Large Animal Models for Simulating Physiology of Transfusion of Red Cell Concentrates—A Scoping Review of The Literature
Abstract
1. Introduction
1.1. Transfusion Therapy and Red Blood Cell Storage Lesion
1.2. Importance of Animal Models in Biomedical Research
2. Material and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Assessment of Quality of Study
3. Results
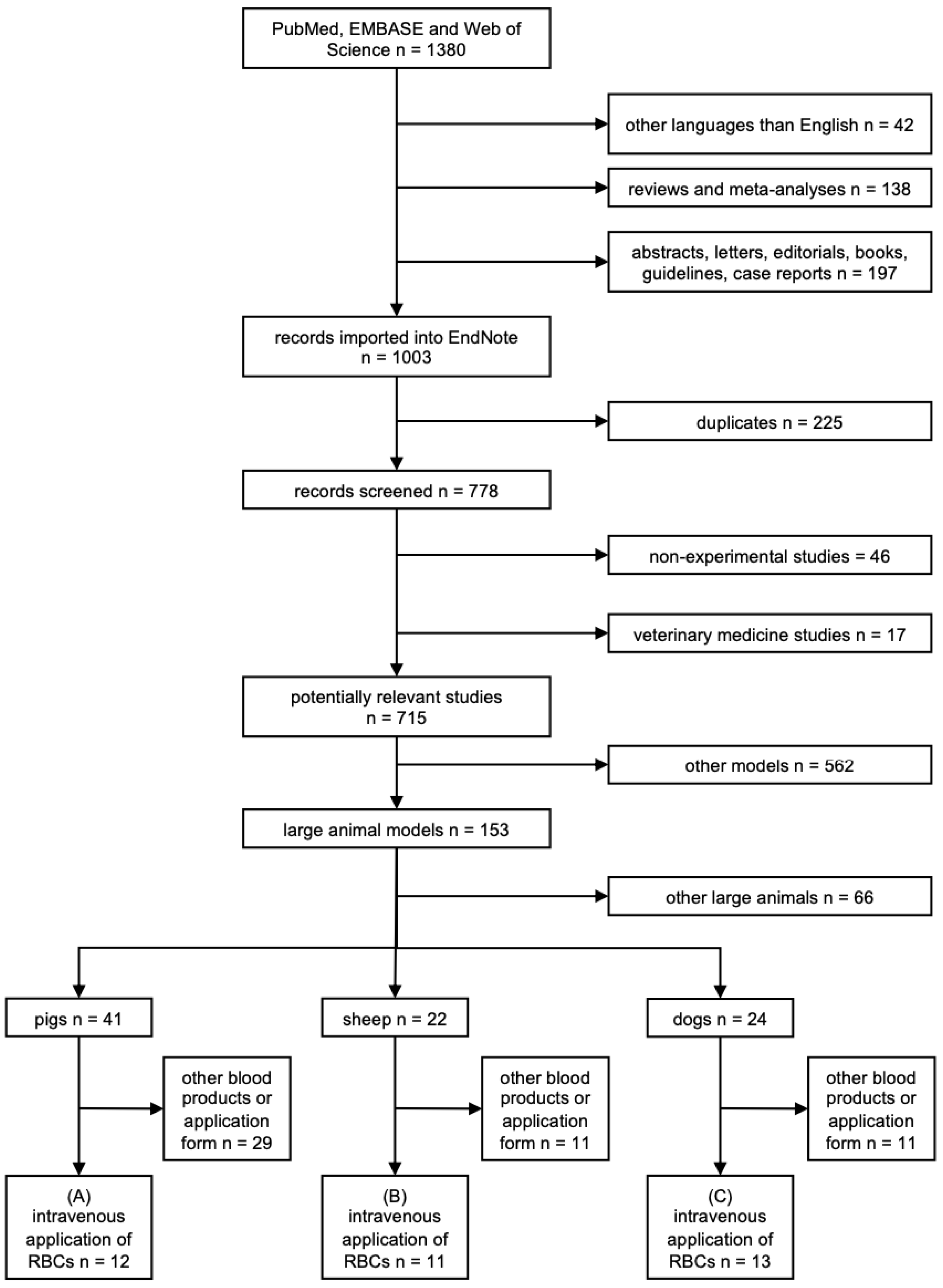
3.1. Search Results
3.2. Basic Study Characteristics
- (A)
- In 12 out of 36 studies, a total of 320 pigs were included, with the number of animals ranging from five to 60 per study [15,20,21,22,23,24,25,26,27,28,29,30]. With a majority of six studies, transfusion of red cell concentrates was most frequently carried out in female swine [15,22,25,26,29,30]. Four studies, however, did not provide any data on the gender of the animals [20,21,27,28]. Only two papers investigating the effects of different volume replacements in asphyxia and hemorrhagic shock model of 32 h old newborn piglets reported data on age [20,21]. In nine out of 12 studies, animals received erythrocyte concentrates without the combination of other blood products [15,20,21,22,23,24,25,26,30]. Fresh frozen plasma or lyophilized plasma, however, was applied in a ratio of 1:1 with packed red blood cells in the remaining three studies [27,28,29]. Of the included publications, three studies reported an application of porcine erythrocytes as a top-load transfusion in healthy swine [22,24,25]. With a total of eight out of 12 studies, transfusion was carried out most frequently in a model of hemorrhagic shock [15,20,21,23,27,28,29,30].
- (B)
- Eleven of the 36 included publications, with a total of 478 sheep, reported a similar heterogeneity in group sizes varying from 10 to 253 animals per study [31,32,33,34,35,36,37,38,39,40,41]. Only two studies of the ones that provided data on the gender of animals used male sheep (34, 35). Six out of 8 studies reported data on animal age, and sheep were most frequently older than three months [31,32,34,35,36,37]. Only two publications stated the use of much younger animals [40,41]. In 5 out of the 11 studies, ovine erythrocytes were transfused as a top-load model [31,33,34,37,38]. Other transfusion models, such as hemorrhagic shock or anemia, were comparatively less frequently used [35,36,39,40,41]. All 11 studies applied ovine red blood cell concentrates without the combination of other blood products [31,32,33,34,35,36,37,38,39,40,41]. However, the study by Muenster et al. exposed erythrocyte concentrates to oxygen or nitric oxide prior to transfusion [21].
