Impact of Anterior Malposition and Bone Cement Augmentation on the Fixation Strength of Cephalic Intramedullary Nail Head Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens and Study Groups
2.2. Specimens Preparation
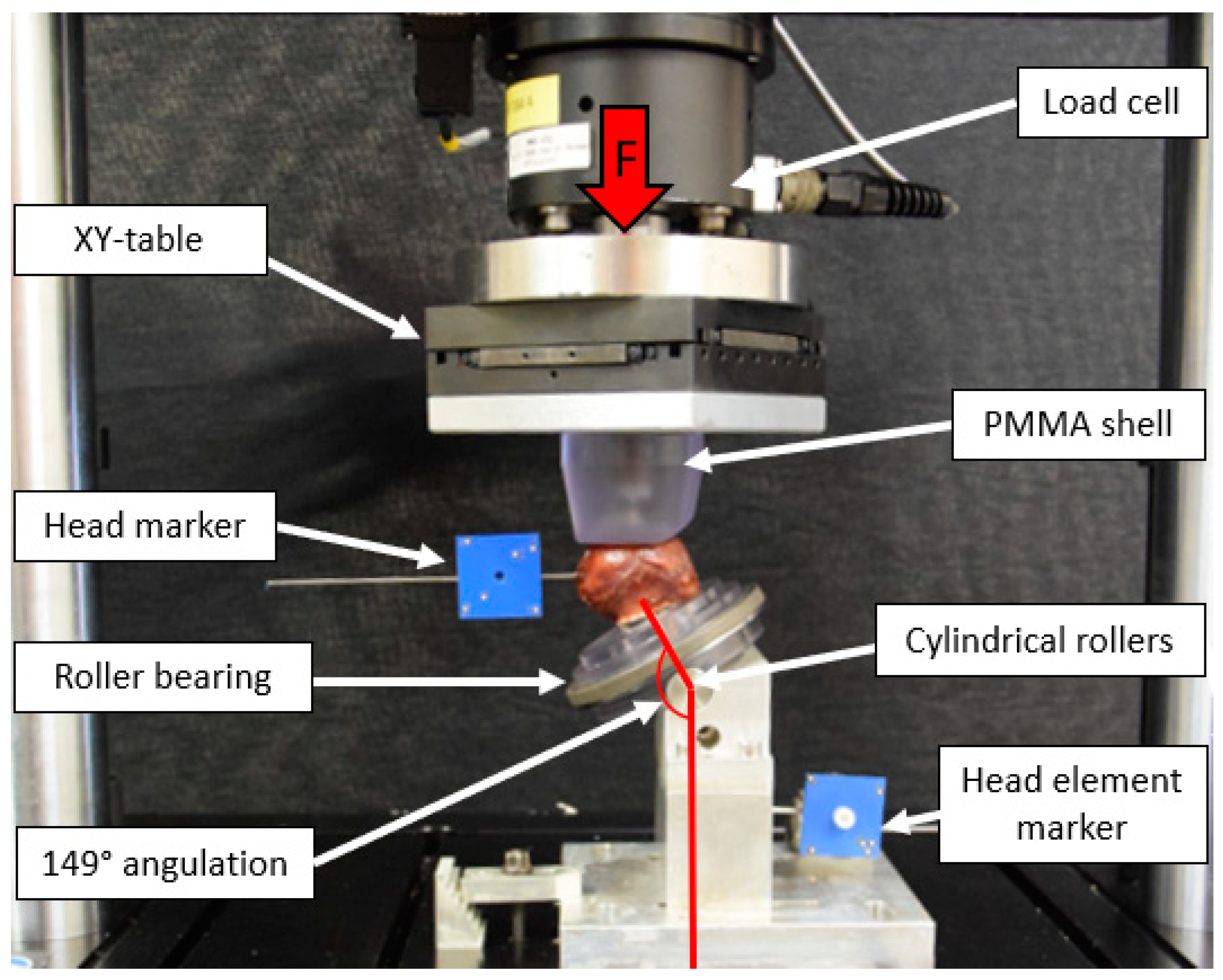
2.3. Test Setup
2.4. Loading Protocol
2.5. Data Acquisition and Analysis
2.6. Statistical Analysis
3. Results
3.1. Morphometrics
3.2. Initial Stiffness
3.3. Varus Deformation, Femoral Head Rotation, Implant Migration and Implant Cut-Out at Predefined Cycles
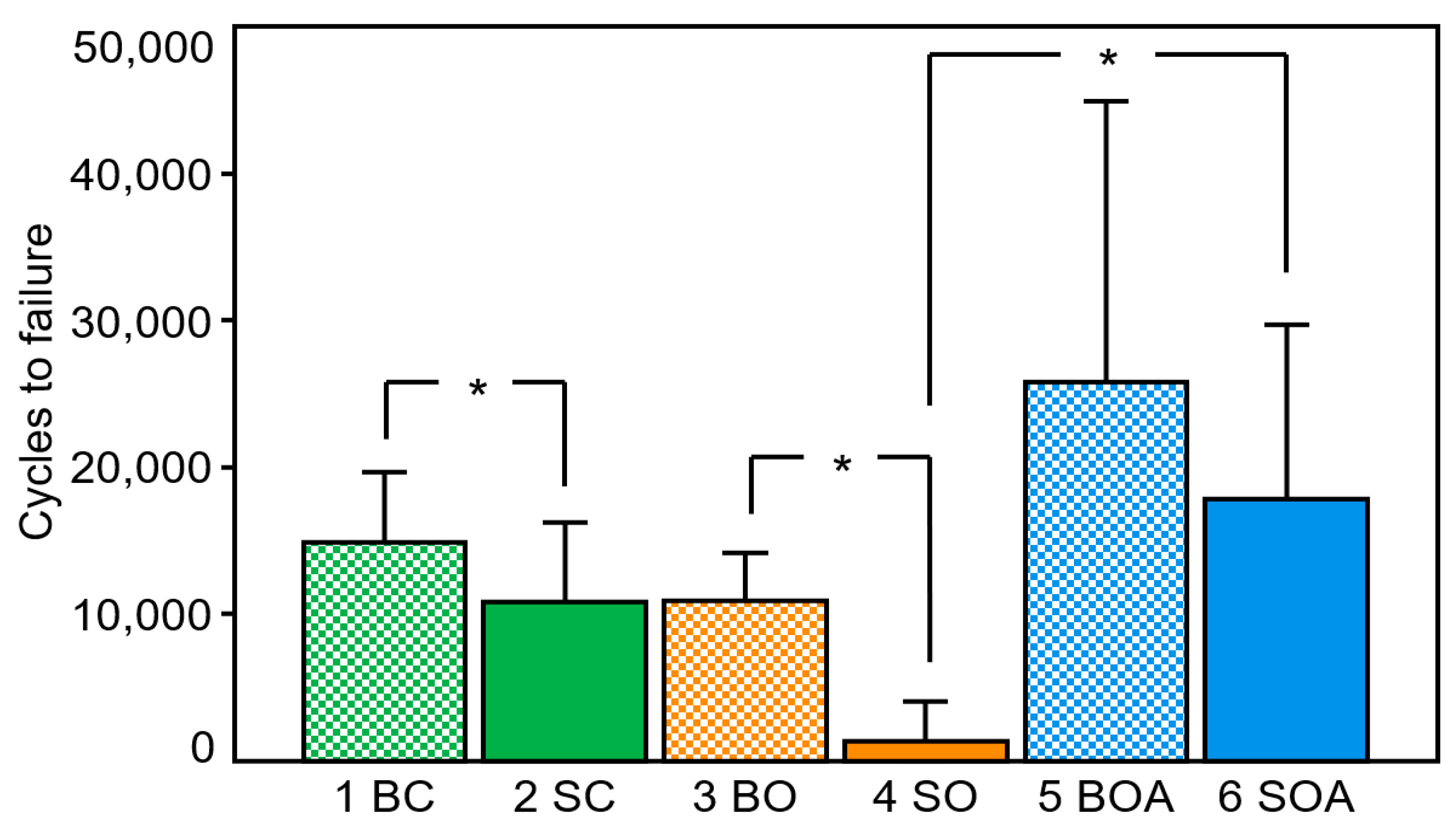
3.4. Cycles to Clinical Failure
3.5. Catastrophic Failure Modes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knobe, M.; Siebert, C.H. [Hip fractures in the elderly: Osteosynthesis versus joint replacement]. Orthopade 2014, 43, 314–324. [Google Scholar] [CrossRef]
- Parker, M.J.; Handoll, H.H. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Szita, J.; Cserhati, P.; Bosch, U.; Manninger, J.; Bodzay, T.; Fekete, K. Intracapsular femoral neck fractures: The importance of early reduction and stable osteosynthesis. Injury 2002, 33, C41–C46. [Google Scholar] [CrossRef]
- Lorich, D.G.; Geller, D.S.; Nielson, J.H. Osteoporotic pertrochanteric hip fractures: Management and current controversies. Instr. Course Lect. 2004, 53, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, K.K.; Fang, C.K.; Chen, C.M.; Su, Y.P.; Wu, H.F.; Chiu, F.Y. Risk factors in cutout of sliding hip screw in intertrochanteric fractures: An evaluation of 937 patients. Int. Orthop. 2010, 34, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Baumgaertner, M.R.; Solberg, B.D. Awareness of tip-apex distance reduces failure of fixation of trochanteric fractures of the hip. J. Bone Jt. Surg. Br. 1997, 79, 969–971. [Google Scholar] [CrossRef]
