Abstract
Background and Objectives: In pre-eclampsia, restricted blood supply due to the lack of trophoblastic cell invasion and spiral artery remodeling is responsible for adverse pregnancies and maternal outcomes, which is added to by maternal undernutrition. This study was designed to observe the effect of multiple nutritional micronutrient supplements on the pregnancy outcomes of underweight pre-eclamptic women. To investigate the effects of lipid-based multiple micr supplementations (LNS-PLW) on pregnancy and maternal outcomes in underweight primigravida pre-eclamptic women. Materials and Methods: A total of 60 pre-eclamptic, underweight primigravida women from the antenatal units of tertiary care hospitals in the Khyber Pakhtunkhwa Province, Pakistan, were randomly divided into two groups (Group 1 and Group 2). The participants of both groups were receiving routine treatment for pre-eclampsia: iron (60 mgs) and folic acid (400 ug) IFA daily. Group 2 was given an additional sachet of 75 gm LNS-PLW daily till delivery. The pregnancy outcomes of both groups were recorded. The clinical parameters, hemoglobin, platelet count, and proteinuria were measured at recruitment. Results: The percentage of live births in Group 2 was 93% compared to 92% in Group 1. There were more normal vaginal deliveries (NVDs) in Group 2 compared to Group 1 (Group 2, 78% NVD; group 1, 69% NVD). In Group 1, 4% of the participants developed eclampsia. The frequency of cesarean sections was 8/26 (31%) in Group 1 and 6/28 (22%) in Group 2. The number of intrauterine deaths (IUDs) was only 1/28 (4%) in Group 2, while it was 2/26 (8%) in Group 1. The gestational age at delivery significantly improved with LNS-PLW supplementation (Group 2, 38.64 ± 0.78 weeks; Group 1, 36.88 ± 1.55 weeks, p-value 0.006). The Apgar score (Group 2, 9.3; Group 1, 8.4) and the birth weight of the babies improved with maternal supplementation with LNS-PLW (Group 2, 38.64 ± 0.78 weeks: Group 1, 36.88 ± 1.55; p-value 0.003). There was no significant difference in systolic blood pressure, while diastolic blood pressure (Group 2, 89.57 ± 2.08 mmHg; Group 1, 92.17 ± 5.18 mmHg, p-value 0.025) showed significant improvement with LNS-PLW supplementation. The hemoglobin concentration increased with the LNS-PLW supplement consumed in Group 2 (Group 2, 12.15 ± 0.78 g/dL; Group 1, 11.39 ± 0.48 g/dL, p-value < 0.001). However, no significant difference among the platelet counts of the two groups was observed. Conclusions: The pregnancy and maternal outcomes of underweight pre-eclamptic women can be improved by the prenatal daily supplementation of LNS-PLW during pregnancy, along with IFA and regular antenatal care and follow-up.
1. Introduction
Pre-eclampsia is the most common hypertensive disorder in pregnancy. About 3–5% of first pregnancies are complicated by pre-eclampsia globally, with a near 15% recurrence rate in subsequent pregnancies [1]. It is characterized by hypertension, proteinuria, and edema ≥20 weeks of gestation in women previously normotensive [2,3]. Pre-eclampsia is a multisystem disorder with a placental origin and creates immediate and long-term fetal and maternal complications. It is documented as one cause of maternal mortality, morbidity, and stillbirths [4,5,6]. In Pakistan, approximately 19% of maternal mortality is attributed to pre-eclampsia [7], rating only third after postpartum hemorrhage and sepsis [4]. Pre-eclampsia may be subclassified as mild and severe, depending upon the presence of hypertension [1]: mild pre-eclampsia with BP (140–159/90–109 mmHg) and severe pre-eclampsia with BP (≥160/110 mmHg) [8]. On the bases of gestational age, pre-eclampsia can also be subclassified as being early-onset (≤34 weeks) due to fetal disorders, primarily through imperfect placentation. Late-onset (≥34 weeks) is due to maternal disorders, lowered maternal thresholds, and excessive physiological placentation [4]. Pre-eclampsia occurs due to abnormal trophoblastic invasion, causing reduced placental perfusion and the replacement of maternal arteriole endothelial cells through an imbalance between the angiogenic and antiangiogenic factors. This, in turn, leads to the damage and dysfunction of the endothelium of maternal arteries, leading to hypertension (due to vasoconstriction), edema due to increased vascular permeability, and proteinuria (due to glomerular damage) [1]. The angiogenic factors are essential for the vessel’s health and for new vessel formation and organ development. In pre-eclampsia, the high levels of soluble fms-like tyrosine kinase-1(sFIt-1), soluble Endoglin (sEng), and antiangiogenic factors affect the levels of placental growth factor (PIGF), transforming growth factor β (TGEβ) and vascular endothelial growth factor, respectively [1]. These low levels lead to endothelial dysfunction and the clinical characteristics of pre-eclampsia [6]. The placental growth factor (PIGF) and soluble fms-like tyrosine kinase-1(sFIt-1) are used to differentiate pre-eclampsia from other diseases, like chronic hypertension and kidney diseases [5]. The pathophysiology of pre-eclampsia occurs due to abnormal trophoblastic invasions, causing reduced placental perfusion and the replacement of maternal arteriole endothelial cells through an imbalance between the angiogenic and antiangiogenic factors [5].
