Pseudophakic Cystoid Macular Oedema (PCME) Prevention in Patients with Non-Proliferative Diabetic Retinopathy (NPDR)—Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
Study Design
3. Statistical Analysis
4. Results
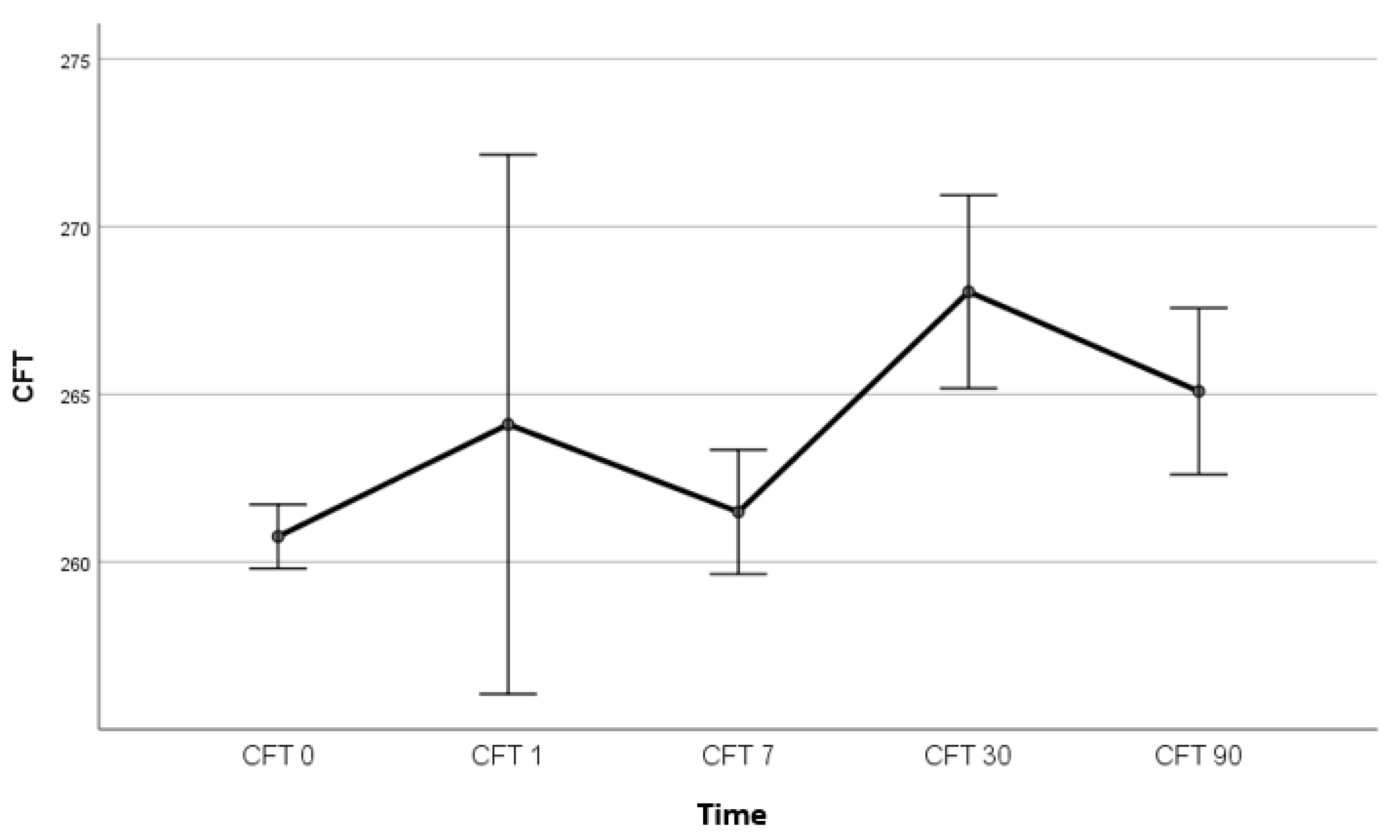
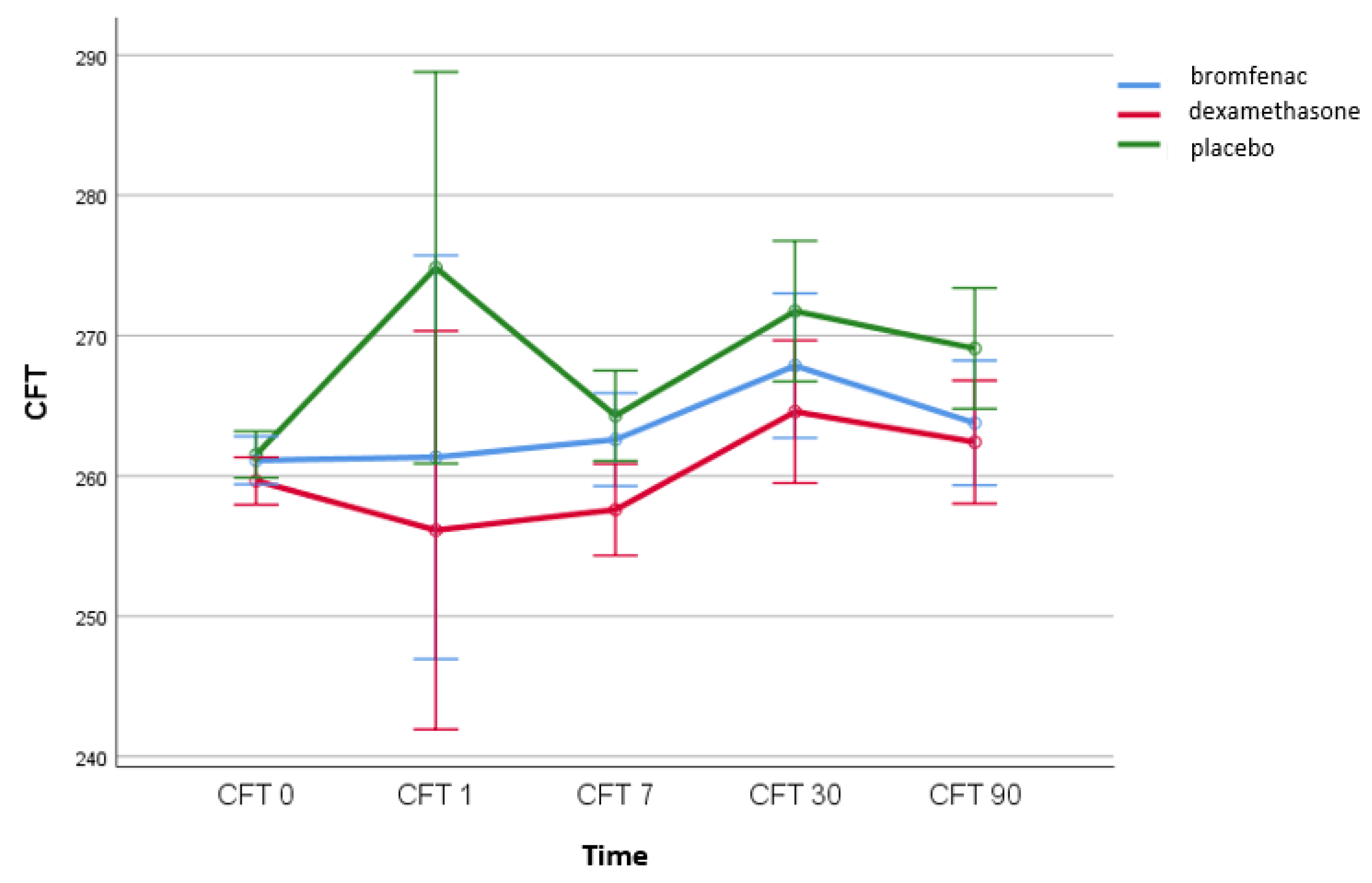
4.1. The Effect of Topical Bromfenac and Dexamethasone on the Incidence of Pseudophakic Cystoid Macular Oedema
4.2. The Correlation between IL-6 Levels and CFT
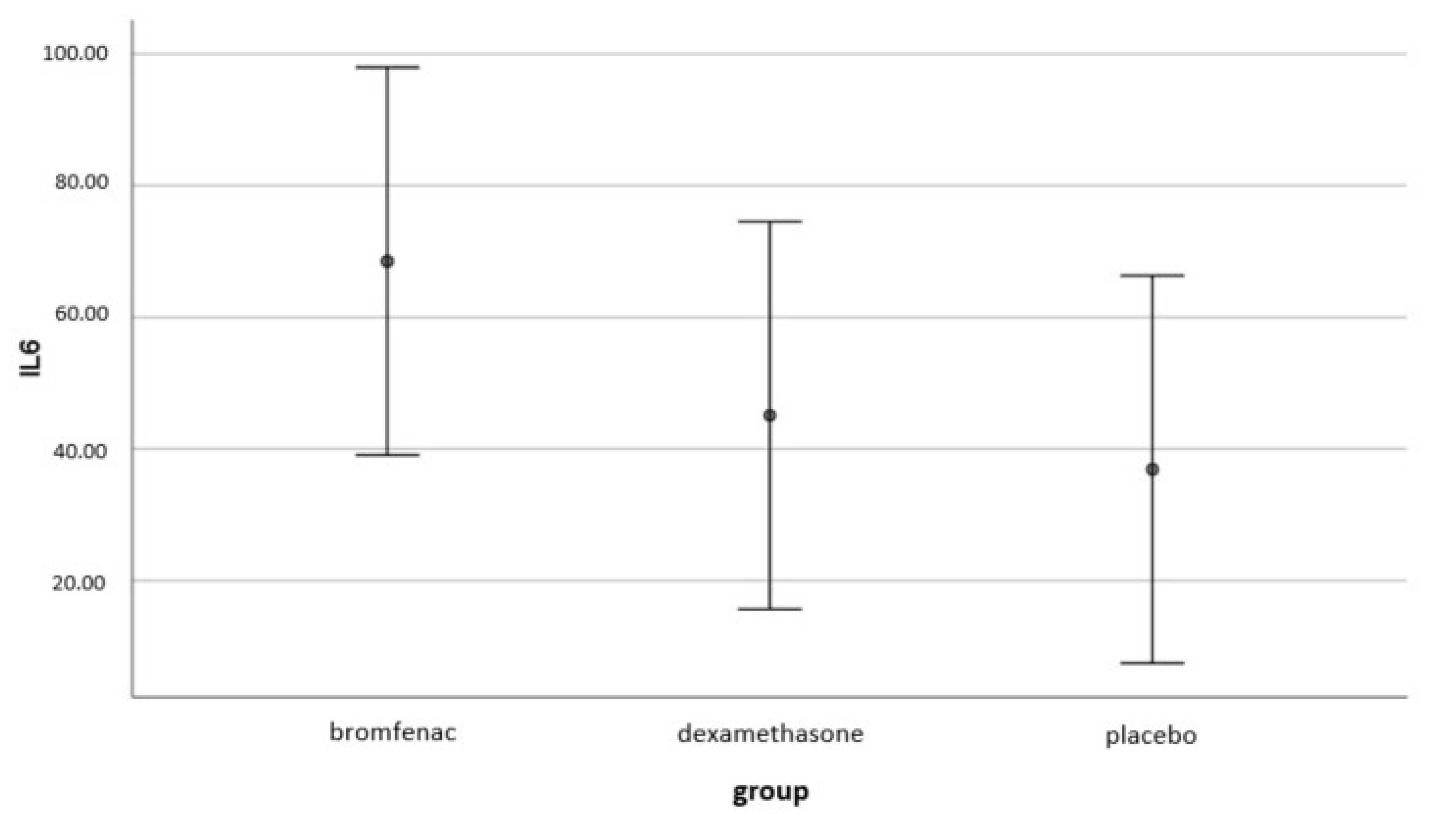
4.3. The Effect of Topical Bromfenac and Dexamethasone on Intraocular Interleukin-6 (IL-6) Concentrations
4.4. Differences in Age and in DM Duration (Risk Factors for PCME)
4.5. Intraocular Pressure (IOP) and Best-Corrected Visual Acuity (BCVA)
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diabetic Retinopathy Clinical Research Network Authors/Writing Committee; Baker, C.W.; Almukhtar, T.; Bressler, N.M.; Glassman, A.R.; Grover, S.; Kim, S.J.; Murtha, T.J.; Rauser, M.E.; Stockdale, C. Macular edema after cataract surgery in eyes without preoperative central-involved diabetic macular edema. JAMA Ophthalmol. 2013, 131, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Modjtahedi, B.S.; Paschal, J.F.; Batech, M.; Luong, T.Q.; Fong, D.S. Perioperative Topical Nonsteroidal Anti-inflammatory Drugs for Macular Edema Prophylaxis Following Cataract Surgery. Am. J. Ophthalmol. 2017, 176, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Belair, M.-L.; Bressler, N.M.; Dunn, J.P.; Thorne, J.E.; Kedhar, S.R.; Jabs, D.A. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina 2008, 28, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cai, L.; Sun, Z.; Ye, H.; Fan, Q.; Zhang, K.; Lu, W.; Lu, Y. Risk factors for and diagnosis of pseudophakic cystoid macular edema after cataract surgery in diabetic patients. J. Cataract Refract. Surg. 2017, 43, 207–214. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Baskaran, P.; Talwar, B.; Venkatesh, R. Prospective, Randomized Study Comparing the Effect of 0.1% Nepafenac and 0.4% Ketorolac Tromethamine on Macular Thickness in Cataract Surgery Patients With Low Risk for Cystoid Macular Edema. Asia Pac. J. Ophthalmol. 2015, 4, 216–220. [Google Scholar] [CrossRef]