- Rupprecht, M.; Grossterlinden, L.; Ruecker, A.H.; de Oliveira, A.N.; Sellenschloh, K.; Nuchtern, J.; Puschel, K.; Morlock, M.; Rueger, J.M.; Lehmann, W. A comparative biomechanical analysis of fixation devices for unstable femoral neck fractures: The Intertan versus cannulated screws or a dynamic hip screw. J. Trauma 2011, 71, 625–634. [Google Scholar] [CrossRef]
- Lenich, A.; Vester, H.; Nerlich, M.; Mayr, E.; Stockle, U.; Fuchtmeier, B. Clinical comparison of the second and third generation of intramedullary devices for trochanteric fractures of the hip--Blade vs screw. Injury 2010, 41, 1292–1296. [Google Scholar] [CrossRef]
- Knobe, M.; Altgassen, S.; Maier, K.J.; Gradl-Dietsch, G.; Kaczmarek, C.; Nebelung, S.; Klos, K.; Kim, B.S.; Gueorguiev, B.; Horst, K.; et al. Screw-blade fixation systems in Pauwels three femoral neck fractures: A biomechanical evaluation. Int. Orthop. 2018, 42, 409–418. [Google Scholar] [CrossRef]
- Sermon, A.; Zderic, I.; Khatchadourian, R.; Scherrer, S.; Knobe, M.; Stoffel, K.; Gueorguiev, B. Bone cement augmentation of femoral nail head elements increases their cut-out resistance in poor bone quality—A biomechanical study. J. Biomech. 2021, 118, 110301. [Google Scholar] [CrossRef]
- Roderer, G.; Scola, A.; Schmolz, W.; Gebhard, F.; Windolf, M.; Hofmann-Fliri, L. Biomechanical in vitro assessment of screw augmentation in locked plating of proximal humerus fractures. Injury 2013, 44, 1327–1332. [Google Scholar] [CrossRef]
- Wahnert, D.; Hofmann-Fliri, L.; Richards, R.G.; Gueorguiev, B.; Raschke, M.J.; Windolf, M. Implant augmentation: Adding bone cement to improve the treatment of osteoporotic distal femur fractures: A biomechanical study using human cadaver bones. Medicine 2014, 93, e166. [Google Scholar] [CrossRef] [PubMed]
- Knobe, M.; Bettag, S.; Kammerlander, C.; Altgassen, S.; Maier, K.J.; Nebelung, S.; Prescher, A.; Horst, K.; Pishnamaz, M.; Herren, C.; et al. Is bone-cement augmentation of screw-anchor fixation systems superior in unstable femoral neck fractures? A biomechanical cadaveric study. Injury 2019, 50, 292–300. [Google Scholar] [CrossRef]