Pre-eclampsia is (7.5%) more common among nulliparous women than parous women [7]. A young age, being underweight, and multiple pregnancies are also non-specific risk factors of pre-eclampsia [9]. Moreover, malnutrition, undernutrition, high-fat/fiber diets, and micronutrient deficiency are risk factors for pre-eclampsia [10,11,12]. The nutritional status of pregnant women plays a vital role in fetal growth and development [13,14]. In low-income countries, maternal undernutrition and anemia are the common causes of maternal mortality and morbidity. Women during pregnancy are at risk of nutritional deficiencies because of the added burden of the metabolism of the fetus and the growing placenta [1]. The deficiency of macro- and micronutrients is common among women of child-bearing age in developing countries [8]. The association between multiple micronutrient deficiencies and the development of pre-eclampsia has also been reported [14,15]. Therefore, undernutrition during pregnancy affects not only the mother’s health but also the health of the growing fetus. In order to balance and improve the micronutrient deficiencies and pregnancy outcomes, iron, folic acid, calcium, and multiple micronutrient supplements are used during pregnancy and before conception [4,5]. In order to improve maternal nutrition and pregnancy outcomes, lipid-based multiple micronutrient supplements are given to underweight pregnant and lactating mothers (LNS-PLW) in underdeveloped countries [6,7]. The benefits of lipid-based multiple micronutrient supplementation have been assessed in normotensive undernourished women [6]. The LNS-PLW is a peanut-based product from the World Food Program (WFP) that is packed in a ready-to-use sachet of 75 gm, having an energy of 400 kcal (Table 1). It is provided to childbearing-aged women to improve maternal nutrition and pregnancy outcomes in underdeveloped countries like Pakistan [1,8].

Table 1.
Chemical composition of (LNS-PLW).
The World Health Organization has recommended that pre-eclampsia screening should be a part of routine antenatal investigation. Most of the routine antenatal investigations are done for the clinical condition which has already been present like screening for viral disease or ultrasound-based screening for morphological abnormalities of the fetus. There is no predictive screening for diseases that have not yet occurred or developed, for example, pre-eclampsia and eclampsia. The outcomes of such a condition are alarming once it develops; therefore, the early detection and prevention of prophylactic therapy may be beneficial. This would enable the clinician to start timely preventive measures, antenatal care, and prophylactic treatment [16,17]. Pre-eclampsia screening has no real cost or side effect from its implementation; therefore, it must be part of the routine antenatal investigation or examination [18]. There are different screening strategies for pre-eclampsia, ranging from simple maternal history analysis to early screening biomarkers.
The screening approaches, which are in practice nowadays, include a detailed maternal history [18], the measurement of mean arterial blood pressure of the pregnant mother, performing Doppler ultrasounds on uterine arteries [19], the identification of serum cardio-metabolic biomarkers [20], the identification of novel markers, like cell-free DNA [21], multiple approaches, etc. [22]. The management of pre-eclampsia is divided into three stages: prevention, early detection, and treatment. Dietary counseling, stopping smoking, the management of pre-existing hepatic and renal diseases, giving low-dose aspirin before 12 weeks of pregnancy, calcium supplementation, antihypertensive drugs, and magnesium sulfate are all prescribed for the prevention of eclampsia. Moreover, during hospitalization due to severe pre-eclampsia (for the termination of the pregnancy), a cesarean section may help in the management of the condition [23].