- Singh, R.; Alpern, L.; Jaffe, G.J.; Lehmann, R.P.; Lim, J.; Reiser, H.J.; Sall, K.; Walters, T.; Sager, D. Evaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathy. Clin. Ophthalmol. 2012, 6, 1259–1269. [Google Scholar] [CrossRef]
- Pollack, A.; Staurenghi, G.; Sager, D.; Mukesh, B.; Reiser, H.; Singh, R.P. Prospective randomised clinical trial to evaluate the safety and efficacy of nepafenac 0.1% treatment for the prevention of macular oedema associated with cataract surgery in patients with diabetic retinopathy. Br. J. Ophthalmol. 2017, 101, 423–427. [Google Scholar] [CrossRef]
- Shorstein, N.H.; Liu, L.; Waxman, M.D.; Herrinton, L.J. Comparative Effectiveness of Three Prophylactic Strategies to Prevent Clinical Macular Edema after Phacoemulsification Surgery. Ophthalmology 2015, 122, 2450–2456. [Google Scholar] [CrossRef]
- Sarfraz, M.H.; Haq, R.I.U.; Mehboob, M.A. Effect of topical nepafenac in prevention of macular edema after cataract surgery in patients with non-proliferative diabetic retinopathy. Pak. J. Med. Sci. 2017, 33, 210–214. [Google Scholar] [CrossRef]
- McCafferty, S.; Harris, A.; Kew, C.; Kassm, T.; Lane, L.; Levine, J.; Raven, M. Pseudophakic cystoid macular edema prevention and risk factors; prospective study with adjunctive once daily topical nepafenac 0.3% versus placebo. BMC Ophthalmol. 2017, 17, 16. [Google Scholar] [CrossRef]
- Perente, I.; Utine, C.A.; Ozturker, C.; Cakir, M.; Kaya, V.; Eren, H.; Kapran, Z.; Yilmaz, O.F. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Curr. Eye Res. 2007, 32, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, Y.; Kim, I.K. Pseudophakic cystoid macular edema. Curr. Opin. Ophthalmol. 2012, 23, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kusbeci, T.; Eryigit, L.; Yavaş, G.; Inan, U.U. Evaluation of cystoid macular edema using optical coherence tomography and fundus fluorescein angiography after uncomplicated phacoemulsification surgery. Curr. Eye Res. 2012, 37, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Sigler, E.J.; Randolph, J.C.; Kiernan, D.F. Longitudinal analysis of the structural pattern of pseudophakic cystoid macular edema using multimodal imaging. Graefes. Arch. Clin. Exp. Ophthalmol. 2016, 254, 43–51. [Google Scholar] [CrossRef]