- Baumgaertner, M.R.; Curtin, S.L.; Lindskog, D.M.; Keggi, J.M. The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J. Bone Jt. Surg. Am. 1995, 77, 1058–1064. [Google Scholar] [CrossRef]
- Kuzyk, P.R.; Zdero, R.; Shah, S.; Olsen, M.; Waddell, J.P.; Schemitsch, E.H. Femoral head lag screw position for cephalomedullary nails: A biomechanical analysis. J. Orthop. Trauma 2012, 26, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Puthezhath, K.; Jayaprakash, C. Is calcar referenced tip-apex distance a better predicting factor for cutting out in biaxial cephalomedullary nails than tip-apex distance? J. Orthop. Surg. 2017, 25, 2309499017727920. [Google Scholar] [CrossRef] [PubMed]
- Tosounidis, T.H.; Castillo, R.; Kanakaris, N.K.; Giannoudis, P.V. Common complications in hip fracture surgery: Tips/tricks and solutions to avoid them. Injury 2015, 46, S3–S11. [Google Scholar] [CrossRef]
- Sermon, A.; Hofmann-Fliri, L.; Zderic, I.; Agarwal, Y.; Scherrer, S.; Weber, A.; Altmann, M.; Knobe, M.; Windolf, M.; Gueorguiev, B. Impact of Bone Cement Augmentation on the Fixation Strength of TFNA Blades and Screws. Medicina 2021, 57, 899. [Google Scholar] [CrossRef] [PubMed]
- Pastor, T.; Zderic, I.; Gehweiler, D.; Gardner, M.J.; Stoffel, K.; Richards, G.; Knobe, M.; Gueorguiev, B. Biomechanical analysis of recently released cephalomedullary nails for trochanteric femoral fracture fixation in a human cadaveric model. Arch. Orthop. Trauma Surg. 2021, 142, 3787–3796. [Google Scholar] [CrossRef]
- Zderic, I.; Oh, J.K.; Stoffel, K.; Sommer, C.; Helfen, T.; Camino, G.; Richards, G.; Nork, S.E.; Gueorguiev, B. Biomechanical Analysis of the Proximal Femoral Locking Compression Plate: Do Quality of Reduction and Screw Orientation Influence Construct Stability? J. Orthop. Trauma 2018, 32, 67–74. [Google Scholar] [CrossRef]