- (C)
- In 13 out of the 36 included studies, a total of 440 dogs were included, with only three studies describing a repeated use of the same animals [14,42,43,44,45,46,47,48,49,50,51,52,53]. Study populations showed similar ranges compared to the other two species, with studies using six to 76 dogs per study [14,42,43,44,45,46,47,48,49,50,51,52,53]. In three out of 13 publications, male and female dogs were included in the same study [42,50,53]. For all studies in pigs and sheep, animals of only one sex were used for each study. However, the studies utilizing canine models were markedly dominated by male dogs [42,50,51,52,53]. Beagles were preferably chosen as a breed, with seven publications providing data in this regard [14,43,44,45,46,47,48]. Reported age mostly varied between 1 and 2.5 years, with only three studies deviating from this range [14,42,43,44,45,46,47,48,49,50,53]. Of the included studies, six publications reported the transfusion of canine red blood cell concentrates in a model of pneumonia [14,43,45,46,47,48]. Furthermore, three studies described the use of a hemorrhagic shock model [44,51,52]. However, two papers reported an application of erythrocytes in a top-load transfusion model, and none of the reviewed studies used healthy dogs [14,42,43,44,45,46,47,48,49,50,51,52,53].
| Author [Reference] [Year] | Place of Study | Control Group [Number] | Intervention Group [Number] | Strain of Animal | Gender | Weight (kg) | Type of Model | Objective of Study | Drop-Outs | |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Animals [Group] | Reason | |||||||||
| Weber et al. [20] [2019] | GER | Sham [6] | RBC [9] 0.9% saline [6] | N/A | N/A | 1.220 [1.060–1.495] | Asphyxia/hemorrhagic shock | Effects of different volume replacements on lung injury | N/A | N/A |
| Weber et al. [21] [2019] | GER | Sham [6] | RBC [9] 0.9% saline [6] | N/A | N/A | 1.220 [1.060–1.495] | Asphyxia/hemorrhagic shock | Inflammatory and cardiac consequences of neonatal AH | N/A | N/A |
| Wozniak et al. [22] [2018] | UK | Sham [6] | D14 RBC [10] D14 Wash. RBC [6] D14 Rej. RBC [10] | LW × LR | female | 50–70 | RBC transfusion | Effects of washing/rejuvenation of RBCs on storage lesion associated kidney and lung injury | 4 [2 D14 RBC; 2 D14 Rej. RBC] | Refractory hypoxemia or cardiovascular instability |
| Biagini et al. [23] [2018] | BRA | RL [8] | D14 RBC [8] | Agroceres® pigs | male | 68 [± 3.3] | Hemorrhagic shock | Effects of RBC transfusion on cardiopulmonary function and inflammation | N/A | N/A |
| Watts et al. [15] [2015] | UK | 0.9% saline [9] | RBC:FFP [9] RBC [6] | LW × LR | female | 43–56 | Polytrauma /hemorrhagic shock | Prehospital resuscitation with blood products as a possibility to avoid ATC | N/A | N/A |
| Biagini et al. [24] [2014] | BRA | N/A | RBC [5] | Agroceres® pigs | male | 37–38 a 60 b | RBC transfusion | Viability of swine RBCs stored for 14 days | 1 [N/A] | Hypoxia |
| Patel et al. [25] [2013] | UK | Sham [7] | D14 RBC [6] D42 RBC [7] | FLW × LR | female | 50–70 | RBC transfusion | Transfusion of allogeneic RBC as a cause of PD Effects of RBC storage duration on severity of PD | 4 [2 D14; 2 CPB + D42] | Cardiovascular instability or refractory hypoxemia |
| CPB [7] CPB + D42 [9] | Post-cardiac surgery acute lung injury | Effects of RBC transfusion on pulmonary function and interaction with CPB | ||||||||
| Patel et al. [26] [2011] | UK | Sham [9] | CPB [7] Sham + RBC [8] CPB + RBC [7] | FLW × LR | female | 50–70 | Post-cardiac surgery acute kidney injury | Effects of anemia therapy with allogenic RBC transfusion during CPB | N/A | N/A |
| Spoerke et al. [27] [2010] | USA | N/A | FFP [8] LP [8] FFP:RBC [8] LP:RBC [8] | Yorkshire crossbred swine | N/A | N/A | Polytrauma /hemorrhagic shock | Effect of RBCs on clotting parameters | N/A | N/A |
| Spoerke et al. [28] [2009] | USA | FFP [8] | LP [8] FFP:RBC [8] LP:RBC [8] | Yorkshire crossbred swine | N/A | N/A | Polytrauma /hemorrhagic shock | Effects of resuscitation with LP on clotting factor activity and coagulopathy correction | N/A | N/A |
| Alam et al. [29] [2009] | USA | Sham [6] | FWB [14] Hextend [14] FFP:RBC [13] FFP [13] | Yorkshire swine | female | 40 [±5] | Polytrauma /hemorrhagic shock | Resuscitation with blood components as a possibility to reverse coagulopathy | N/A | N/A |
| Buchholz et al. [30] [1999] | USA | CPDA-1 RBC [5] | Adsol RBC [5] | Pitman-Moore mini-pigs | female | 90–133 | Hemorrhagic shock | Effects of massive infusion of Adsol and CPDA-1 on metabolism and circulation | 2 [CDPA-1] | Cardiorespiratory arrest |
| Author [Reference] [Year] | Place of Study | Control Group [Number] | Intervention Group [Number] | Strain of Animal | Gender | Age (Months) | Weight (kg) | Type of Model | Objective of Study | Drop-Outs | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Animals [Group] | Reason | |||||||||||
| Muenster et al. [31] [2016] | USA | FRBC + O2 [4] FRBC + NO [8] | SRBC + O2 [8] SRBC + NO [9] Washed SRBC [5] | Polypay | N/A | 3–4 | 33 [±2] | RBC transfusion | Effects of NO treatment and washing of RBCs before transfusion | N/A | N/A | |
| McDonald et al. [32] [2015] | AUS | Sham [4] S-ALI [7] ECMO [7] | S-ALI + ECMO [8] S-ALI + ECMO + RBC [14] | Border Leicester-SAMM cross | N/A | 12–36 | 48.6 [±6] | S-ALI/ECMO | Effects of S-ALI, ECMO and RBC transfusion on oxidative stress and plasma selenium levels | N/A | N/A | |
| McCutcheon et al. [33] [2015] | UK | RBC [7] | P-CAPT RBC [7] | ARQ/ ARQ PrP | N/A | N/A | N/A | RBC transfusion | Efficacy of the P-CAPT prion removal filter | 1 [RBC] | N/A | |
| Simonova et al. [34] [2014] | AUS | N/A | D5 RBC [6] D38 RBC [6] | Merino | male | 12–36 | 40.7 [±1.7] | RBC transfusion | Development of an ovine RBC transfusion model in comparison to humans | N/A | N/A | |