- Flach, A.J. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans. Am. Ophthalmol. Soc. 1998, 96, 557–634. [Google Scholar]
- Munk, M.R.; Jampol, L.M.; Simader, C.; Huf, W.; Mittermüller, T.J.; Jaffe, G.J.; Schmidt-Erfurth, U. Differentiation of Diabetic Macular Edema From Pseudophakic Cystoid Macular Edema by Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6724–6733. [Google Scholar] [CrossRef]
- Kim, S.J.; Equi, R.; Bressler, N.M. Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology 2007, 114, 881–889. [Google Scholar] [CrossRef]
- Yao, Y.; Li, R.; Du, J.; Long, L.; Li, X.; Luo, N. Interleukin-6 and Diabetic Retinopathy: A Systematic Review and Meta-Analysis. Curr. Eye Res. 2019, 44, 564–574. [Google Scholar] [CrossRef]
- Funatsu, H.; Yamashita, H.; Ikeda, T.; Mimura, T.; Eguchi, S.; Hori, S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology 2003, 110, 1690–1696. [Google Scholar] [CrossRef]
- Hoekzema, R.; Verhagen, C.; van Haren, M.; Kijlstra, A. Endotoxin-induced uveitis in the rat. The significance of intraocular interleukin-6. Investig. Ophthalmol. Vis. Sci. 1992, 33, 532–539. [Google Scholar]
- Malecaze, F.; Chollet, P.; Cavrois, E.; Vita, N.; Arné, J.L.; Ferrara, P. Role of interleukin 6 in the inflammatory response after cataract surgery. An experimental and clinical study. Arch. Ophthalmol. 1991, 109, 1681–1683. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, H.; Yamashita, H.; Noma, H.; Shimizu, E.; Mimura, T.; Hori, S. Prediction of macular edema exacerbation after phacoemulsification in patients with nonproliferative diabetic retinopathy. J. Cataract. Refract. Surg. 2002, 28, 1355. [Google Scholar] [CrossRef]
- Funatsu, H.; Yamashita, H.; Noma, H.; Mimura, T.; Yamashita, T.; Hori, S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am. J. Ophthalmol. 2002, 133, 70–77. [Google Scholar] [CrossRef]