- Konstantinidis, L.; Papaioannou, C.; Hirschmuller, A.; Pavlidis, T.; Schroeter, S.; Sudkamp, N.P.; Helwig, P. Intramedullary nailing of trochanteric fractures: Central or caudal positioning of the load carrier? A biomechanical comparative study on cadaver bones. Injury 2013, 44, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, M.; Bosworth, D.M.; Thompson, F.R.; Wilson, H.J., Jr.; Ishizuka, T. A ten-year analysis of intertrochanteric fractures of the femur. J. Bone Jt. Surg. Am. 1959, 41, 1399–1408. [Google Scholar] [CrossRef]
- Sermon, A.; Boner, V.; Boger, A.; Schwieger, K.; Boonen, S.; Broos, P.L.; Richards, R.G.; Windolf, M. Potential of polymethylmethacrylate cement-augmented helical proximal femoral nail antirotation blades to improve implant stability—A biomechanical investigation in human cadaveric femoral heads. J. Trauma Acute Care Surg. 2012, 72, E54–E59. [Google Scholar] [CrossRef] [PubMed]
- Sommers, M.B.; Roth, C.; Hall, H.; Kam, B.C.; Ehmke, L.W.; Krieg, J.C.; Madey, S.M.; Bottlang, M. A laboratory model to evaluate cutout resistance of implants for pertrochanteric fracture fixation. J. Orthop. Trauma 2004, 18, 361–368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bergmann, G.; Deuretzbacher, G.; Heller, M.; Graichen, F.; Rohlmann, A.; Strauss, J.; Duda, G.N. Hip contact forces and gait patterns from routine activities. J. Biomech. 2001, 34, 859–871. [Google Scholar] [CrossRef]
- Sermon, A.; Hofmann-Fliri, L.; Richards, R.G.; Flamaing, J.; Windolf, M. Cement augmentation of hip implants in osteoporotic bone: How much cement is needed and where should it go? J. Orthop. Res. 2014, 32, 362–368. [Google Scholar] [CrossRef]