| Fung et al. [35] [2013] | AUS | 0.9% saline [2] HA 4% [2] | D5 RBC [5] D38 RBC [5] | Merino | male | 12–36 | 40.2 [±2.9] | Hemorrhagic shock | Comparison of fresh versus old RBC transfusion | N/A | N/A | |
| Baron et al. [36] [2013] | USA | WB [6] | RBC [6] RBC + NO [5] | Polypay | N/A | 3–4 | 28–35 | Hemorrhagic shock | Adverse effects of RBC transfusion after prolonged storage and possible prevention by NO inhalation | N/A | N/A | |
| Lacroux et al. [37] [2012] | FRA | Sham [5] | WB [5] RBC [5] Plasma [5] Buffy-Coat [5] RBC LD [5] Plasma LD [5] RBC LD/PR1 [5] RBC LD/PR2 [5] | VRQ/ VRQ | N/A | 6–10 | N/A | RBC transfusion | Potential of blood products to transmit scrapie through transfusion route and the efficacy of LD and LD/PR filters in risk reduction | N/A | N/A | |
| McCutcheon et al. [38] [2011] | UK | WB [9] | WB [8] a RBC [9] a Plasma [9] a Buffy-Coat [8] a Platelets [9] a | RBC [28] b Plasma [29] b Buffy-Coat [29] b Platelets [28] b RBC LD [29] b Plasma LD [29] b Platelets LD [29] b | ARQ/ ARQ PrP | N/A | N/A | N/A | RBC transfusion | Risk of different blood components to transmit CJD via transfusion | N/A | N/A |
| Jonker et al. [39] [2011] | USA | D10 Sham [5] | D10 Anemia [6] D20 Sham [7] D20 Anemia + RBC [7] | Mixed western | N/A | 109–129 GA | 2.4 c,* 3.7 d,* | Anemia | Effects of RBC transfusion on the growth and proliferation of cardiomyocytes | N/A | N/A | |
| Vane et al. [40] [2002] | USA | RL [6] | DCLHb [6] RBC [7] | Merino | N/A | N/A | 31.1 [±1.2] | Anemia | Comparison of the systemic effects of DCLHb and RBC transfusion | 2 [DCLHb] | Hemodynamic instability or dysfunctional hindlimbs | |
| Widness et al. [41] [2000] | USA | N/A | RBC [10] | Mixed Dorset and Suffolk | N/A | 5.7 [±0.2] days | 5.74 [±0.25] | Anemia | Cardiovascular and metabolic responses after RBC transfusion | N/A | N/A | |
| Author [Reference] [Year] | Place of Study | Control Group [Number] | Intervention Group [Number] | Strain of Animal | Gender [Number] | Age (Years) | Weight (kg) | Type of Model | Objective of Study | Drop-Outs | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Animals [Group] | Reason [Number] | ||||||||||
| Callan et al. [42] [2021] | USA | AIHA FRBC [20] ITP FRBC [1] | AIHA SRBC [18] ITP SRBC [1] | mix, labrador, other | male [18] female [22] | 2.8–14.5 | 8–72 | RBC transfusion | Association of prolonged storage of RBCs with hemolysis and cytokine response | 8 [2 FRBC; 6 SRBC] | Thromb. disease [2] SIRS [2] MDOS [2] Sepsis [1] |
| Remy et al. [43] [2019] | USA | Human albumin + D42 RBC [9] | Human Hp + D42 RBC [9] | beagles | N/A | 1.5–2.5 (18–30 months) | 9–12.5 | Pneumonia/ Sepsis | Interaction of CFH levels and haptoglobin | N/A | N/A |
| 0.9% saline [4] | Human Hp [4] | ||||||||||
| Suffredini et al. [14] [2017] | USA | D7 RBC [18] | Iron sucrose [13] Ferumoxytol [11] | beagles | N/A | 1–2 | 9–12.5 | Pneumonia /Anemia | Comparison of fresh RBCs and potential risks of iron therapy | N/A | N/A |
| Solomon et al. [44] [2015] | USA | N/A | D7 RBC [6] D42 RBC [6] | beagles | N/A | 1–2.2 (12–28 months) | 9–12.5 | Hemorrhagic shock | Adverse effects of prolonged stored RBCs | N/A | N/A |
| Cortes-Puch et al. [45] [2015] | USA | D7 RBC 5-10mL [6] D7 RBC 20-40mL [6] D7 RBC 60–80mL [6] | D42 RBC 5-10mL [6] D42 RBC 20-40mL [6] D42 RBC 60–80mL [6] | beagles | N/A | 1–2.2 (12–28 months) | 9–12.5 | Pneumonia | Effects of different volumes on transfusion risks of prolonged stored RBCs | N/A | N/A |
| UW D14 RBC [7] UW D21 RBC [6] UW D28 RBC [4] UW D35 RBC [3] | W D14 RBC [7] W D21 RBC [6] W D28 RBC [4] W D35 RBC [3] | Influence of storage time on the transfusion of washed vs. unwashed RBCs | |||||||||
| Wang et al. [46] [2014] | USA | D7 RBC S.a.0 [4] D42 RBC S.a.0 [4] | D7 RBC S.a.1 [4] D42 RBC S.a.1 [4] D7 RBC S.a.1.25 [12] D42 RBC S.a.1.25 [12] D7 RBC S.a. > 1.5 [4] D42 RBC S.a. > 1.5 [4] | beagles | N/A | 1–2.2 (12–28 months) | 10–15 | Pneumonia | Contributing effects of the bacterial dose on transfusion risks of prolonged stored RBCs | N/A | N/A |
| Cortes-Puch et al. [47] [2014] | USA | UW D7 RBC [6] UW D42 RBC [6] | W D7 RBC [6] W D42 RBC [6] | beagles | N/A | 1–2.2 (12–28 months) | 9–12.5 | Pneumonia | Effects of washing on CFH and iron levels during different storage times | N/A | N/A |
| Solomon et al. [48] [2013] | USA | D7 RBC [8] | D42 RBC [8] | beagles | N/A | 1–2.2 (12–28 months) | 10–15 | Pneumonia | Viability of commercially available RBCs | N/A | N/A |
| N/A | S.a.1 [8] S.a. 1.25 [8] S.a. 1.5 [4] S.a. 2.0 [4] | Bacterial dose finding | |||||||||
| D7 RBC S.a.1.25 [12] | D42 RBC S.a.1.25 [12] | Influence of storage duration on mortality | |||||||||
| Standl et al. [49] [2003] | GER | D21 RBC [6] | HBOC-201 [6] | foxhound | N/A | 20 ± 5 months | 29 ± 4 | Anemia | Tissue oxygenation potential of the alternative carrier HBOC-201 | N/A | N/A |
| Standl et al. [50] [1996] | GER | D0 RBC [8] | D21 RBC [8] HBOC [8] | foxhound | male [15] female [9] | 2 ± 0.5 | 30 ± 14 | Anemia | Comparison of tissue oxygenation potential of stored RBCs, freshly donated blood and HBOC | N/A | N/A |
| Lucas et al. [51] [1996] | USA | RL + RBC [N/A] | FFP + RL + RBC [N/A] | N/A | male [22] | N/A | 9–25 | Hemorrhagic shock | Efficiency of resuscitation with FFPs in preventing coagulopathy | N/A | N/A |
| Ross et al. [52] [1990] | USA | Control [6] | RBC [6] | mongrel | male [6] | N/A | 15 | Hemorrhagic shock | Effects of severe blood loss on the kinetics of the complement system | N/A | N/A |
| LeBlanc and Edwards [53] [1986] | USA | WB [19] | RBC [19] | N/A | male [23] female [15] | 3–14 days | 0.57 ± 0.12 a 0.50 ± 0.11 b | RBC transfusion | Effect of acute polycythemia on the disappearance rate of fibrinogen | N/A | N/A |
