoulou, S.; Tsiouda, T.; Tsekitsidi, E.; Arvaniti, K.; Miziou, A.; Tsingerlioti, Z.; Apostolidis, G.; Ntiloudis, R.; et al. Lung Cancer, Treatment and Nutritional Status. Mol. Clin. Oncol. 2021, 15, 248. [Google Scholar] [CrossRef]
- Purcell, S.A.; Wallengren, O.; Baracos, V.E.; Lundholm, K.; Iresjö, B.-M.; Chu, Q.S.C.; Ghosh, S.S.; Prado, C.M. Determinants of Change in Resting Energy Expenditure in Patients with Stage III/IV Colorectal Cancer. Clin. Nutr. 2020, 39, 134–140. [Google Scholar] [CrossRef]
- Zampino, M.; AlGhatrif, M.; Kuo, P.-L.; Simonsick, E.M.; Ferrucci, L. Longitudinal Changes in Resting Metabolic Rates with Aging Are Accelerated by Diseases. Nutrients 2020, 12, 3061. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary Patterns: A Mediterranean Diet Score and Its Relation to Clinical and Biological Markers of Cardiovascular Disease Risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A Study of Concurrent and Construct Validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Lai, C.-H.; Lin, C.-Y.; Tsai, Y.-H.; Chang, Y.-C.; Chen, H.-C.; Tseng, C.-C.; Chang, H.-C.; Huang, K.-T.; Chen, Y.-C.; et al. Body Mass Index, Weight Loss, and Mortality Risk in Advanced-Stage Non-Small Cell Lung Cancer Patients: A Focus on EGFR Mutation. Nutrients 2021, 13, 3761. [Google Scholar] [CrossRef] [PubMed]
- Le-Rademacher, J.; Lopez, C.; Wolfe, E.; Foster, N.R.; Mandrekar, S.J.; Wang, X.; Kumar, R.; Adjei, A.; Jatoi, A. Weight Loss over Time and Survival: A Landmark Analysis of 1000+ Prospectively Treated and Monitored Lung Cancer Patients. J. Cachexia Sarcopenia Muscle 2020, 11, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Degens, J.H.R.J.; Dingemans, A.C.; Willemsen, A.C.H.; Gietema, H.A.; Hurkmans, D.P.; Aerts, J.G.; Hendriks, L.E.L.; Schols, A.M.W.J. The Prognostic Value of Weight and Body Composition Changes in Patients with Non-small-cell Lung Cancer Treated with Nivolumab. J. Cachexia Sarcopenia Muscle 2021, 12, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Isenring, E.; Gough, K.; Krishnasamy, M. The Prevalence of Weight Loss during (Chemo)Radiotherapy Treatment for Lung Cancer and Associated Patient- and Treatment-Related Factors. Clin. Nutr. 2014, 33, 1074–1080. [Google Scholar] [CrossRef]
- The SCRINIO Working Group; Bozzetti, F.; Mariani, L.; Lo Vullo, S.; Amerio, M.L.; Biffi, R.; Caccialanza, R.; Capuano, G.; Correja, I.; Cozzaglio, L.; et al. The Nutritional Risk in Oncology: A Study of 1,453 Cancer Outpatients. Support Care Cancer 2012, 20, 1919–1928. [Google Scholar] [CrossRef]
- Schneider, S.M.; Veyres, P.; Pivot, X.; Soummer, A.-M.; Jambou, P.; Filippi, J.; van Obberghen, E.; Hébuterne, X. Malnutrition Is an Independent Factor Associated with Nosocomial Infections. Br. J. Nutr. 2004, 92, 105–111. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body Composition as an Independent Determinant of 5-Fluorouracil–Based Chemotherapy Toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef] [PubMed]
- Matias, C.N.; Nunes, C.L.; Francisco, S.; Tomeleri, C.M.; Cyrino, E.S.; Sardinha, L.B.; Silva, A.M. Phase Angle Predicts Physical Function in Older Adults. Arch. Gerontol. Geriatr. 2020, 90, 104151. [Google Scholar] [CrossRef]