- Funatsu, H.; Noma, H.; Mimura, T.; Eguchi, S.; Hori, S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009, 116, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.K.; Kim, S.-W.; Oh, J.; Lee, T.S.; Huh, K. Inflammatory and angiogenic factors in the aqueous humor and the relationship to diabetic retinopathy. Curr. Eye Res. 2010, 35, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K.; Knudtson, M.D.; Wong, T.Y.; Tsai, M.Y. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch. Ophthalmol. 2006, 124, 87–94. [Google Scholar] [CrossRef]
- Chu, L.; Wang, B.; Xu, B.; Dong, N. Aqueous cytokines as predictors of macular edema in non-diabetic patients following uncomplicated phacoemulsification cataract surgery. Mol. Vis. 2013, 19, 2418–2425. [Google Scholar]
- Laursen, S.B.; Erichsen, J.H.; Holm, L.M.; Kessel, L. Prevention of macular edema in patients with diabetes after cataract surgery. J. Cataract Refract. Surg. 2019, 45, 854–869. [Google Scholar] [CrossRef]
- Alnagdy, A.A.; Abouelkheir, H.Y.; El-Khouly, S.E.; Tarshouby, S.M. Impact of topical nonsteroidal anti-inflammatory drugs in prevention of macular edema following cataract surgery in diabetic patients. Int. J. Ophthalmol. 2018, 11, 616–622. [Google Scholar] [CrossRef]
- Matsumura, T.; Iwasaki, K.; Arimura, S.; Takeda, R.; Takamura, Y.; Inatani, M. Topical bromfenac reduces multiple inflammatory cytokines in the aqueous humour of pseudophakic patients. Sci. Rep. 2021, 11, 6018. [Google Scholar] [CrossRef]
- Olson, R.J.; Braga-Mele, R.; Chen, S.H.; Miller, K.M.; Pineda, R.; Tweeten, J.P.; Musch, D.C. Cataract in the Adult Eye Preferred Practice Pattern®. Ophthalmology 2017, 124, P1–P119. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Lehmann, R.; Martel, J.; Jong, K.; Pollack, A.; Tsorbatzoglou, A.; Staurenghi, G.; Cervantes, G.C.-C.; Alpern, L.; Modi, S.; et al. Nepafenac 0.3% after Cataract Surgery in Patients with Diabetic Retinopathy: Results of 2 Randomized Phase 3 Studies. Ophthalmology 2017, 124, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Staurenghi, G.; Pollack, A.; Adewale, A.; Walker, T.M.; Sager, D.; Lehmann, R. Efficacy of nepafenac ophthalmic suspension 0.1% in improving clinical outcomes following cataract surgery in patients with diabetes: An analysis of two randomized studies. Clin. Ophthalmol. 