Relationship between Outerbridge Scale and Chondropathy Femorotibial Joint in Relation to Gender and Age—The Use of 1.5T and 3.0T MRI Scanners
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Description of the Participants
2.3. Inclusion and Exclusion Criteria
2.4. Evaluation of Cartilage Chondromalacia
2.5. Image Acquisition
2.6. Statistical Analysis
3. Results
3.1. Age
3.2. BMI
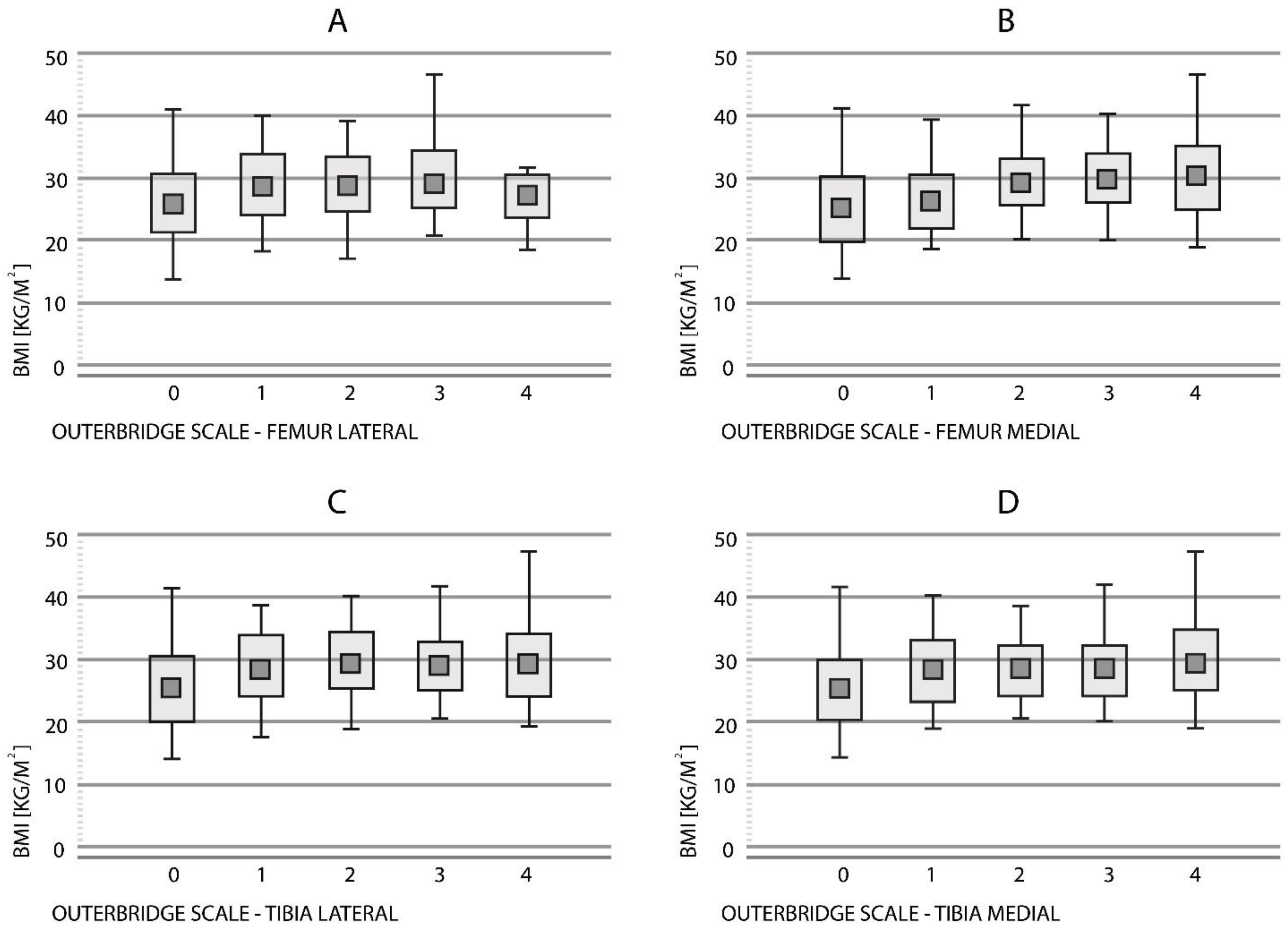
3.2.1. Femur Lateral
3.2.2. Femur Medial
3.2.3. Tibia Lateral
3.2.4. Tibia Medial
3.3. Gender
3.3.1. Women
3.3.2. Men
3.4. Type of Scanners (1.5T vs. 3.0T)
3.5. Logistic Regression of Age, BMI, Sex and MRI Scanner Type on Outerbridge Score
4. Discussion
4.1. Gender
4.2. Aging
4.3. BMI
4.4. MRI and the Assessment of Cartilage Chondromalacia
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carballo, C.; Nakagawa, Y.; Sekiya, I.; Rodeo, S. Basic Science of Articular Cartilage. Clin. Sport. Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Ekman, S.; Carlson, C. The Pathophysiology of Osteochondrosis. Veterinary Clinics of North America. Small Anim. Pract. 1998, 28, 17–32. [Google Scholar] [CrossRef]
- Wong, B.; Bae, W.; Chun, J.; Gratz, K.; Lotz, M.; Robert, L. Sah Biomechanics of cartilage articulation: Effects of lubrication and degeneration on shear deformation. Arthritis Rheum. 2008, 58, 2065–2074. [Google Scholar] [CrossRef]
- Sacitharan, P.; Vincent, T. Cellular ageing mechanisms in osteoarthritis. Mamm. Genome 2016, 27, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Slattery, C.; Kweon, C. Classifications in Brief: Outerbridge Classification of Chondral Lesions. Clin. Orthop. Relat. Res. 2018, 476, 2101–2104. [Google Scholar] [CrossRef]
- Reed, M.; Villacis, D.; Hatch, G.; Burke, W.; Colletti, P.; Narvy, S.; Mirzayan, R.; Vangsness, C. 3.0-Tesla MRI and Arthroscopy for Assessment of Knee Articular Cartilage Lesions. Orthopedics 2013, 36, e1060–e1064. [Google Scholar] [CrossRef]
- Burge, A.; Potter, H.; Argentieri, E. Magnetic Resonance Imaging of Articular Cartilage within the Knee. J. Knee Surg. 2018, 31, 155–165. [Google Scholar] [CrossRef]
- Schreiner, A.; Stoker, A.; Bozynski, C.; Kuroki, K.; Stannard, J.; Cook, J. Clinical Application of the Basic Science of Articular Cartilage Pathology and Treatment. J. Knee Surg. 2020, 33, 1056–1068. [Google Scholar] [CrossRef]
- Jungmann, P.; Welsch, G.; Brittberg, M.; Trattnig, S.; Braun, S.; Imhoff, A.; Salzmann, G. Magnetic Resonance Imaging Score and Classification System (AMADEUS) for Assessment of Preoperative Cartilage Defect Severity. Cartilage 2016, 8, 272–282. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhao, F. Comparison of 1.5- and 3.0-T magnetic resonance imaging for evaluating lesions of the knee. Medicine 2018, 97, e12401. [Google Scholar] [CrossRef]