3.3. Preparation and Storage of Erythrocytes
- (A)
- Eight studies reported data on porcine erythrocytes preparation and storage, with a total number of 145 donor animals (Table 4). Group sizes varied immensely between the publications: a single donor animal was used in a pilot study by Biagini et al., and 60 donor animals were used by Alam et al. [24,29]. Three of the remaining four studies did not provide any information on preparation or storage [20,21,27]. Spoerke et al. only reported data on the strain of animals (Yorkshire crossbred swine) as well as on the preparation of plasma for lyophilization [28]. Overall, in most cases, whole blood collection was performed using citrate-phosphate-dextrose (CPD) as an anticoagulant followed by centrifugation and storage in sucrose-adenosine-glucose-mannitol (SAG-M) solution [15,22,25,26]. Only two studies stated to have used citrate-phosphate-dextrose-adenine-1 (CPDA-1) [23,24]. With CPD and CPDA-1 being the most commonly used anticoagulants, Buchholz et al. investigated the use of Adsol, an alternative electrolyte mixture that contains mannitol (750 mg/unit), as well as increased amounts of glucose (2.2 g/unit compared to 1.6 g/unit for CPD and 2.01 g/unit for CPDA-1), and reported no evidence of inappropriate osmotic diuresis or hyperglycemia, but showed significant hypocalcemia, arterial hypotension, and elevated blood glucose concentrations in CPDA-1 animals [30]. As summarized in Table 4, porcine red blood cell concentrates were stored at a temperature of 4 °C in all eight studies, with the exception of the studies of Biagini et al., who allowed a range of storage temperatures from 2 to 8 °C [15,22,23,24,25,26,29,30]. Storage time was reported to be 14 days in five studies by Patel et al., including the second group of 42-day-old red blood cells [15,22,23,24,25]. Two publications described the use of prepared erythrocyte concentrates immediately or within 24 h after collection [29,30]. Only one paper reported a mean storage time of 37 days [26]. Procedures to reduce storage lesions, such as supernatant removal by mechanical washing (CATS; Fresenius, Germany) or a combination of washing and rejuvenation with a solution rich in inosine (Rejuveso solution; Zimmer Biomer, USA) before application, were exclusively demonstrated by Wozinak et al. [22].
- (B)
- In 11 publications, ovine erythrocytes were collected from a total of 175 donor animals, and 61 of these donor animals were previously infected with prions (Table S1) [31,33,34,35,36,37,38,39,40]. Two out of these 11 studies, however, did not provide any information on the number of donor sheep [32,41]. Similarly to porcine red blood cell concentrates, sucrose-adenosine-glucose-mannitol (SAG-M) was most frequently used as an additive solution [32,34,35,38]. Only Muenster et al. and Baron et al. described the use of Adsol [31,36]. With a mean of 35 to 42 days for the prolonged storage groups in five out of 11 studies, maximum storage time differed compared to the porcine studies that used 14 days of storage for the prolonged storage groups [31,32,34,35,36]. Only Vane et al. described similar storage times for their ovine studies as compared to porcine transfusion models with 8–10 days [40]. Washing procedures (COBE 2991 Cell Processing Set, TERUMO BCT, Lakewood, CO) to possibly reduce storage lesions were exclusively performed in a study by Muenster et al. [31].
- (C)
- As shown in Table S2, only three publications provided data on the number of donor animals which came to a total of 28 dogs [49,50,52]. Ten studies reported no information in this regard but showed a clear predominance of allogenic donors [14,42,43,44,45,46,47,48,51,53]. With five to 42 days, a significantly broader range in storage time was used among dogs and thus deviated from the 14 days majorly described in group A [14,42,43,44,45,46,47,48,49,50]. In addition, the systematic analysis indicated differences in the use of phosphate-adenine-glucose-guanosine-saline-mannitol (PAGGS-M) as an additive solution by the majority of canine studies [49,50]. However, it should be noted that data were only available in three out of 13 publications [48,49,50].
3.4. Storage-Related Changes in Red Blood Cell Units
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tobian, A.A.R.; Ness, P.M. Red Cells—Aging Gracefully in the Blood Bank. N. Engl. J. Med. 2016, 375, 1995–1997. [Google Scholar] [CrossRef]
- Yoshida, T.; Prudent, M.; D’Alessandro, A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019, 17, 27–52. [Google Scholar]
- Tinmouth, A.; Fergusson, D.; Yee, I.C.; Hébert, P.C. Clinical consequences of red cell storage in the critically ill. Transfusion 2006, 46, 2014–2027. [Google Scholar] [CrossRef]
- Donadee, C.; Raat, N.J.; Kanias, T.; Tejero, J.; Lee, J.S.; Kelley, E.E.; Zhao, X.; Liu, C.; Reynolds, H.; Azarov, I.; et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 2011, 124, 465–476. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E.; Veldman, T.H.; Doctor, A.; Telen, M.J.; Ortel, T.L.; Reid, T.S.; Mulherin, M.A.; Zhu, H.; Buck, R.D.; Califf, R.M.; et al. Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. USA 2007, 104, 17063–17068. [Google Scholar] [CrossRef]
- Hovav, T.; Yedgar, S.; Manny, N.; Barshtein, G. Alteration of red cell aggregability and shape during blood storage. Transfusion 1999, 39, 277–281. [Google Scholar] [CrossRef]
- Sut, C.; Tariket, S.; Chou, M.L.; Garraud, O.; Laradi, S.; Hamzeh-Cognasse, H.; Seghatchian, J.; Burnouf, T.; Cognasse, F. Duration of red blood cell storage and inflammatory marker generation. Blood Transfus. 2017, 15, 145–152. [Google Scholar] [PubMed]