2017, 11, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Xu, B.; Wang, B.; Chu, L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol. Vis. 2013, 19, 1734–1746. [Google Scholar] [PubMed]
- Chen, J.L.; Bhat, P.; Lobo-Chan, A.-M. Perioperative Management of Uveitic Cataracts. Adv. Ophthalmol. Optom. 2019, 4, 325–339. [Google Scholar] [CrossRef]
- Gaynes, B.I.; Onyekwuluje, A. Topical ophthalmic NSAIDs: A discussion with focus on nepafenac ophthalmic suspension. Clin. Ophthalmol. 2008, 2, 355–368. [Google Scholar] [CrossRef]
- Kim, S.J.; Flach, A.J.; Jampol, L.M. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv. Ophthalmol. 2010, 55, 108–133. [Google Scholar] [CrossRef]
- Leamingsurveys. Survey of US ASCRS Members. 2012. Available online: http://www.analeyz.com/NEWAnaleyz%20ASCRS%202012.htm (accessed on 1 September 2022).
- Karkhur, S.; Verma, V.; Gupta, R.; Sharma, B. Commentary: Newer non-steroidal anti-inflammatory drugs for postoperative management in phacoemulsification. Are topical corticosteroids still obligatory? Indian J. Ophthalmol. 2022, 70, 433–434. [Google Scholar] [CrossRef]
- Chylack, L.T., Jr.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.Y. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Zur, D.; Loewenstein, A. Postsurgical Cystoid Macular Edema. Dev. Ophthalmol. 2017, 58, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Ramezani, A.; Nikkhah, H.; Yaseri, M. The effect of topical sodium diclofenac on macular thickness in diabetic eyes after phacoemulsification: A randomized controlled trial. Int. Ophthalmol. 2017, 37, 13–18. [Google Scholar] [CrossRef]
- Kim, S.J.; Patel, S.N.; Sternberg, P., Jr. Routine Use of Nonsteroidal Anti-inflammatory Drugs with Corticosteroids in Cataract Surgery: Beneficial or Redundant? Ophthalmology 2016, 123, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, L.; Bujtar, E.; Castoldi, D.; Torrazza, C.; Orzalesi, N. Effectiveness of diclofenac eyedrops in reducing inflammation and the incidence of cystoid macular edema after cataract surgery. J. Cataract Refract. Surg. 1996, 22 (Suppl. 1), 794–799. [Google Scholar] [CrossRef]