- Wang, L.; Denniston, M.; Lee, S.; Galuska, D.; Lowry, R. Long-term Health and Economic Impact of Preventing and Reducing Overweight and Obesity in Adolescence. J. Adolesc. Health 2010, 46, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Oliveria, S.; Felson, D.; Cirillo, P.; Reed, J.; Walker, A. Body Weight, Body Mass Index, and Incident Symptomatic Osteoarthritis of the Hand, Hip, and Knee. Epidemiology 1999, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Blagojevic, M.; Jinks, C.; Jeffery, A.; Jordan, K. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2010, 18, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Abbate, L.; Stevens, J.; Schwartz, T.; Renner, J.; Helmick, C.; Jordan, J. Anthropometric Measures, Body Composition, Body Fat Distribution, and Knee Osteoarthritis in Women*. Obesity 2006, 14, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.; Gerhardsson de Verdier, M.; Rollof, J.; Nilsson, P.; Engström, G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: A population-based prospective cohort study. Ann. Rheum. Dis. 2008, 68, 490–496. [Google Scholar] [CrossRef]
- Bedson, J.; Croft, P. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskelet. Disord. 2008, 9, 116. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C. Body mass index and risk of knee osteoarthritis: Systematic review and meta-analysis of prospective studies. BMJ Open 2015, 5, e007568. [Google Scholar] [CrossRef]
- Rai, M.; Sandell, L.; Barrack, T.; Cai, L.; Tycksen, E.; Tang, S.; Silva, M.; Barrack, R. A Microarray Study of Articular Cartilage in Relation to Obesity and Severity of Knee Osteoarthritis. Cartilage 2020, 4, 458–472. [Google Scholar] [CrossRef]
- Sieroń, D.; Jabłońska, I.; Lukoszek, D.; Szyluk, K.; Meusburger, H.; Delimpasis, G.; Kostrzewa, M.; Platzek, I.; Christe, A. Knee Diameter and Cross-Section Area Measurements in MRI as New Promising Methods of Chondromalacia Diagnosis-Pilot Study. Medicina 2022, 58, 1142. [Google Scholar] [CrossRef]
- Park, C.; Song, K.; Kim, J.; Lee, S. Retrospective evaluation of outcomes of bone peg fixation for osteochondral lesion of the talus. Bone Jt. J. 2020, 102-B, 1349–1353. [Google Scholar] [CrossRef]
- Slimi, F.; Zribi, W.; Trigui, M.; Amri, R.; Gouiaa, N.; Abid, C.; Rebai, M.; Boudawara, T.; Jebahi, S.; Keskes, H. The effectiveness of platelet-rich plasma gel on full-thickness cartilage defect repair in a rabbit model. Bone Jt. Res. 2021, 10, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.; Clune, J.; Walker, J.; Gloth, P. Suitability of EGCG as a Means of Stabilizing a Porcine Osteochondral Xenograft. J. Funct. Biomater. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.; Barawi, A.; Wang, H.; Roelofs, A.; Kaneva, M.; Guan, Z.; Lydon, H.; Thomas, B.; Thorup, A.; Fernandez, B.; et al. Agrin induces long-term osteochondral regeneration by supporting repair morphogenesis. Sci. Transl. Med. 2020, 12, eaax9086. [Google Scholar] [CrossRef] [PubMed]