Pre-eclampsia is more common in primigravida, and it is associated with micronutrient deficiencies. Our country is facing a lack of a proper diet, especially for women of childbearing age, due to poverty. Nutritional deficiency is one of the risk factors of pre-eclampsia. To our knowledge, little evidence is available on the effect of LNS-PLW in pre-eclamptic pregnancy and maternal outcomes in our region. The prevalence of undernutrition at childbearing age is quite high; therefore, the current study was designed and conducted to assess the impact of lipid-based multiple micronutrient supplementation (LNS-PLW) on the maternal and pregnancy outcomes of pre-eclamptic underweight women during their first pregnancy.
2. Methods
After screening, 463 pre-eclamptic women from April 2018-December 2019 (Figure 1) we recruited, considering primigravida pre-eclamptic women whose BMI was less than the requirement for their gestational age retrospectively on their first antenatal visit from their antenatal record file. The BMI is calculated by using a computer pregnancy weight gain calculator. This study was conducted in the tertiary health care facilities of Peshawar (Lady Reading Hospital, Hayatabad Medical Complex) and Swat (Civil Hospital Matta), Khyber Pakhtunkhwa (KP) province of Pakistan. The trial was approved by Khyber Medical University, by the Advanced Study and Research Board, and the Ethical Committee (No: DIR/KMU-AS&RB/EN/000527-18 August 2016) (DIR/KMU = EB/EN/000314 on 27 October 2016). The participants were divided by using a computer randomizer (version 3.0) (Medical University of Graz, Institute for Medical Informatics, Statistics and Documentation Auenbruggerplatz 2/5, A-8036 Graz, Austria) into two groups (Group 1 and Group 2) and informed consent was obtained from them. The participants of both groups received conventional treatment for pre-eclampsia and IFA daily. The Group 2 participants received an additional 75 gm sachet of high-energy LNS-PLW supplement daily till delivery. One participant of Group 2 disliked the taste of LNS-PLW, and another participant lost contact and follow-up, so they dropped out. Two participants in Group 1 refused to continue, and two participants lost contact and dropped out. Therefore, 26 participants from Group 1 and 28 participants from Group 2 completed the follow-up, and we collected their pregnancy and maternal outcomes.
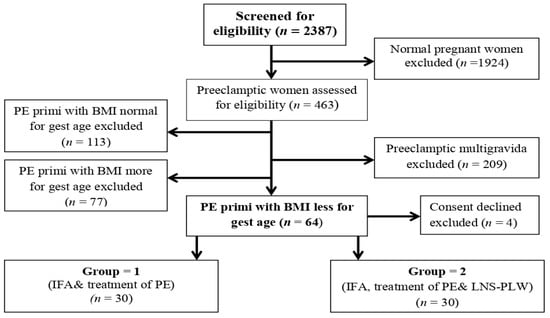
Figure 1.
Flow chart showing the enrollment of the participants and intervention allocation. Abbreviations: PE: Preeclampsia, BMI: Basal metabolic index, IFA: Iron folic acid, LNS-PLW: Lipid based nutritional supplements for pregnant and lactating women.
2.1. Data Collection
The socioeconomic data, family, and personal histories were recorded by predesigned questionnaires given to each participant. The age, gestational age, anthropometric measurements (height, weight, BMI), systolic and diastolic blood pressure, and the proteinuria of the participants were measured. About 5 mL of blood was taken from each participant at the time of enrollment and then delivered via an aseptic technique. The hemoglobin and platelet counts were measured by Sysmex XE-2100 hematology analyzer (Sysmex Corporation, Shisumekkusu Kabushiki-gaisha, Tokyo, Japan). The LNS-PLW was provided weekly and the empty sachets were collected to check their compliance. The leftovers in the sachet were measured. The principal researcher reminded each participant, telephonically, on alternate days to take their medication and LNS-PLW supplement. Their weight was measured by a Beurer digital glass weight scale-GS 200 Allium (Lessingstraße 10 b 89231 Neu-Ulm, Germany), and for their height, a portable stadiometer (Seca Leicester 214) (Hammer Steindamm 9-25. 22089 Hamburg, Germany) was used. The BMI kg/m2 of the participants was measured. At 10 min intervals, the blood pressure of the patients was measured three times by using a standard mercury sphygmomanometer, and the mean was noted. Proteinuria was measured via the dipstick method. The mode of delivery was also recorded.
2.2. Statistical Analysis
The Shapiro–Wilk test was used for normalizing the data; it was found to be normally distributed. The demographic data were analyzed as descriptive statistics, and the values were presented as mean ± standard deviation. The data of the two groups were compared by student t-testing. For the statistical analyses, we used SPSS software 22 (IBM, Armonk, NY, USA), and p < 0.05 was considered significant.