- Zanetti, F.R.; Fulco, E.A.M.; Chaves, F.R.P.; da Costa Pinto, A.P.; Arieta, C.E.L.; Lira, R.P.C. Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery: A randomized trial. Indian J. Ophthalmol. 2012, 60, 277–281. [Google Scholar] [CrossRef]
- Simone, J.N.; Pendelton, R.A.; Jenkins, J.E. Comparison of the efficacy and safety of ketorolac tromethamine 0.5% and prednisolone acetate 1% after cataract surgery. J. Cataract. Refract. Surg. 1999, 25, 699–704. [Google Scholar] [CrossRef]
- Schoenberger, S.D.; Kim, S.J. Nonsteroidal anti-inflammatory drugs for retinal disease. Int. J. Inflamm. 2013, 2013, 281981. [Google Scholar] [CrossRef]
- Schultz, R.O.; Van Horn, D.L.; Peters, M.A.; Klewin, K.M.; Schutten, W.H. Diabetic keratopathy. Trans. Am. Ophthalmol. Soc. 1981, 79, 180–199. [Google Scholar]
- Wielders, L.H.P.; Schouten, J.S.A.G.; Winkens, B.; van den Biggelaar, F.J.H.M.; Veldhuizen, C.A.; Murta, J.C.N.; Goslings, W.R.O.; Kohnen, T.; Tassignon, M.-J.; Joosse, M.V.; et al. Randomized controlled European multicenter trial on the prevention of cystoid macular edema after cataract surgery in diabetics: ESCRS PREMED Study Report 2. J. Cataract Refract. Surg. 2018, 44, 836–847. [Google Scholar] [CrossRef]

| Pair of Variables | Dexamethasone | |||
|---|---|---|---|---|
| Valid N | Spearman R | t(N-2) | p-Value | |
| IL6 & CFT -7 | 30 | 0.315 | 1.755 | 0.090 |
| IL6 & CFT 0 | 30 | 0.265 | 1.455 | 0.157 |
| IL6 & CFT 1 | 30 | 0.370 | 2.110 | 0.044 |
| IL6 & CFT 7 | 30 | 0.376 | 2.148 | 0.040 |
| IL6 & CFT 30 | 30 | 0.465 | 2.778 | 0.010 |
| IL6 & CFT 90 | 30 | 0.340 | 1.911 | 0.066 |
| Pair of Variables | Dexamethasone | |||
|---|---|---|---|---|
| Valid N | Spearman R | t(N-2) | p-Value | |
| IL6 & CFT 0 | 30 | 0.171 | 0.920 | 0.366 |
| IL6 & CFT 1 | 30 | 0.309 | 1.718 | 0.097 |
| IL6 & CFT 7 | 30 | 0.170 | 0.913 | 0.369 |