- Matada, M.; Holi, M.; Raman, R.; Jayaramu Suvarna, S. Visualization of Cartilage from Knee Joint Magnetic Resonance Images and Quantitative Assessment to Study the Effect of Age, Gender and Body Mass Index (BMI) in Progressive Osteoarthritis (OA). Curr. Med. Imaging Former. Curr. Med. Imaging Rev. 2019, 15, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Schwartz, T.; Helmick, C.; Renner, J.; Tudor, G.; Koch, G.; Dragomir, A.; Kalsbeek, W.; Luta, G.; Jordan, J. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008, 59, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Grotle, M.; Hagen, K.; Natvig, B.; Dahl, F.; Kvien, T. Obesity and osteoarthritis in knee, hip and/or hand: An epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet. Disord. 2008, 9, 132. [Google Scholar] [CrossRef]
- Ding, C.; Cicuttini, F.; Blizzard, L.; Scott, F.; Jones, G. A longitudinal study of the effect of sex and age on rate of change in knee cartilage volume in adults. Rheumatology 2006, 46, 273–279. [Google Scholar] [CrossRef]
- Berry, P.; Wluka, A.; Davies-Tuck, M.; Wang, Y.; Strauss, B.; Dixon, J.; Proietto, J.; Jones, G.; Cicuttini, F. The relationship between body composition and structural changes at the knee. Rheumatology 2010, 49, 2362–2369. [Google Scholar] [CrossRef]
- Tsai, C.L.; Liu, T.K. Osteoarthritis in women: Its relationship to estrogen and current trends. Life Sci. 1992, 50, 1737–1744. [Google Scholar] [CrossRef]
- Wluka, A. Users of oestrogen replacement therapy have more knee cartilage than non-users. Ann. Rheum. Dis. 2001, 60, 332–336. [Google Scholar] [CrossRef]
- McKean, K.; Landry, S.; Hubley-Kozey, C.; Dunbar, M.; Stanish, W.; Deluzio, K. Gender differences exist in osteoarthritic gait. Clin. Biomech. 2007, 22, 400–409. [Google Scholar] [CrossRef]
- Hanna, F.; Teichtahl, A.; Wluka, A.; Wang, Y.; Urquhart, D.; English, D.; Giles, G.; Cicuttini, F. Women have increased rates of cartilage loss and progression of cartilage defects at the knee than men. Menopause 2009, 16, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.; Sun, H. Events in Articular Chondrocytes with Aging. Curr. Osteoporos. Rep. 2011, 9, 196–201. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M. Sex differences in osteoarthritis of the hip and knee. J. Am. Acad. Orthop. Surg. 2007, 15, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R. The Role of Aging in the Development of Osteoarthritis. Trans. Am. Clin. Clim. Assoc. 2017, 128, 44–54. [Google Scholar]