- Hsiao, M.-Y.; Chang, K.-V.; Wu, W.-T.; Huang, K.-C.; Han, D.-S. Grip Strength and Demographic Variables Estimate Appendicular Muscle Mass Better Than Bioelectrical Impedance in Taiwanese Older Persons. J. Am. Med. Dir. Assoc. 2021, 22, 760–765. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Macchia, P.; Di Somma, C.; Falco, A.; Savanelli, M.; Colao, A.; Savastano, S. Mediterranean Diet and Phase Angle in a Sample of Adult Population: Results of a Pilot Study. Nutrients 2017, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.T.; Davies, J.W.L.; Burns, H.J.G. The Relationship Between Resting Energy Expenditure and Weight Loss in Benign and Malignant Disease: Ann. Surg. 1986, 203, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Takemura, Y.; Sasaki, M.; Goto, K.; Takaoka, A.; Ohi, A.; Kurihara, M.; Nakanishi, N.; Nakano, Y.; Hanaoka, J. Energy Metabolism and Nutritional Status in Hospitalized Patients with Lung Cancer. J. Clin. Biochem Nutr. 2016, 59, 122–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wall, B.A.; Galvão, D.A.; Fatehee, N.; Taaffe, D.R.; Spry, N.; Joseph, D.; Newton, R.U. Reduced Cardiovascular Capacity and Resting Metabolic Rate in Men with Prostate Cancer Undergoing Androgen Deprivation: A Comprehensive Cross-Sectional Investigation. Adv. Urol. 2015, 2015, 976235. [Google Scholar] [CrossRef]
- Talwar, D.; Ha, T.K.; Scott, H.R.; Cooney, J.; Fell, G.S.; O’Reilly, D.S.; Lean, M.E.; McMillan, D.C. Effect of Inflammation on Measures of Antioxidant Status in Patients with Non-Small Cell Lung Cancer. Am. J. Clin. Nutr. 1997, 66, 1283–1285. [Google Scholar] [CrossRef][Green Version]
- Suh, S.-Y.; LeBlanc, T.W.; Shelby, R.A.; Samsa, G.P.; Abernethy, A.P. Longitudinal Patient-Reported Performance Status Assessment in the Cancer Clinic Is Feasible and Prognostic. JOP 2011, 7, 374–381. [Google Scholar] [CrossRef]
- Purcell, S.A.; Elliott, S.A.; Ryan, A.M.; Sawyer, M.B.; Prado, C.M. Accuracy of a Portable Indirect Calorimeter for Measuring Resting Energy Expenditure in Individuals With Cancer. J. Parenter. Enter. Nutr. 2019, 43, 145–151. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Du, Y.; Wu, X.; Chang, Y.; Li, W.; Liu, Y.; Hu, W.; Zhao, J. The Clinical Application Value of Phase Angle of Six Parts in Nutritional Evaluation of Tumor Patients. Support Care Cancer 2022, 30, 7983–7989. [Google Scholar] [CrossRef]
| Total (n = 82) | ||
|---|---|---|
| Mean or Median | SD or 25th–75th | |
| Age (years) | 65.8 | 9.1 |
| Pack years | 75.5 | 47.5–102.5 |
| BMI (kg/m2) | 26.9 | 5.0 |
| Waist circumference (cm) | 105.0 | 96.0–120.0 |
| Hip circumference (cm) | 104.0 | 98.0–111.2 |
| Waist-to-hip ratio | 1.04 | 0.94–1.10 |
| MedDietScore | 31.0 | 29.0–33.0 |
| Frequency (n) | % | |
| Chemotherapy (C) | 13 | 15.7 |
| Immunotherapy (I) | 11 | 13.3 |
| C + I | 21 | 25.3 |
| C +targeted treatment * | 5 | 6.0 |
| C+ Radiotherapy (R) | 19 | 22.9 |
| I + R | 4 | 4.8 |
| R + P + I + P | 3 | 3.6 |
| C + R + P | 1 | 1.2 |
| Baseline | Follow Up | ||
|---|---|---|---|
| Mean ± SD or Median, 25th–75th | Mean ± SD or Median, 25th–75th | p | |