| IL6 & CFT 30 | 30 | 0.609 | 4.064 | 0.000 |
| IL6 & CFT 90 | 30 | 0.465 | 2.781 | 0.010 |
| IL6 & CFT 0 | 30 | 0.171 | 0.920 | 0.366 |
| Pollack 2017 [7] | Nepafenac 0.1%+ Dexamethasone 0.1% | Dexamethasone 0.1% | p = 0.01 |
|---|---|---|---|
| Singh 2012 [6] | Nepafenac 0.1%+ prednisolone | Prednisolone (Omnipred) | p < 0.001 |
| Singh 2017 [32] | Nepafenac 0.3%+ prednisolone | Prednisolone (Omnipred) | p < 0.001 |
| Singh 2017 [33] | Nepafenac 0.1%+ prednisolone | Prednisolone (Omnipred) | p = 0.001; p = 0.018 |
| Sarfraz 2017 [9] | Nepafenac 0.1%+ prednisolone 0.1% | 1% Prednisolone Acetate | p < 0.05 |
| Entezari 2017 [44] | Diclofenac 0.1%+ corticosteroid | Corticosteroid (not specified) | p = 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jukić, A.; Kasalica Žužul, R.; Pavan, J.; Lovrić, M.; Kozmar, A.; Plavec, D.; Kuzman, T.; Kalauz, M.; Jukić, T. Pseudophakic Cystoid Macular Oedema (PCME) Prevention in Patients with Non-Proliferative Diabetic Retinopathy (NPDR)—Randomized Controlled Trial. Medicina 2022, 58, 1667. https://doi.org/10.3390/medicina58111667
Jukić A, Kasalica Žužul R, Pavan J, Lovrić M, Kozmar A, Plavec D, Kuzman T, Kalauz M, Jukić T. Pseudophakic Cystoid Macular Oedema (PCME) Prevention in Patients with Non-Proliferative Diabetic Retinopathy (NPDR)—Randomized Controlled Trial. Medicina. 2022; 58(11):1667. https://doi.org/10.3390/medicina58111667
Chicago/Turabian StyleJukić, Anđela, Rajka Kasalica Žužul, Josip Pavan, Mila Lovrić, Ana Kozmar, Davor Plavec, Tomislav Kuzman, Miro Kalauz, and Tomislav Jukić. 2022. "Pseudophakic Cystoid Macular Oedema (PCME) Prevention in Patients with Non-Proliferative Diabetic Retinopathy (NPDR)—Randomized Controlled Trial" Medicina 58, no. 11: 1667. https://doi.org/10.3390/medicina58111667
APA StyleJukić, A., Kasalica Žužul, R., Pavan, J., Lovrić, M., Kozmar, A., Plavec, D., Kuzman, T., Kalauz, M., & Jukić, T. (2022). Pseudophakic Cystoid Macular Oedema (PCME) Prevention in Patients with Non-Proliferative Diabetic Retinopathy (NPDR)—Randomized Controlled Trial. Medicina, 58(11), 1667. https://doi.org/10.3390/medicina58111667