- Gutierrez, K.; Dicks, N.; Glanzner, W.G.; Agellon, L.B.; Bordignon, V. Efficacy of the porcine species in biomedical research. Front. Genet. 2015, 6, 293. [Google Scholar] [CrossRef] [PubMed]
- Eggel, M.; Würbel, H. Internal consistency and compatibility of the 3Rs and 3Vs principles for project evaluation of animal research. Lab Anim. 2021, 55, 233–243. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique: Methuen; Methuen & Co., Limited: London, UK, 1959. [Google Scholar]
- Rothman, A.; Wiencek, R.G.; Davidson, S.; Evans, W.N.; Restrepo, H.; Sarukhanov, V.; Mann, D. Challenges in the development of chronic pulmonary hypertension models in large animals. Pulm. Circ. 2017, 7, 156–166. [Google Scholar] [CrossRef][Green Version]
- Gandolfi, F.; Vanelli, A.; Pennarossa, G.; Rahaman, M.; Acocella, F.; Brevini, T.A. Large animal models for cardiac stem cell therapies. Theriogenology 2011, 75, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.M. Modeling of asthma, COPD and cystic fibrosis in sheep. Pulm. Pharmacol. Ther. 2008, 21, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Suffredini, D.A.; Xu, W.; Sun, J.; Barea-Mendoza, J.; Solomon, S.B.; Brashears, S.L.; Perlegas, A.; Kim-Shapiro, D.B.; Klein, H.G.; Natanson, C.; et al. Parenteral irons versus transfused red blood cells for treatment of anemia during canine experimental bacterial pneumonia. Transfusion 2017, 57, 2338–2347. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.; Nordmann, G.; Brohi, K.; Midwinter, M.; Woolley, T.; Gwyther, R.; Wilson, C.; Poon, H.; Kirkman, E. Evaluation of prehospital blood products to attenuate acute coagulopathy of trauma in a model of severe injury and shock in anesthetized pigs. Shock 2015, 44 (Suppl. S1), 138–148. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Donovan, K.; Seeley, C.; Dickson, E.A.; Palmer, A.J.R.; Doree, C.; Brunskill, S.; Reid, J.; Acheson, A.G.; Sugavanam, A.; et al. Risk of Infection Associated with Administration of Intravenous Iron: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2133935. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Palmer, A.J.R.; Fisher, S.A.; Rahman, S.M.; Brunskill, S.; Doree, C.; Reid, J.; Sugavanam, A.; Stanworth, S.J. What is the effect of perioperative intravenous iron therapy in patients undergoing non-elective surgery? A systematic review with meta-analysis and trial sequential analysis. Perioper. Med. 2018, 7, 30. [Google Scholar] [CrossRef]
- Gourlay, T.; Simpson, C.; Robertson, C.A. Development of a portable blood salvage and autotransfusion technology to enhance survivability of personnel requiring major medical interventions in austere or military environments. J. R. Army Med. Corps 2018, 164, 96–102. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Weber, B.; Mendler, M.R.; Lackner, I.; von Zelewski, A.; Höfler, S.; Baur, M.; Braun, C.K.; Hummler, H.; Schwarz, S.; Pressmar, J.; et al. Lung injury after asphyxia and hemorrhagic shock in newborn piglets: Analysis of structural and inflammatory changes. PLoS ONE 2019, 14, e0219211. [Google Scholar] [CrossRef]
- Weber, B.; Mendler, M.R.; Lackner, I.; Pressmar, J.; Haffner-Luntzer, M.; Hofler, S.; Braun, C.K.; Hummler, H.; Schwarz, S.; Kalbitz, M.; et al. Tissue damage in the heart after cardiac arrest induced by asphyxia and hemorrhage in newborn pigs. Pediatr. Res. 2019, 86, 709–718. [Google Scholar] [CrossRef]
- Wozniak, M.J.; Qureshi, S.; Sullo, N.; Dott, W.; Cardigan, R.; Wiltshire, M.; Nath, M.; Patel, N.N.; Kumar, T.; Goodall, A.H.; et al. A Comparison of Red Cell Rejuvenation versus Mechanical Washing for the Prevention of Transfusion-Associated Organ Injury in Swine. Anesthesiology 2018, 128, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Biagini, S.; Dale, C.S.; Real, J.M.; Moreira, E.S.; Carvalho, C.R.R.; Schettino, G.P.P.; Wendel, S.; Azevedo, L.C.P. Short-term effects of stored homologous red blood cell transfusion on cardiorespiratory function and inflammation: An experimental study in a hypovolemia model. Braz. J. Med. Biol. Res. 2018, 51, e6258. [Google Scholar] [CrossRef] [PubMed]
- Biagini, S.; Costa, P.A.; Wendel, S.; Schettino, G.; Azevedo, L.C. In vitro and in vivo validation of stored swine erythrocyte viability to establish an experimental model of homologous red blood cell transfusion: A pilot study. Rev. Bras. De Ter. Intensiv. 2014, 26, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.N.; Lin, H.; Jones, C.; Walkden, G.; Ray, P.; Sleeman, P.A.; Angelini, G.D.; Murphy, G.J. Interactions of cardiopulmonary bypass and erythrocyte transfusion in the pathogenesis of pulmonary dysfunction in swine. Anesthesiology 2013, 119, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.N.; Lin, H.; Toth, T.; Welsh, G.I.; Jones, C.; Ray, P.; Satchell, S.C.; Sleeman, P.; Angelini, G.D.; Murphy, G.J. Reversal of anemia with allogenic RBC transfusion prevents post-cardiopulmonary bypass acute kidney injury in swine. Am. J. Physiol.—Ren. Physiol. 2011, 301, F605-14. [Google Scholar] [CrossRef]
- Spoerke, N.J.; Van, P.Y.; Differding, J.A.; Zink, K.A.; Cho, S.D.; Muller, P.J.; Karahan, Z.A.; Sondeen, J.L.; Holcomb, J.B.; Schreiber, M.A. Red Blood Cells Accelerate the Onset of Clot Formation in Polytrauma and Hemorrhagic Shock. J. Trauma-Injury Infect. Crit. Care 2010, 69, 1054–1059. [Google Scholar] [CrossRef]
- Spoerke, N.; Zink, K.; Cho, S.D.; Differding, J.; Muller, P.; Karahan, A.; Sondeen, J.; Holcomb, J.B.; Schreiber, M. Lyophilized Plasma for Resuscitation in a Swine Model of Severe Injury. Arch. Surg. 2009, 144, 829–834. [Google Scholar] [CrossRef]