- Schopper, C.; Keck, K.; Zderic, I.; Migliorini, F.; Link, B.C.; Beeres, F.J.P.; Babst, R.; Nebelung, S.; Eschbach, D.; Knauf, T.; et al. Screw-blade fixation systems for implant anchorage in the femoral head: Horizontal blade orientation provides superior stability. Injury 2021, 52, 1861–1867. [Google Scholar] [CrossRef]
- Goffin, J.M.; Pankaj, P.; Simpson, A.H.; Seil, R.; Gerich, T.G. Does bone compaction around the helical blade of a proximal femoral nail anti-rotation (PFNA) decrease the risk of cut-out?: A subject-specific computational study. Bone Jt. Res. 2013, 2, 79–83. [Google Scholar] [CrossRef]
- Windolf, M.; Muths, R.; Braunstein, V.; Gueorguiev, B.; Hanni, M.; Schwieger, K. Quantification of cancellous bone-compaction due to DHS Blade insertion and influence upon cut-out resistance. Clin. Biomech. 2009, 24, 53–58. [Google Scholar] [CrossRef]
- Chapman, T.; Zmistowski, B.; Krieg, J.; Stake, S.; Jones, C.M.; Levicoff, E. Helical Blade Versus Screw Fixation in the Treatment of Hip Fractures With Cephalomedullary Devices: Incidence of Failure and Atypical “Medial Cutout”. J. Orthop. Trauma 2018, 32, 397–402. [Google Scholar] [CrossRef]
- O’Neill, F.; Condon, F.; McGloughlin, T.; Lenehan, B.; Coffey, J.C.; Walsh, M. Dynamic hip screw versus DHS blade: A biomechanical comparison of the fixation achieved by each implant in bone. J. Bone Jt. Surg. Br. 2011, 93, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.C.; Gorczyca, J.T.; Kates, S.; Ketz, J.; Soles, G.; Humphrey, C.A. Radiographic Review of Helical Blade Versus Lag Screw Fixation for Cephalomedullary Nailing of Low-Energy Peritrochanteric Femur Fractures: There is a Difference in Cutout. J. Orthop. Trauma 2017, 31, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Kammerlander, C.; Hem, E.S.; Klopfer, T.; Gebhard, F.; Sermon, A.; Dietrich, M.; Bach, O.; Weil, Y.; Babst, R.; Blauth, M. Cement augmentation of the Proximal Femoral Nail Antirotation (PFNA)—A multicentre randomized controlled trial. Injury 2018, 49, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Sermon, A.; Boner, V.; Schwieger, K.; Boger, A.; Boonen, S.; Broos, P.; Richards, G.; Windolf, M. Biomechanical evaluation of bone-cement augmented Proximal Femoral Nail Antirotation blades in a polyurethane foam model with low density. Clin. Biomech. 