| Weight (Kg) | 78.0, 70–87.2 | 77.0, 68.0–87.0 | 0.07 |
| BMI (kg/m2) | 26.9 ± 5.0 | 26.9± 5.7 | 0.9 |
| Waist circumference (cm) | 105.0, 96.0–120.0 | 107.0, 97.0–120.0 | 0.7 |
| Total body fat (%) | 27.8 ± 7.1 | 28.5 ± 7.3 | 0.2 |
| Total lean mass (kg) | 57.4 ± 10.6 | 56.6 ± 10.60 | 0.1 |
| Total body water (%) | 55.6 ± 7.4 | 54.8 ± 8.5 | 0.4 |
| Extracellular water (%) | 24.2, 22.1–26.6 | 23.8, 21.8–26.4 | 0.2 |
| Intracellular water (%) | 30.4, 29.0–32.7 | 30.1, 28.4–31.6 | 0.009 |
| PhA (o) | 5.1 ± 0.8 | 4.9 ± 0.8 | <0.0001 |
| Resting metabolic rate (Kcal) | 1869 ± 414 | 1743 ± 367 | 0.002 |
| VO2 (mL/min) | 267.7 ± 60.7 | 248.3 ± 53.3 | 0.002 |
| Ventilation rate (L/min) | 9.98 ± 2.08 | 9.41 ± 2.28 | 0.03 |
| Respiratory frequency (breaths/min) | 19.35 ± 4.78 | 19.50 ± 4.03 | 0.7 |
| Fraction of exhaled oxygen (%) | 17.7 ± 0.44 | 17.7 ± 0.54 | 0.8 |
| Baseline | Follow Up | p | |
|---|---|---|---|
| Mean ± SD or Median, 25th–75th | Mean ± SD or Median, 25th–75th | ||
| Subjects with weight loss (n = 37) | |||
| BMI (kg/m2) | 27.4 ± 5.4 | 26.1 ± 5.2 | <0.0001 |
| Total body fat (%) | 29.38 ± 9.04 | 29.37 ± 6.01 | 0.9 |
| Waist circumference (cm) | 101.0, 95.5–118.5 | 106.0, 97.0–119.0 | 0.8 |
| Hips circumference (cm) | 103.0, 98.0–113.0 | 102.0, 97.5–109.0 | 0.06 |
| PhA (o) | 5.15 ± 0.75 | 4.85 ± 0.73 | <0.0001 |
| Resting metabolic rate (Kcal) | 1862 ± 419 | 1797 ± 375 | 0.2 |
| VO2 (mL/min) | 265.5 ± 62.9 | 255.9± 54.8 | 0.2 |
| Ventilation rate (L/min) | 9.79 ± 2.14 | 9.81 ± 2.31 | 0.9 |
| Respiratory frequency (breaths/min) | 18.81 ± 5.64 | 19.91 ± 4.61 | 0.1 |
| Fraction of exhaled oxygen (%) | 17.74 ± 0.50 | 17.7 ± 0.57 | 0.4 |
| Subjects with stable weight (n = 27) | |||
| BMI (kg/m2) | 27.5 ± 5.5 | 27.1 ± 5.5 | 1.0 |
| Total body fat (%) | 26.62 ± 8.32 | 26.7 ± 8.85 | 0.6 |
| Waist circumference (cm) | 110.0, 96.0–122.0 | 107.0, 94.0–122.0 | 0.3 |
| Hips circumference (cm) | 106.0, 98.0–113.0 | 105.0, 93.0–110.0 | 0.3 |
| PhA (o) | 5.16 ± 0.90 | 4.93 ± 0.89 | <0.0001 |
| Resting metabolic rate (Kcal) | 1767 ± 385 | 1650 ± 351 | 0.05 |
| VO2 (mL/min) | 254.2 ± 55.2 | 234.8 ± 50.8 | 0.04 |
| Ventilation rate (L/min) | 9.6 ± 2.0 | 8.9 ± 2.2 | 0.03 |
| Respiratory frequency (breaths/min) | 19.0 ± 3.8 | 18.9 ± 3.7 | 0.8 |
| Fraction of exhaled oxygen (%) | 17.8 ± 0.46 | 17.8 ± 0.60 | 0.8 |
| Subjects with weight gain (n = 18) | |||
| BMI (kg/m2) | 25.7, 24.1–27.2 | 25.3, 26.9–29.2 | <0.0001 |
| Total body fat (%) | 26.68 ± 4.92 | 29.61 ± 7.41 | 0.08 |
| Waist circumference (cm) | 107.0, 100.0–115.7 | 108.0, 101.0–114.5 | 0.2 |
| Hips circumference (cm) | 101.5, 98.2–110.2 | 105.5, 101.2–110.0 | 0.3 |
| PhA (o) | 5.30 ± 0.86 | 4.96 ± 0.85 | 0.03 |
| Resting metabolic rate (Kcal) | 2035 ± 415 | 1771 ± 365 | 0.03 |
| VO2 (mL/min) | 292.2 ± 59.80 | 253.2 ± 53.0 | 0.02 |
| Ventilation rate (L/min) | 10.8 ± 1.91 | 9.2 ± 2.1 | 0.04 |
| Respiratory frequency (breaths/min) | 20.8 ± 3.9 | 19.4 ± 3.08 | 0.1 |
| Fraction of exhaled oxygen (%) | 17.7 ± 0.26 | 17.6 ± 0.39 | 0.4 |
| Weight Change (kg) | BMI Change (kg/m²) | Waist Circumference Change (cm) | Fat Change (%) | Lean Mass Change (kg) | TBW Change (%) | ECW Change (%) | Phase Angle Change (°) | ||