3. Results
The mean age of the participants (Group 1: 22.1 ± 3.3 years; Group 2: 23.3 ± 3.6 years, p-value 0.17) ranged from 15–35 years. Their BMI at the first antenatal visit was calculated retrospectively from their antenatal record (Group 1: 19.6 ± 1.3; Group 2: 19.7 ± 0.9, p-value 0.84). While the BMI kg/m2 at enrollment was recorded as Group 1: 22.6 ± 1.5 kg/m2; Group 2, 22.5 ± 1.3 kg/m2, p-value 0.459. The gestational age (from the first antenatal visit) was calculated retrospectively from participants’ antenatal records (Group 1: 19.8 ± 3.5 weeks; Group 2: 20.5 ± 3.1 weeks, p-value 0.45), while the gestational age at enrollment was 30.1 ± 2 weeks for Group 1 and 29.8 ± 2.3 weeks for Group 2 (p-value 0.59). The baseline systolic blood pressure was 146.7 ± 8.9 mmHg for Group 1 and 144.7 ± 6.8 mmHg for Group 2 (p-value 0.63), and the diastolic blood pressure was 95.7 ± 5.2 mmHg for Group 1 and 95.5 ± 4.4 mmHg for Group 2 (p-value 0.89). The proteinuria recorded via the dipstick method at enrollment was as follows: Group 1: 1.8 ± 0.7; Group 2: 1.9 ± 0.7 (p-value 0.34). The baseline hemoglobin concentration was as follows: Group 1: 10.5 ± 0.8 g/dL; Group 2: 10.5 ± 0.9 g/dL (p-value 0.92). The platelets count was as follows: Group 1: 202,166.67 ± 57,770.70/uL; Group 2: 196,200.00 ± 53,832.05/uL (p-value 0.68), taken during recruitment, as shown in Table 2.

Table 2.
Comparison of baseline parameters of the study groups.
The socioeconomic data of the study participants were collected; it was noted that 49% of participants belonged to the low-income group, 28% to the middle-income group, and only 23% belonged to the high-income group. The literacy rate among the participants was also found to be low: 76.66% of women in the control group and 83.33 % in the LNS-PLW group were found to be illiterate.
Maternal Follow-Up at Delivery
The supplements consumed by the two groups during the prenatal periods (IFA by Group 1: 6.8 ± 1.9 weeks; IFA and LNS-PLW by Group 2: 8.8 ± 1.9 weeks; p-value 0.00).
The live birth in Group 2 was 93%, while in Group 1, it was 92%. The number of normal vaginal deliveries in Group 2 was higher: Group 1: 69% NVD; Group 2:78% NVD. In Group 1, 4% of the participants developed eclampsia as shown in Table 3.

Table 3.
Effect of supplementation on maternal and pregnancy outcomes.
The gestational age at delivery was higher in Group 2 (Group 1: 36.9 ± 1.6 weeks; Group 2: 38.6 ± 0.8 weeks, p = 0.006).
The blood pressure was calculated after delivery; the systolic BP of both groups was the same (Group 1: 135.7 ± 11.2 mmHg; Group 2: 131.3 ± 4.6 mmHg, p-value 0.07), while the diastolic BP (Group 1: 92.2 ± 5.2 mmHg; Group 2: 89.6 ± 2.1 mmHg, p-value 0.025) showed significant improvement in the LNS-PLW-consuming Group 2 as shown in Table 4.

Table 4.
Clinical and hematological parameters of study groups at delivery.
The blood samples were collected from the women after delivery and during their stay in the hospital in order to compare the hemoglobin concentration. Hemoglobin concentration significantly improved with LNS-PLW supplementation (Group 1: 11.4 ± 0.5 g/dL; Group 2: 12.2 ± 0.8 g/dL, p-value 0.001).
4. Discussion
Pre-eclampsia is a common pregnancy-related complication that occurs in childbearing-age women. It is responsible for poor maternal and fetal outcomes, fetal and maternal mortality, and morbidity [15]. A balance nutritional intake during pregnancy is responsible for the proper functioning of bodily systems [24,25]. Pregnancy is a condition for which appropriate and balanced food intake is needed not only for the mother but also for the growing fetus. Maternal undernutrition and malnutrition increase the risk of pre-eclampsia, gestational hypertension, anemia, premature delivery, fetal and maternal mortality, and morbidity [26,27].