2012, 27, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Erhart, J.; Unger, E.; Schefzig, P.; Varga, P.; Trulson, I.; Gormasz, A.; Trulson, A.; Reschl, M.; Hagmann, M.; Vecsei, V.; et al. Rotational Stability of Scaphoid Osteosyntheses: An In Vitro Comparison of Small Fragment Cannulated Screws to Novel Bone Screw Sets. PLoS ONE 2016, 11, e0156080. [Google Scholar] [CrossRef]
- Von der Linden, P.; Gisep, A.; Boner, V.; Windolf, M.; Appelt, A.; Suhm, N. Biomechanical evaluation of a new augmentation method for enhanced screw fixation in osteoporotic proximal femoral fractures. J. Orthop. Res. 2006, 24, 2230–2237. [Google Scholar] [CrossRef]
- Rompen, I.F.; Knobe, M.; Link, B.C.; Beeres, F.J.P.; Baumgaertner, R.; Diwersi, N.; Migliorini, F.; Nebelung, S.; Babst, R.; van de Wall, B.J.M. Cement augmentation for trochanteric femur fractures: A meta-analysis of randomized clinical trials and observational studies. PLoS ONE 2021, 16, e0251894. [Google Scholar] [CrossRef]
- Fensky, F.; Nuchtern, J.V.; Kolb, J.P.; Huber, S.; Rupprecht, M.; Jauch, S.Y.; Sellenschloh, K.; Puschel, K.; Morlock, M.M.; Rueger, J.M.; et al. Cement augmentation of the proximal femoral nail antirotation for the treatment of osteoporotic pertrochanteric fractures—A biomechanical cadaver study. Injury 2013, 44, 802–807. [Google Scholar] [CrossRef]
- Hofmann-Fliri, L.; Nicolino, T.I.; Barla, J.; Gueorguiev, B.; Richards, R.G.; Blauth, M.; Windolf, M. Cement augmentation of implants--no general cure in osteoporotic fracture treatment. A biomechanical study on non-displaced femoral neck fractures. J. Orthop. Res. 2016, 34, 314–319. [Google Scholar] [CrossRef]