- Alam, H.B.; Bice, L.M.; Butt, M.U.; Cho, S.D.; Dubick, M.A.; Duggan, M.; Englehart, M.S.; Holcomb, J.B.; Morris, M.S.; Prince, M.D.; et al. Testing of blood products in a polytrauma model: Results of a multi-institutional randomized preclinical trial. J. Trauma. 2009, 67, 856–864. [Google Scholar] [CrossRef]
- Buchholz, D.H.; Borgia, J.F.; Ward, M.; Miripol, J.E.; Simpson, J.M. Comparison of Adsol and CPDA-1 blood preservatives during simulated massive resuscitation after hemorrhage in swine. Transfusion 1999, 39, 998–1004. [Google Scholar] [CrossRef]
- Muenster, S.; Beloiartsev, A.; Yu, B.; Du, E.; Abidi, S.; Dao, M.; Fabry, G.; Graw, J.A.; Wepler, M.; Malhotra, R.; et al. Exposure of Stored Packed Erythrocytes to Nitric Oxide Prevents Transfusion-associated Pulmonary Hypertension. Anesthesiology 2016, 125, 952–963. [Google Scholar] [CrossRef][Green Version]
- McDonald, C.I.; Fung, Y.L.; Shekar, K.; Diab, S.D.; Dunster, K.R.; Passmore, M.R.; Foley, S.R.; Simonova, G.; Platts, D.; Fraser, J.F. The impact of acute lung injury, ECMO and transfusion on oxidative stress and plasma selenium levels in an ovine model. J. Trace Elem. Med. Biol. 2015, 30, 4–10. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, S.; Alejo Blanco, A.R.; Tan, B.C.; González, L.; Martin, S.; Mallinson, G.; Appleford, N.E.; Turner, M.L.; Manson, J.C.; Houston, E.F. A prion reduction filter does not completely remove endogenous prion infectivity from sheep blood. Transfusion 2015, 55, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Simonova, G.; Tung, J.P.; Fraser, J.F.; Do, H.L.; Staib, A.; Chew, M.S.; Dunster, K.R.; Glenister, K.M.; Jackson, D.E.; Fung, Y.L. A comprehensive ovine model of blood transfusion. Vox Sang. 2014, 106, 153–160. [Google Scholar] [CrossRef]
- Fung, Y.L.; Tung, J.P.; Foley, S.R.; Simonova, G.; Thom, O.; Staib, A.; Collier, J.; Dunster, K.R.; Solano, C.; Shekar, K.; et al. Stored blood transfusion induces transient pulmonary arterial hypertension without impairing coagulation in an ovine model of nontraumatic haemorrhage. Vox Sang. 2013, 105, 150–158. [Google Scholar] [CrossRef]
- Baron, D.M.; Beloiartsev, A.; Nakagawa, A.; Martyn, T.; Stowell, C.P.; Malhotra, R.; Mayeur, C.; Bloch, K.D.; Zapol, W.M. Adverse effects of hemorrhagic shock resuscitation with stored blood are ameliorated by inhaled nitric oxide in lambs. Crit. Care Med. 2013, 41, 2492–2501. [Google Scholar] [CrossRef]
- Lacroux, C.; Bougard, D.; Litaise, C.; Simmons, H.; Corbiere, F.; Dernis, D.; Tardivel, R.; Morel, N.; Simon, S.; Lugan, S.; et al. Impact of leucocyte depletion and prion reduction filters on TSE blood borne transmission. PLoS ONE 2012, 7, e42019. [Google Scholar] [CrossRef]
- McCutcheon, S.; Alejo Blanco, A.R.; Houston, E.F.; de Wolf, C.; Tan, B.C.; Smith, A.; Groschup, M.H.; Hunter, N.; Hornsey, V.S.; MacGregor, I.R.; et al. All clinically-relevant blood components transmit prion disease following a single blood transfusion: A sheep model of vCJD. PLoS ONE 2011, 6, e23169. [Google Scholar] [CrossRef]
- Jonker, S.S.; Scholz, T.D.; Segar, J.L. Transfusion effects on cardiomyocyte growth and proliferation in fetal sheep after chronic anemia. Pediatr. Res. 2011, 69, 485–490. [Google Scholar] [CrossRef]
- Vane, L.A.; Funston, J.S.; Kirschner, R.; Harper, D.; Deyo, D.J.; Traber, D.L.; Traber, L.L.; Kramer, G.C. Comparison of transfusion with DCLHb or pRBCs for treatment of intraoperative anemia in sheep. J. Appl. Physiol. 2002, 92, 343–353. [Google Scholar] [CrossRef][Green Version]
- Widness, J.A.; Lowe, L.S.; Bell, E.F.; Burmeister, L.F.; Mock, D.M.; Kistard, J.A.; Bard, H. Adaptive responses during anemia and its correction in lambs. J. Appl. Physiol. 2000, 88, 1397–1406. [Google Scholar] [CrossRef][Green Version]
- Callan, M.B.; Thawley, V.J.; Marryott, K.A.; Shabro, A.; Fernando, S.; Kahn, S.; Hudson, K.E.; Hod, E.A. Hemolytic anemia blunts the cytokine response to transfusion of older red blood cells in mice and dogs. Transfusion 2021, 61, 3309–3319. [Google Scholar] [CrossRef]
- Remy, K.E.; Cortes-Puch, I.; Sun, J.; Feng, J.; Lertora, J.J.; Risoleo, T.; Katz, J.; Basu, S.; Liu, X.; Perlegas, A.; et al. Haptoglobin therapy has differential effects depending on severity of canine septic shock and cell-free hemoglobin level. Transfusion 2019, 59, 3628–3638. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.B.; Cortes-Puch, I.; Sun, J.; Remy, K.E.; Wang, D.; Feng, J.; Khan, S.S.; Sinchar, D.; Kim-Shapiro, D.B.; Klein, H.G.; et al. Transfused older stored red blood cells improve the clinical course and outcome in a canine lethal hemorrhage and reperfusion model. Transfusion 2015, 55, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Puch, I.; Remy, K.E.; Solomon, S.B.; Sun, J.; Wang, D.; Al-Hamad, M.; Kelly, S.M.; Sinchar, D.; Bellavia, L.; Kanias, T.; et al. In a canine pneumonia model of exchange transfusion, altering the age but not the volume of older red blood cells markedly alters outcome. Transfusion 2015, 55, 2564–2575. [Google Scholar] [CrossRef]
- Wang, D.; Cortés-Puch, I.; Sun, J.; Solomon, S.B.; Kanias, T.; Remy, K.E.; Feng, J.; Alimchandani, M.; Quezado, M.; Helms, C.; et al. Transfusion of older stored blood worsens outcomes in canines depending on the presence and severity of pneumonia. Transfusion 2014, 54, 1712–1724. [Google Scholar] [CrossRef]
- Cortés-Puch, I.; Wang, D.; Sun, J.; Solomon, S.B.; Remy, K.E.; Fernandez, M.; Feng, J.; Kanias, T.; Bellavia, L.; Sinchar, D.; et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood 2014, 123, 1403–1411. [Google Scholar] [CrossRef]