In this study, the hemoglobin concentration significantly increased in Group 2 when compared with Group 1. This may be due to the 10 mg of extra iron in the LNS-PLW supplement with routine IFA (60 mg iron and 400 ug folic acid). In contradiction with findings, a study was conducted in Malawi, in which three groups of women were supplemented from ≤20 weeks of gestation with IFA (containing 60 mg of iron), MMN (containing 20 mg of iron), and LNS (containing 20 mg of iron); at 36 weeks of gestation, the researchers compared the women’s hemoglobin levels and reported higher concentrations of hemoglobin in the IFA group [28]. Another study conducted in Bangladesh compared pregnant women (≤20 weeks of gestation) who were receiving either IFA (60 mg of iron and 400 ug of folic acid) or LNS (containing 20 mg of iron). They reported a higher concentration of hemoglobin in the IFA group [29]. Similarly, a study conducted on Ghanaian women reported higher hemoglobin concentrations in women using IFA (containing 60 mg of iron) when compared to those given LNS (containing 20 mg of iron) from ≤20 weeks of gestation [30]. The difference in our study was that, along with IFA (60 mg of iron), we provided an extra 10 mg of iron in the LNS-PLW as well, whereas, in the above studies, the researchers compared three groups of women with different supplements, i.e., IFA, MMN, or LNS individually.
The diastolic blood pressure of the LNS-PLW consumers in the current study decreased significantly at delivery, while no difference was observed for their systolic blood pressure. The LNS-PLW used in the current study was peanut-based, and 80% of its fat content is composed of omega-3 (60%) and omega-6 (20%). Several meta-analyses and trials reported the beneficial effects of ω-3 polyunsaturated fatty acid supplementation on blood pressure control and cardioprotective measures [31]. Moreover, omega-3 may also improve endothelial and vascular functioning and arterioles stiffness [32]. Few other studies conducted on underweight pregnant women showed no difference in systolic and diastolic blood pressure regarding LNS and IFA consumers, presumably due to the subjects being normotensive women [33,34].
Pregnancy outcomes were compared. It was observed that the number of preterm deliveries (<37 weeks) was 35% in Group 1, while all the women on LNS-PLW supplementation delivered after 37 weeks of gestation. The study reported that pre-eclampsia babies are usually preterm [35]. However, a study conducted by Wen et al., 2018, reported that supplementation with folic acid had no effect on babies’ gestational age and contradicts our finding [36]. The difference in our observations may be due to the additional intake of the LNS-PLW supplement along with IFA, whereas the study of Wen et al. assessed the effects of folic acid only [36].
The gestational age of our participants at enrollment (in both groups) was found to be below 34 weeks, meaning all were in the early-onset pre-eclampsia class. In the current study, it was observed that the numbers of live births are higher in the LNS-PLW consumers. Moreover, the number of intrauterine deaths (IUDs) is lower in the supplemented group. These findings are in line with another study that reported that supplementation in pre-eclampsia reduces IUD [37]. It was reported that multiple micronutrients (MMNs) supplementations reduced the risk of preterm birth, while LNS supplementation showed no positive effects on the term of pregnancy [13,27]. The number of NVDs was higher in Group 2 compared to Group 1, while 31% of the women in Group 1 had a caesarean section. It is documented that the rate of cesarean section increases with the severity of pre-eclampsia [38], and also micronutrient-deficient women have a greater risk of cesarean delivery [39]. Similarly, Kiattisak et al., 2018, reported that pre-eclamptic women had a higher occurrence of cesarean delivery [35,40]. In contrast to our findings, a study compared the pregnancy outcome of IFA and LNS consumer groups and found no significant difference in pregnancy and fetal outcomes regarding cesarean delivery in rural Bangladesh [41].
The strength of the current study is the participant’s compliance and cooperation in maintaining high-quality assurance during supplementation and data collection. Moreover, the low dropout rate was made possible by the main researcher herself collecting the data and following the participants.
This study has some limitations; firstly, the women in both groups were pre-eclamptic, and for comparison, normal pregnant women were not recruited. Secondly, due to time and resource constraints, we analyzed a small number of participants in only three antenatal units.
5. Conclusions
The prenatal use of LNS-PLW daily, along with IFA and regular follow-up, can improve pregnancies and maternal outcomes by increasing the live birth and term of the babies and decreasing maternal complications, like eclampsia and the number of deliveries by cesarean section.