- Brandt, E.; Verdonschot, N.; van Vugt, A.; van Kampen, A. Biomechanical analysis of the percutaneous compression plate and sliding hip screw in intracapsular hip fractures: Experimental assessment using synthetic and cadaver bones. Injury 2006, 37, 979–983. [Google Scholar] [CrossRef]

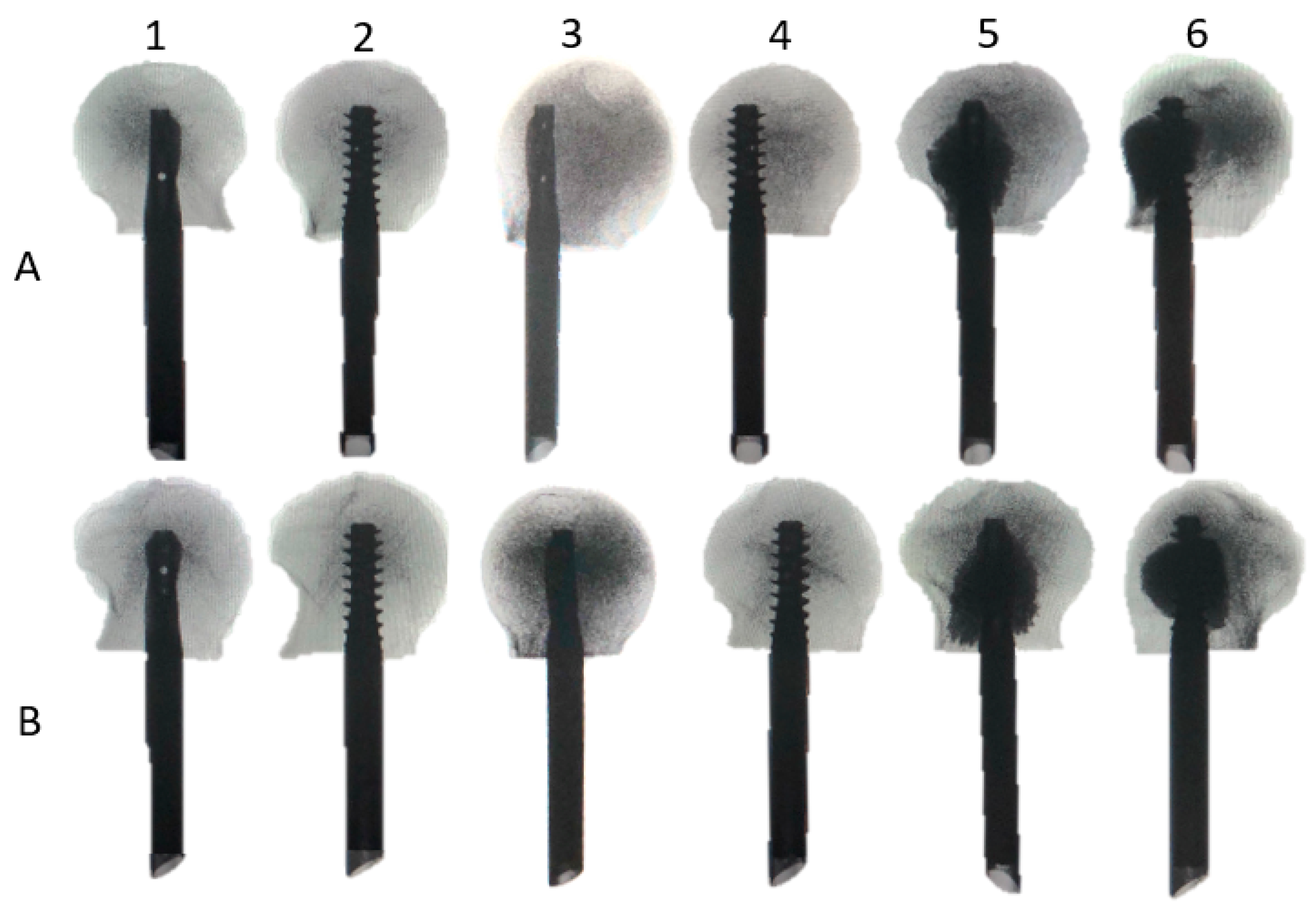
| Parameter | Cycles | Study Groups | |||||
|---|---|---|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | |||||
| 1 Blade Centre– Centre | 2 Screw Centre– Centre | 3 Blade Off-Centre | 4 Screw Off-Centre | 5 Blade Off-Centre Augmented | 6 Screw Off-Centre Augmented | ||
| Varus deformation [deg] | 2000 | 1.75 ± 0.67 | 2.17 ± 0.90 | 1.20 ± 1.02 | 3.33 ± 2.07 | 1.06 ± 0.40 | 1.47 ± 0.51 |
| 4000 | 2.16 ± 0.82 | 2.91 ± 1.49 | 1.61 ± 1.45 | 5.15 ± 2.58 | 1.20 ± 0.50 | 1.74 ± 0.69 | |
| 6000 | 2.53 ± 0.90 | 3.77 ± 2.07 | 2.25 ± 1.88 | – | 1.37 ± 0.65 | 2.09 ± 1.00 | |
| 8000 | 3.01 ± 1.05 | 4.89 ± 2.62 | 3.12 ± 2.37 | – | 1.51 ± 0.85 | – | |
| 10,000 | 3.44 ± 1.55 | 6.08 ± 3.07 | 5.24 ± 6.29 | – | 1.69 ± 1.05 | – | |
| Femoral head rotation [deg] | 2000 | 1.03 ± 1.90 | 0.84 ± 1.74 | 0.68 ± 0.42 | 25.60 ± 17.02 | 0.54 ± 0.31 | 1.60 ± 1.67 |
| 4000 | 1.32 ± 1.93 | 2.24 ± 5.23 | 1.35 ± 0.76 | 30.97 ± 25.50 | 0.66 ± 0.39 | 2.92 ± 3.50 | |
| 6000 | 1.65 ± 2.09 | 3.73 ± 7.71 | 2.73 ± 1.45 | – | 0.84 ± 0.51 | 5.82 ± 7.81 | |
| 8000 | 2.55 ± 3.68 | 6.20 ± 9.79 | 5.88 ± 3.42 | – | 1.22 ± 0.76 | – | |
| 10,000 | 5.03 ± 7.78 | 8.32 ± 11.44 | 10.74 ± 10.77 | – | 2.02 ± 1.55 | – | |
| Implant migration [mm] | 2000 | 0.09 ± 0.11 | 0.16 ± 0.15 | 0.60 ± 0.02 | 0.40 ± 0.30 | 0.06 ± 0.06 | 0.07 ± 0.02 |
| 4000 | 0.20 ± 0.31 | 0.24 ± 0.20 | 0.10 ± 0.03 | 0.37 ± 0.39 | 0.08 ± 0.06 | 0.09 ± 0.04 | |
| 6000 | 0.31 ± 0.42 | 0.31 ± 0.25 | 0.30 ± 0.27 | – | 0.11 ± 0.07 | 0.14 ± 0.08 | |
| 8000 | 0.64 ± 0.89 | 0.48 ± 0.31 | 0.59 ± 0.42 | – | 0.19 ± 0.11 | – | |