|---|---|---|---|---|---|---|---|---|---|
| Weight change (kg) | rho | 0.998 | 0.193 | 0.322 | 0.534 | −0.314 | −0.252 | −0.01 | |
| p | <0.001 | 0.08 | 0.003 | <0.001 | 0.004 | 0.02 | 0.9 | ||
| BMI change (kg/m²) | rho | 0.998 | 0.199 | 0.328 | 0.527 | −0.320 | −0.260 | −0.02 | |
| p | <0.001 | 0.07 | 0.003 | <0.001 | 0.003 | 0.01 | 0.8 | ||
| Waist circumference change (cm) | rho | 0.193 | 0.199 | 0.268 | −0.01 | −0.14 | −0.03 | −0.08 | |
| p | 0.08 | 0.07 | 0.01 | 0.9 | 0.2 | 0.7 | 0.4 | ||
| Total body fat change (%) | rho | 0.322 | 0.328 | 0.268 | −0.359 | −0.749 | −0.615 | −0.12 | |
| p | 0.003 | 0.003 | 0.01 | <0.001 | <0.001 | <0.001 | 0.2 | ||
| Lean mass change (kg) | rho | 0.534 | 0.527 | −0.01 | −0.359 | 0.202 | 0.147 | 0.201 | |
| p | <0.001 | <0.001 | 0.9 | <0.001 | 0.06 | 0.1 | 0.07 | ||
| TBW change (%) | rho | −0.314 | −0.320 | −0.14 | −0.749 | 0.202 | 0.831 | 0.137 | |
| p | 0.004 | 0.003 | 0.2 | <0.001 | 0.06 | <0.001 | 0.2 | ||
| ECW change (%) | rho | −0.252 | −0.260 | −0.03 | −0.615 | 0.147 | 0.831 | 0.116 | |
| p | 0.02 | 0.01 | 0.7 | <0.001 | 0.1 | <0.001 | 0.2 | ||
| PhA (o) | rho | −0.01 | −0.02 | −0.08 | −0.117 | 0.201 | 0.137 | 0.116 | |
| p | 0.9 | 0.8 | 0.4 | 0.2 | 0.07 | 0.22 | 0.2 | ||
| Mediterranean diet score | rho | −0.12 | −0.13 | −0.04 | −0.154 | −0.01 | 0.093 | 0.171 | 0.251 |
| p | 0.2 | 0.2 | 0.7 | 0.1 | 0.9 | 0.4 | 0.1 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detopoulou, P.; Tsiouda, T.; Pilikidou, M.; Palyvou, F.; Tsekitsidi, E.; Mantzorou, M.; Pezirkianidou, P.; Kyrka, K.; Methenitis, S.; Voulgaridou, G.; et al. Changes in Body Weight, Body Composition, Phase Angle, and Resting Metabolic Rate in Male Patients with Stage IV Non-Small-Cell Lung Cancer Undergoing Therapy. Medicina 2022, 58, 1779. https://doi.org/10.3390/medicina58121779
Detopoulou P, Tsiouda T, Pilikidou M, Palyvou F, Tsekitsidi E, Mantzorou M, Pezirkianidou P, Kyrka K, Methenitis S, Voulgaridou G, et al. Changes in Body Weight, Body Composition, Phase Angle, and Resting Metabolic Rate in Male Patients with Stage IV Non-Small-Cell Lung Cancer Undergoing Therapy. Medicina. 2022; 58(12):1779. https://doi.org/10.3390/medicina58121779
Chicago/Turabian StyleDetopoulou, Paraskevi, Theodora Tsiouda, Maria Pilikidou, Foteini Palyvou, Eirini Tsekitsidi, Maria Mantzorou, Persefoni Pezirkianidou, Krystallia Kyrka, Spyridon Methenitis, Gavriela Voulgaridou, and et al. 2022. "Changes in Body Weight, Body Composition, Phase Angle, and Resting Metabolic Rate in Male Patients with Stage IV Non-Small-Cell Lung Cancer Undergoing Therapy" Medicina 58, no. 12: 1779. https://doi.org/10.3390/medicina58121779
APA StyleDetopoulou, P., Tsiouda, T., Pilikidou, M., Palyvou, F., Tsekitsidi, E., Mantzorou, M., Pezirkianidou, P., Kyrka, K., Methenitis, S., Voulgaridou, G., Zarogoulidis, P., Oikonomidou, R., Matthaios, D., Porpodis, Κ., Giannakidis, D., & Papadopoulou, S. K. (2022). Changes in Body Weight, Body Composition, Phase Angle, and Resting Metabolic Rate in Male Patients with Stage IV Non-Small-Cell Lung Cancer Undergoing Therapy. Medicina, 58(12), 1779. https://doi.org/10.3390/medicina58121779