Author Contributions
Conceptualization, N.S., S.F. and R.N.; Data curation, H.Z.; Formal analysis, N.S. and H.Z.; Funding acquisition, F.F.; Investigation, M.Z.; Methodology, N.S., H.Z. and F.F.; Project administration, R.N.; Resources, M.A.M. and M.Z.; Supervision, R.N.; Writing—original draft, N.S. and M.Z.; Writing—review & editing, M.A.M., R.N., S.F. and F.F. All authors have read and agreed to the published version of the manuscript.
Funding
The publication charges for this article are partially borne from Khyber Medical University Publication Fund (No.DIR//ORIC/Ref/22/00004).
Institutional Review Board Statement
The trial was approved by Khyber Medical University, by the Advanced Study and Research Board, and the Ethical Committee (No: DIR/KMU-AS&RB/EN/000527-18 August 2016) (DIR/KMU = EB/EN/000314 on 27 October 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated or analyzed during this work are included in this published article. Also, all data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.; Biniwale, P.; Deshpande, H.; Sundari, T.; Govindarajan, M.; Kannan, J.; Mandrupkar, G.; Datar, N.; Pandya, M.; Balamba, P. Diagnosis, management and care of hypertensive disorders of pregnancy (HDP): An Indian Expert Opinion. Indian J. Obstet. Gynecol. Res. 2019, 6, 122–132. [Google Scholar] [CrossRef]
- Baqai, S.M.; Rahim, R.; Ala, H.; Tarar, S.H.; Waqar, F.; Yasmeen, H.; Waheed, A. Society of Obstetricians and Gynaecologists Pakistan (SOGP) Hypertensive Disorders in Pregnancy Guidelines-2022. Pak. Armed Forces Med. J. 2022, 72, 731–753. [Google Scholar]
- Paridaens, H.; Gruson, D. (Eds.) Pre-Eclampsia: Overview on the Role of Biomarkers in 2016; Annales de Biologie Clinique: Paris, France, 2017. [Google Scholar]
- Hod, T.; Cerdeira, A.S.; Karumanchi, S.A. Molecular mechanisms of preeclampsia. Cold Spring Harb. Perspect. Med. 2015, 5, a023473. [Google Scholar] [CrossRef] [PubMed]
- Massimiani, M.; Lacko, L.A.; Swanson, C.S.B.; Salvi, S.; Argueta, L.B.; Moresi, S.; Ferrazzani, S.; Gelber, S.E.; Baergen, R.N.; Toschi, N.; et al. Increased circulating levels of Epidermal Growth Factor-like Domain 7 in pregnant women affected by preeclampsia. Transl. Res. 2019, 207, 19–29. [Google Scholar] [CrossRef]
- Shah, S. Hypertensive disorders in pregnancy. In Obstetric and Gynecologic Nephrology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 11–23. [Google Scholar]
- Moori, M.H. A Review of Hypertensive Disorders Management During Pregnancy. Int. J. Med. Investig. 2022, 11, 27–31. [Google Scholar]
- Magee, L.A.; Pels, A.; Helewa, M.; Rey, E.; von Dadelszen, P.; Audibert, F.; Bujold, E.; Côté, A.-M.; Douglas, M.J.; Eastabrook, G. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J. Obstet. Gynaecol. Can. 2014, 36, 416–438. [Google Scholar] [CrossRef]
- Turan, K.; Arslan, A.; Uçkan, K.; Demir, H.; Demir, C. Change of the levels of trace elements and heavy metals in threatened abortion. J. Chin. Med. Assoc. 2019, 82, 554–557. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The role of Fe, Zn, and Cu in pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef]
- Habibi, N.; Grieger, J.A.; Bianco-Miotto, T. A review of the potential interaction of selenium and iodine on placental and child health. Nutrients 2020, 12, 2678. [Google Scholar] [CrossRef]
- Mohammad, N.S.; Nazli, R.; Zafar, H.; Fatima, S. Effects of lipid based Multiple Micronutrients Supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak. J. Med. Sci. 2022, 38, 219. [Google Scholar]
- Lassi, Z.S.; Padhani, Z.A.; Rabbani, A.; Rind, F.; Salam, R.A.; Das, J.K.; Bhutta, Z.A. Impact of dietary interventions during pregnancy on maternal, neonatal, and child outcomes in low-and middle-income countries. Nutrients 2020, 12, 531. [Google Scholar] [CrossRef]