- Marie, P.; Kassem, M. Extrinsic Mechanisms Involved in Age-Related Defective Bone Formation. J. Clin. Endocrinol. Metab. 2011, 96, 600–609. [Google Scholar] [CrossRef]
- Shirazi, R.; Shirazi-Adl, A. Computational biomechanics of articular cartilage of human knee joint: Effect of osteochondral defects. J. Biomech. 2009, 42, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Binder-Macleod, S.; Snyder-Mackler, L. Characterization of the human quadriceps muscle in active elders. Arch. Phys. Med. Rehabil. 2001, 82, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Resorlu, M.; Doner, D.; Karatag, O.; Toprak, C. The relationship between chondromalacia patella, medial meniscal tear and medial periarticular bursitis in patients with osteoarthritis. Radiol. Oncol. 2017, 51, 401–406. [Google Scholar] [CrossRef]
- Pereira, D.; Severo, M.; Ramos, E.; Branco, J.; Santos, R.; Costa, L.; Lucas, R.; Barros, H. Potential role of age, sex, body mass index and pain to identify patients with knee osteoarthritis. Int. J. Rheum. Dis. 2015, 20, 190–198. [Google Scholar] [CrossRef]
- Go, D.; Kim, D.; Guermazi, A.; Crema, M.; Hunter, D.; Hwang, H.; Kim, H. Metabolic obesity and the risk of knee osteoarthritis progression in elderly community residents: A 3-year longitudinal cohort study. Int. J. Rheum. Dis. 2021, 25, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, C.; Liu, B.; Dong, N.; Ding, L.; Li, Y.; Liu, J.; Feng, W.; Qi, X.; Jin, X. An update on the association between metabolic syndrome and osteoarthritis and on the potential role of leptin in osteoarthritis. Cytokine 2020, 129, 155043. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Steinbach, L.; Zhao, J.; Stehling, C.; Ma, C.; Link, T. Comparative study of imaging at 3.0 T versus 1.5 T of the knee. Skelet. Radiol. 2009, 38, 761–769. [Google Scholar] [CrossRef]
- Mandell, J.; Rhodes, J.; Shah, N.; Gaviola, G.; Gomoll, A.; Smith, S. Routine clinical knee MR reports: Comparison of diagnostic performance at 1.5 T and 3.0 T for assessment of the articular cartilage. Skelet. Radiol. 2017, 46, 1487–1498. [Google Scholar] [CrossRef]
- Vasconcelos, F.; Cordeiro, B.; Rech, C.; Petroski, E. Sensitivity and specificity of the body mass index for the diagnosis of overweight/obesity in elderly. Cad. Saude Publica 2010, 26, 1519–1527. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Grade | Macroscopy | MRI |
|---|---|---|
| Grade 0 | Normal cartilage | Normal cartilage |
| Grade 1 | Rough surface; chondral softening; focal thickening | Inhomogeneous; high signal; surface intact; cartilage swelling |
| Grade 2 | Irregular surface defects; <50% of cartilage thickness | Superficial ulceration, fissuring, fibrillation; <50% of cartilage thickness |