- Solomon, S.B.; Sun, J.; Kanias, T.; Feng, J.; Helms, C.C.; Solomon, M.A.; Alimchandani, M.; Quezado, M.; Gladwin, M.T.; Kim-Shapiro, D.B.; et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood 2013, 121, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Standl, T.; Freitag, M.; Burmeister, M.A.; Horn, E.P.; Wilhelm, S.; Schulte am Esch, J. Hemoglobin-based oxygen carrier HBOC-201 provides higher and faster increase in oxygen tension in skeletal muscle of anemic dogs than do stored red blood cells. J. Vasc. Surg. 2003, 37, 859–865. [Google Scholar] [CrossRef][Green Version]
- Standl, T.; Horn, P.; Wilhelm, S.; Greim, C.; Freitag, M.; Freitag, U.; Sputtek, A.; Jacobs, E.; Esch, J.S.A. Bovine haemoglobin is more potent than autologous red blood cells in restoring muscular tissue oxygenation after profound isovolaemic haemodilution in dogs. J. Can. Anesth. 1996, 43, 714–723. [Google Scholar] [CrossRef]
- Lucas, C.E.; Ledgerwood, A.M.; Saxe, J.M.; Dombi, G.; Lucas, W.F. Plasma supplementation is beneficial for coagulation during severe hemorrhagic shock. Am. J. Surg. 1996, 171, 399–404. [Google Scholar] [CrossRef]
- Ross, M.; England, D.; Webb, H.; Hoover, E.L. Effects of plasmapheresis and autotransfusion on complement recovery in dogs. J. Surg Res. 1990, 49, 30–33. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.H.; Edwards, J.D. Acute polycythemia increases the disappearance rate of clottable fibrinogen in the newborn dog. Pediatr. Res. 1986, 20, 151–154. [Google Scholar] [CrossRef]
- Council of Europe. Guide to the Preparation, Use and Quality Assurance of Blood Components, 16th ed.; Council of Europe: Strasbourg, France, 2011. [Google Scholar]
- García-Roa, M.; Del Carmen Vicente-Ayuso, M.; Bobes, A.M.; Pedraza, A.C.; González-Fernández, A.; Martín, M.P.; Sáez, I.; Seghatchian, J.; Gutiérrez, L. Red blood cell storage time and transfusion: Current practice, concerns and future perspectives. Blood Transfus. 2017, 15, 222–231. [Google Scholar] [PubMed]
- Lagerberg, J.W.; Korsten, H.; Van Der Meer, P.F.; De Korte, D. Prevention of red cell storage lesion: A comparison of five different additive solutions. Blood Transfus. 2017, 15, 456–462. [Google Scholar]
- Ozment, C.P.; Mamo, L.B.; Campbell, M.L.; Lokhnygina, Y.; Ghio, A.J.; Turi, J.L. Transfusion-related biologic effects and free hemoglobin, heme, and iron. Transfusion 2013, 53, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, B.; Rajbhandary, S.; Kleinman, S.; Harris, A.; Kamani, N. Trends in United States blood collection and transfusion: Results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion 2016, 56, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Anastasiadi, A.T.; Drossos, P.V.; Karadimas, D.G.; Valsami, S.; Stamoulis, K.E.; Papassideri, I.S.; Politou, M.; Antonelou, M.H.; Kriebardis, A.G. Sex-related aspects of the red blood cell storage lesion. Blood Transfus. 2021, 19, 224–236. [Google Scholar]
- Bush, J.A.; Berlin, N.I.; Jensen, W.N.; Brill, A.B.; Cartwright, G.E.; Wintrobe, M.M. Erythrocyte life span in growing swine as determined by glycine-2-C14. J. Exp. Med. 1955, 101, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Almizraq, R.J.; Holovati, J.L.; Acker, J.P. Characteristics of Extracellular Vesicles in Red Blood Concentrates Change with Storage Time and Blood Manufacturing Method. Transfus. Med. Hemother. 2018, 45, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.M.; Yu, B.; Lei, C.; Bagchi, A.; Beloiartsev, A.; Stowell, C.P.; Steinbicker, A.U.; Malhotra, R.; Bloch, K.D.; Zapol, W.M. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology 2012, 116, 637–647. [Google Scholar] [CrossRef]
- Simonova, G.; Wellburn, R.; Fung, Y.L.; Fraser, J.F.; Tung, J.P. Ovine red cell concentrates for transfusion research—Is the storage lesion comparable to human red cell concentrates? Vox Sang. 2021, 116, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Tchir, J.D.; Acker, J.P.; Holovati, J.L. Rejuvenation of ATP during storage does not reverse effects of the hypothermic storage lesion. Transfusion 2013, 53, 3184–3191. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Pajic-Lijakovic, I.; Gural, A. Deformability of Stored Red Blood Cells. Front. Physiol. 2021, 12, 722896. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.; Myrand-Lapierre, M.E.; Ang, R.R.; Duffy, S.P.; Scott, M.D.; Ma, H. Microfluidic deformability analysis of the red cell storage lesion. J. Biomech. 2015, 48, 4065–4072. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, L.; Schulzki, T.; Goede, J.S.; Hayes, J.; Reinhart, W.H. Erythrocyte storage in hypertonic (SAGM) or isotonic (PAGGSM) conservation medium: Influence on cell properties. Vox Sang. 2008, 95, 280–287. [Google Scholar] [CrossRef]
- Remy, K.E.; Cortés-Puch, I.; Solomon, S.B.; Sun, J.; Pockros, B.M.; Feng, J.; Lertora, J.J.; Hantgan, R.R.; Liu, X.; Perlegas, A.; et al. Haptoglobin improves shock, lung injury, and survival in canine pneumonia. JCI Insight 2018, 3, e123013. [Google Scholar] [CrossRef]
- Applefeld, W.N.; Wang, J.; Solomon, S.B.; Sun, J.; Klein, H.G.; Natanson, C. RBC Storage Lesion Studies in Humans and Experimental Models of Shock. Appl. Sci. 2020, 10, 1838. [Google Scholar] [CrossRef]
- Rydal, M.P.; Bhattarai, S.; Nielsen, J.P. An Experimental Model for Iron Deficiency Anemia in Sows and Offspring Induced by Blood Removal during Gestation. Animals 2021, 11, 2848. [Google Scholar] [CrossRef]
- Heidbüchel, K.; Raabe, J.; Baldinger, L.; Hagmüller, W.; Bussemas, R. One Iron Injection Is Not Enough-Iron Status and Growth of Suckling Piglets on an Organic Farm. Animals 2019, 9, 651. [Google Scholar] [CrossRef]
- Stocchi, V.; Magnani, M.; Novelli, G.; Dachà, M.; Fornaini, G. Pig red blood cell hexokinase: Evidence for the presence of hexokinase types II and III, and their purification and characterization. Arch. Biochem. Biophys. 1983, 226, 365–376. [Google Scholar] [CrossRef]