- Nkamba, D.M.; Vangu, R.; Elongi, M.; Magee, L.A.; Wembodinga, G.; Bernard, P.; Ditekemena, J.; Robert, A. Health facility readiness and provider knowledge as correlates of adequate diagnosis and management of pre-eclampsia in Kinshasa, Democratic Republic of Congo. BMC Health Serv. Res. 2020, 20, 926. [Google Scholar] [CrossRef]
- Plasencia, W.; Maiz, N.; Bonino, S.; Kaihura, C.; Nicolaides, K. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 2007, 30, 742–749. [Google Scholar] [CrossRef]
- Groom, K.M.; McCowan, L.M.; Mackay, L.K.; Lee, A.C.; Said, J.M.; Kane, S.C.; Walker, S.P.; van Mens, T.E.; Hannan, N.J.; Tong, S. Enoxaparin for the prevention of preeclampsia and intrauterine growth restriction in women with a history: A randomized trial. Am. J. Obstet. Gynecol. 2017, 216, 296.e1–296.e14. [Google Scholar]
- Lim, K.; Ramus, R. Preeclampsia; practice essentials, overview, pathophysiology. In Medscape; medscape medical news, located in the heart of Manhattan, steps from Times Square New York. 2018. [Google Scholar]
- Khong, S.L.; Kane, S.C.; Brennecke, S.P.; da Silva Costa, F. First-trimester uterine artery Doppler analysis in the prediction of later pregnancy complications. Dis. Markers 2015, 2015, 679730. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, F.; Ding, Y. Serum screening in first trimester to predict pre-eclampsia, small for gestational age and preterm delivery: Systematic review and meta-analysis. BMC Pregnancy Childbirth 2015, 15, 191. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Akolekar, R.; Mandal, R.; Dong, E.; Xia, J.; Kruger, M.; Wishart, D.S.; Nicolaides, K. First-trimester metabolomic detection of late-onset preeclampsia. Am. J. Obstet. Gynecol. 2013, 208, 58.e1–58.e7. [Google Scholar] [CrossRef]
- Russo, M.L.; Blakemore, K.J. (Eds.) A historical and practical review of first trimester aneuploidy screening. In Seminars in Fetal and Neonatal Medicine; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- English, F.A.; Kenny, L.C.; McCarthy, F.P. Risk factors and effective management of preeclampsia. Integr. Blood Press. Control 2015, 8, 7. [Google Scholar]
- Serbesa, M.L.; Iffa, M.T.; Geleto, M. Factors associated with malnutrition among pregnant women and lactating mothers in Miesso Health Center, Ethiopia. Eur. J. Midwifery 2019, 3, 13. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, D.; Mao, X.; Xia, Y.; Baker, P.N.; Zhang, H. Maternal dietary patterns and pregnancy outcome. Nutrients 2016, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Prakash, A.A.; Das, P.K.; Gupta, S.; Pusdekar, Y.V.; Hibberd, P.L. Maternal anemia and underweight as determinants of pregnancy outcomes: Cohort study in eastern rural Maharashtra, India. BMJ Open 2018, 8, e021623. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.; Fatima, S.; Nazli, R.; Habib, S.H.; Shah, M. Supplementation in primi-gravidas and its effect on maternal, birth and infant outcomes: A randomized controlled trial. Khyber Med. Univ. J. 2021, 13, 187–192. [Google Scholar] [CrossRef]
- Jorgensen, J.M.; Ashorn, P.; Ashorn, U.; Baldiviez, L.M.; Gondwe, A.; Maleta, K.; Nkhoma, M.; Dewey, K.G. Effects of lipid-based nutrient supplements or multiple micronutrient supplements compared with iron and folic acid supplements during pregnancy on maternal haemoglobin and iron status. Matern. Child Nutr. 2018, 14, e12640. [Google Scholar] [CrossRef] [PubMed]
- Matias, S.L.; Mridha, M.K.; Young, R.T.; Hussain, S.; Dewey, K.G. Daily maternal lipid-based nutrient supplementation with 20 mg iron, compared with iron and folic acid with 60 mg iron, resulted in lower iron status in late pregnancy but not at 6 months postpartum in either the mothers or their infants in Bangladesh. J. Nutr. 2018, 148, 1615–1624. [Google Scholar] [CrossRef]