| 10,000 | 1.42 ± 2.20 | 0.96 ± 0.67 | 0.93 ± 0.49 | – | 0.37 ± 0.29 | – | |
| Implant cut-out [mm] | 2000 | 1.09 ± 0.35 | 1.23 ± 0.23 | 1.11 ± 0.53 | 3.53 ± 1.75 | 1.02 ± 0.19 | 1.28 ± 0.38 |
| 4000 | 1.38 ± 0.44 | 1.55 ± 0.42 | 1.38 ± 0.75 | 4.28 ± 2.44 | 1.18 ± 0.25 | 1.51 ± 0.49 | |
| 6000 | 1.63 ± 0.53 | 1.98 ± 0.67 | 1.70 ± 1.02 | – | 1.35 ± 0.35 | 1.76 ± 0.64 | |
| 8000 | 1.90 ± 0.67 | 2.61 ± 1.07 | 2.16 ± 1.32 | – | 1.54 ± 0.43 | – | |
| 10,000 | 2.30 ± 1.12 | 3.53 ± 2.08 | 4.33 ± 5.45 | – | 1.74 ± 0.53 | – | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor, T.; Zderic, I.; Schopper, C.; Haefeli, P.C.; Kastner, P.; Souleiman, F.; Gueorguiev, B.; Knobe, M. Impact of Anterior Malposition and Bone Cement Augmentation on the Fixation Strength of Cephalic Intramedullary Nail Head Elements. Medicina 2022, 58, 1636. https://doi.org/10.3390/medicina58111636
Pastor T, Zderic I, Schopper C, Haefeli PC, Kastner P, Souleiman F, Gueorguiev B, Knobe M. Impact of Anterior Malposition and Bone Cement Augmentation on the Fixation Strength of Cephalic Intramedullary Nail Head Elements. Medicina. 2022; 58(11):1636. https://doi.org/10.3390/medicina58111636
Chicago/Turabian StylePastor, Torsten, Ivan Zderic, Clemens Schopper, Pascal C. Haefeli, Philipp Kastner, Firas Souleiman, Boyko Gueorguiev, and Matthias Knobe. 2022. "Impact of Anterior Malposition and Bone Cement Augmentation on the Fixation Strength of Cephalic Intramedullary Nail Head Elements" Medicina 58, no. 11: 1636. https://doi.org/10.3390/medicina58111636
APA StylePastor, T., Zderic, I., Schopper, C., Haefeli, P. C., Kastner, P., Souleiman, F., Gueorguiev, B., & Knobe, M. (2022). Impact of Anterior Malposition and Bone Cement Augmentation on the Fixation Strength of Cephalic Intramedullary Nail Head Elements. Medicina, 58(11), 1636. https://doi.org/10.3390/medicina58111636