- Magnani, M.; Stocchi, V.; Serafini, N.; Piatti, E.; Dachà, M.; Fornaini, G. Pig red blood cell hexokinase: Regulatory characteristics and possible physiological role. Arch. Biochem. Biophys. 1983, 226, 377–387. [Google Scholar] [CrossRef]
- Dixon, E.; Wilson, B.A. Erythrocyte metabolism: Kinetic and electrophoretic analyses of pig red cell hexokinase. J. Exp. Zool. 1981, 215, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Agar, N.S.; Suzuki, M. Red cell metabolism: A comparative study of some mammalian species. Comp. Biochem. Physiol. B 1984, 79, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.H. Glucose-6-phosphate dehydrogenase and potassium concentration in sheep erythrocytes. Nature 1963, 200, 1334–1335. [Google Scholar] [CrossRef] [PubMed]
- Burr, M.J. The relationship between pH and aerobic glycolysis in human and canine erythrocytes. Comp. Biochem. Physiol. B 1972, 41, 687–694. [Google Scholar] [CrossRef]
- Žura Žaja, I.; Vince, S.; Poljičak Milas, N.; Lobpreis, I.R.A.; Špoljarić, B.; Shek Vugrovečki, A.; Milinković-Tur, S.; Šimpraga, M.; Pajurin, L.; Mikuš, T.; et al. A New Method of Assessing Sheep Red Blood Cell Types from Their Morphology. Animals 2019, 9, 1130. [Google Scholar] [CrossRef]
- Blasi, B.; D’Alessandro, A.; Ramundo, N.; Zolla, L. Red blood cell storage and cell morphology. Transfus. Med. 2012, 22, 90–96. [Google Scholar] [CrossRef]
- Eckermann, J.M.; Buhler, L.H.; Zhu, A.; Dor, F.J.; Awwad, M.; Cooper, D.K. Initial investigation of the potential of modified porcine erythrocytes for transfusion in primates. Xenotransplantation 2004, 11, 18–26. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Anastasiadi, A.T.; Valsami, S.I.; Stamoulis, K.E.; Papageorgiou, E.G.; Politou, M.; Papassideri, I.S.; Kriebardis, A.G.; Antonelou, M.H. Osmotic hemolysis is a donor-specific feature of red blood cells under various storage conditions and genetic backgrounds. Transfusion 2021, 61, 2538–2544. [Google Scholar] [CrossRef]
- Recktenwald, S.M.; Lopes, M.G.M.; Peter, S.; Hof, S.; Simionato, G.; Peikert, K.; Hermann, A.; Danek, A.; van Bentum, K.; Eichler, H.; et al. Erysense, a Lab-on-a-Chip-Based Point-of-Care Device to Evaluate Red Blood Cell Flow Properties with Multiple Clinical Applications. Front. Physiol. 2022, 13, 884690. [Google Scholar] [CrossRef]
- Mock, D.M.; Nalbant, D.; Kyosseva, S.V.; Schmidt, R.L.; An, G.; Matthews, N.I.; Vlaar, A.P.J.; van Bruggen, R.; de Korte, D.; Strauss, R.G.; et al. Development, validation, and potential applications of biotinylated red blood cells for posttransfusion kinetics and other physiological studies: Evidenced-based analysis and recommendations. Transfusion 2018, 58, 2068–2081. [Google Scholar] [CrossRef] [PubMed]
| Author [Reference] [Year] | Donor [Number] | Rate of Infusion (mL/h) | Centrif. [min] | Storage | 24 h In Vivo RBC Survival (%) | Hemoglobin (g/dL) [Baseline] | Free Hemoglobin (mg/dL) [Baseline] | Hematocrit (%) [Baseline] | Hemolysis Index (%) [Baseline] | 2,3-DPG conc. (μmo/g Hb) [Baseline] | ATP conc. (μmol/g Hb) [Baseline] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anticoagulant | Additive Solution [mL/unit] | Temp. (°C) | Time (Days) | |||||||||||
| Wozniak et al. [22] [2018] | allogenic [11] | N/A | N/A | CPD | SAG-M | 4 | 14 | 72 a 71 b 68 c | 14.2 ± 2.3 a 12.8 ± 1.3 b 12.4 ± 0.5 c [14.5 ± 2.45] | 26 a 103 b 6 c [9] | 48.8 ± 7.9 a 49.7 ± 6.5 b 42 ± 14 c [49.4 ± 8.4] | 0.1 ± 0.04 a 1.0 ± 0.02 b 0.1 ± 0.03 c [0.07 ± 0.1] | 6.5 ± 1.6 a 13.8 ± 0.1 b 18.3 ± 7.5 c [14.4 ± 4] | 0.8 ± 0.3 a 1.3 ± 0.1 b 1.8 ± 0.4 c [2.0 ± 0.6] |
| Biagini et al. [23] [2018] | allogenic [16] | 1785 † | 1130 g [16] | CPDA-1 | N/A | 4–8 | 14 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Watts et al. [15] [2015] | allogenic [24] | 1800 † | N/A | CPD | SAG-M | 4 | 14 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Biagini et al. [24] [2014] | allogenic /autologous [1] | N/A | 3300 rpm [16] | CPDA-1 | N/A | 2–6 | 14 | 97.5 | 22.2 ± 1.5 [23.3 ± 1.4] | 112.4 ± 31.4 [31.0 ± 9.3] | 71.1 ± 2.3 [73.3 ± 3.5] | 0.5 ± 0.1 [0.1 ± 0.1] | N/A | N/A |
| Patel et al. [25] [2013] | allogenic [15] | 250 | N/A | CPD | SAG-M | 4 | 14 vs. 42 | N/A | N/A | N/A | 33 a,* 38 d,* [36 *] | 0.3 a,* 0.87 ± 0.59 d [0 *] | 12 a,* 1 d,* [15 *] | 1 a,* 0 d,* [6 *] |
| Patel et al. [26] [2011] | allogenic [8] | 250 | 2000 rpm [5] | CPD | SAG-M | 4 | 37 (34–42) | N/A | 12.1 (0.25) ** [11.7 (0.41) **] | N/A | 37.2 (2.66) ** [35.7 (1.23) **] | 0.87 (0.24) ** [0.01 (0.0) **] | N/A | 0.01 (0.0) ** [3.24 (0.53) **] |
| Alam et al. [29] [2009] | autologous [60] | 3000 † | 5000 rpm [15] | N/A | N/A | 4 | 1 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Buchholz et al. [30] [1999] | autologous [10] | 999 | N/A | CPD | Adsol [63] vs. CPDA-1 [100] | N/A | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berndt, M.; Buttenberg, M.; Graw, J.A. Large Animal Models for Simulating Physiology of Transfusion of Red Cell Concentrates—A Scoping Review of The Literature. Medicina 2022, 58, 1735. https://doi.org/10.3390/medicina58121735
Berndt M, Buttenberg M, Graw JA. Large Animal Models for Simulating Physiology of Transfusion of Red Cell Concentrates—A Scoping Review of The Literature. Medicina. 2022; 58(12):1735. https://doi.org/10.3390/medicina58121735
Chicago/Turabian StyleBerndt, Melanie, Maximilian Buttenberg, and Jan A. Graw. 2022. "Large Animal Models for Simulating Physiology of Transfusion of Red Cell Concentrates—A Scoping Review of The Literature" Medicina 58, no. 12: 1735. https://doi.org/10.3390/medicina58121735
APA StyleBerndt, M., Buttenberg, M., & Graw, J. A. (2022). Large Animal Models for Simulating Physiology of Transfusion of Red Cell Concentrates—A Scoping Review of The Literature. Medicina, 58(12), 1735. https://doi.org/10.3390/medicina58121735