- Adu-Afarwuah, S.; Lartey, A.; Okronipa, H.; Ashorn, P.; Zeilani, M.; Baldiviez, L.M.; Oaks, B.M.; Vosti, S.; Dewey, K.G. Impact of small-quantity lipid-based nutrient supplement on hemoglobin, iron status and biomarkers of inflammation in pregnant Ghanaian women. Matern. Child Nutr. 2017, 13, e12262. [Google Scholar] [CrossRef]
- Bonafini, S.; Giontella, A.; Tagetti, A.; Marcon, D.; Montagnana, M.; Benati, M.; Gaudino, R.; Cavarzere, P.; Karber, M.; Rothe, M. Possible role of CYP450 generated omega-3/omega-6 PUFA metabolites in the modulation of blood pressure and vascular function in obese children. Nutrients 2018, 10, 1689. [Google Scholar] [CrossRef]
- Tousoulis, D.; Plastiras, A.; Siasos, G.; Oikonomou, E.; Verveniotis, A.; Kokkou, E.; Maniatis, K.; Gouliopoulos, N.; Miliou, A.; Paraskevopoulos, T. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis 2014, 232, 10–16. [Google Scholar] [CrossRef]
- Jahan, K.; Roy, S.; Mihrshahi, S.; Sultana, N.; Khatoon, S.; Roy, H.; Datta, L.R.; Roy, A.; Jahan, S.; Khatun, W. Short-term nutrition education reduces low birthweight and improves pregnancy outcomes among urban poor women in Bangladesh. Food Nutr. Bull. 2014, 35, 414–421. [Google Scholar] [CrossRef]
- Abreu, A.M.; Young, R.R.; Buchanan, A.; Lofgren, I.E.; Okronipa, H.E.; Lartey, A.; Ashorn, P.; Adu-Afarwuah, S.; Dewey, K.G.; Oaks, B.M. Maternal Blood Pressure in Relation to Prenatal Lipid-Based Nutrient Supplementation and Adverse Birth Outcomes in a Ghanaian Cohort: A Randomized Controlled Trial and Cohort Analysis. J. Nutr. 2021, 151, 1637–1645. [Google Scholar] [CrossRef]
- Kongwattanakul, K.; Saksiriwuttho, P.; Chaiyarach, S.; Thepsuthammarat, K. Incidence, characteristics, maternal complications, and perinatal outcomes associated with preeclampsia with severe features and HELLP syndrome. Int. J. Women’s Health 2018, 10, 371. [Google Scholar] [CrossRef]
- Wen, S.W.; White, R.R.; Rybak, N.; Gaudet, L.M.; Robson, S.; Hague, W.; Simms-Stewart, D.; Carroli, G.; Smith, G.; Fraser, W.D. Effect of high dose folic acid supplementation in pregnancy on pre-eclampsia (FACT): Double blind, phase III, randomised controlled, international, multicentre trial. BMJ 2018, 362, k3478. [Google Scholar] [CrossRef]
- Ali, A.M.; Alobaid, A.; Malhis, T.N.; Khattab, A.F. Effect of vitamin D3 supplementation in pregnancy on risk of pre-eclampsia–Randomized controlled trial. Clin. Nutr. 2019, 38, 557–563. [Google Scholar] [CrossRef]
- Coppage, K.H.; Polzin, W.J. Severe preeclampsia and delivery outcomes: Is immediate cesarean delivery beneficial? Am. J. Obstet. Gynecol. 2002, 186, 921–923. [Google Scholar] [CrossRef]
- Mosavat, M.; Arabiat, D.; Smyth, A.; Newnham, J.; Whitehead, L. Second-trimester maternal serum vitamin D and pregnancy outcome: The Western Australian Raine cohort study. Diabetes Res. Clin. Pract. 2021, 175, 108779. [Google Scholar] [CrossRef]
- Sanchez, M.P.; Guida, J.P.; Simões, M.; Marangoni-Junior, M.; Cralcev, C.; Santos, J.C.; Dias, T.Z.; Luz, A.G.; Costa, M.L. Can pre-eclampsia explain higher cesarean rates in the different groups of Robson’s classification? Int. J. Gynecol. Obstet. 2021, 152, 339–344. [Google Scholar] [CrossRef]
- Mridha, M.K.; Matias, S.L.; Paul, R.R.; Hussain, S.; Sarker, M.; Hossain, M.; Peerson, J.M.; Vosti, S.A.; Dewey, K.G. Prenatal lipid-based nutrient supplements do not affect pregnancy or childbirth complications or cesarean delivery in Bangladesh: A cluster-randomized controlled effectiveness trial. J. Nutr. 2017, 147, 1776–1784. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).