| Grade 3 | Loss of >50% cartilage thickness | Ulceration fissuring, fibrillation; >50% of depth of cartilage |
| Grade 4 | Cartilage loss | Full thickness chondral wear with exposure of subchondral bone |
| Correlated Variables | N | Rs Spearman | T (N-2) | p |
|---|---|---|---|---|
| Femur Lateral & Age | 324 | 0.69 | 16.9308 | <0.001 |
| Femur Medial & Age | 324 | 0.72 | 18.8962 | <0.001 |
| Tibia Lateral & Age | 324 | 0.71 | 18.3634 | <0.001 |
| Tibia Medial & Age | 324 | 0.74 | 20.0209 | <0.001 |
| Outerbridge | BMI (Mean ± SD) | |||
|---|---|---|---|---|
| Femur Lateral | Tibia Lateral | Femur Medial | Tibia Medial | |
| Grade 0 | 26.0 ± 4.88 | 25.7 ± 4.92 | 25.3 ± 4.47 | 25.4 ± 4.55 |
| Grade 1 | 29.1 ± 5.31 | 28.8 ± 4.97 | 26.4 ± 4.17 | 28.2 ± 4.71 |
| Grade 2 | 29.4 ± 4.49 | 29.6 ± 4.54 | 29.4 ± 3.85 | 28.3 ± 3.97 |
| Grade 3 | 29.6 ± 5.00 | 28.8 ± 4.06 | 29.9 ± 4.90 | 28.3 ± 3.97 |
| Grade 4 | 27.0 ± 3.54 | 29.4 ± 5.33 | 30.3 ± 5.01 | 29.6 ± 4.78 |
| Outerbridge Scale | ||
|---|---|---|
| 1.5T MRI scanner | 3.0T MRI Scanner | |
| Femur Lateral | 1.387 ± 1.306 | 1.225 ± 1.400 |
| Tibia Lateral | 1.581 ± 1.391 | 1.207 ± 1.379 |
| Femur Medial | 1.794 ± 1.528 | 1.367 ± 1.503 |
| Tibia Medial | 1.709 ± 1.503 | 1.260 ± 1.485. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieroń, D.; Jabłońska, I.; Niemiec, P.; Lukoszek, D.; Szyluk, K.; Platzek, I.; Meusburger, H.; Delimpasis, G.; Christe, A. Relationship between Outerbridge Scale and Chondropathy Femorotibial Joint in Relation to Gender and Age—The Use of 1.5T and 3.0T MRI Scanners. Medicina 2022, 58, 1634. https://doi.org/10.3390/medicina58111634
Sieroń D, Jabłońska I, Niemiec P, Lukoszek D, Szyluk K, Platzek I, Meusburger H, Delimpasis G, Christe A. Relationship between Outerbridge Scale and Chondropathy Femorotibial Joint in Relation to Gender and Age—The Use of 1.5T and 3.0T MRI Scanners. Medicina. 2022; 58(11):1634. https://doi.org/10.3390/medicina58111634
Chicago/Turabian StyleSieroń, Dominik, Izabella Jabłońska, Paweł Niemiec, Dawid Lukoszek, Karol Szyluk, Ivan Platzek, Hugo Meusburger, Georgios Delimpasis, and Andreas Christe. 2022. "Relationship between Outerbridge Scale and Chondropathy Femorotibial Joint in Relation to Gender and Age—The Use of 1.5T and 3.0T MRI Scanners" Medicina 58, no. 11: 1634. https://doi.org/10.3390/medicina58111634
APA StyleSieroń, D., Jabłońska, I., Niemiec, P., Lukoszek, D., Szyluk, K., Platzek, I., Meusburger, H., Delimpasis, G., & Christe, A. (2022). Relationship between Outerbridge Scale and Chondropathy Femorotibial Joint in Relation to Gender and Age—The Use of 1.5T and 3.0T MRI Scanners. Medicina, 58(11), 1634. https://doi.org/10.3390/medicina58111634








