Osteoclast and Sclerostin Expression in Osteocytes in the Femoral Head with Risedronate Therapy in Patients with Hip Fractures: A Retrospective Comparative Study
Abstract
1. Introduction
2. Materials and Methods
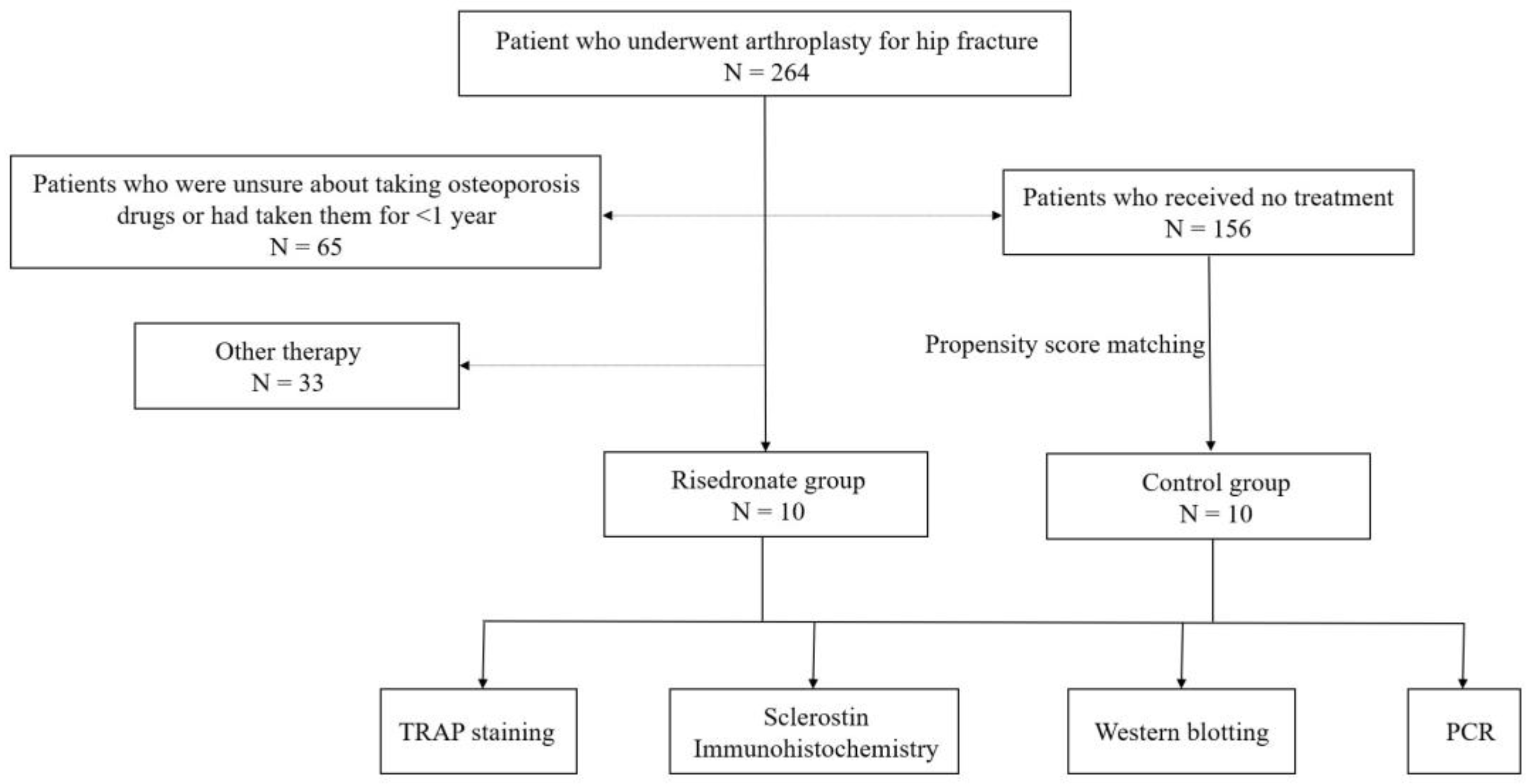
2.1. Specimen Collection
2.2. Histological Analysis of Decalcified Bone Tissue
2.3. TRAP Staining
2.4. Sclerostin Immunohistochemistry
2.5. Western Blotting
2.6. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.7. Statistical Analysis
3. Results
3.1. Specimen Collection
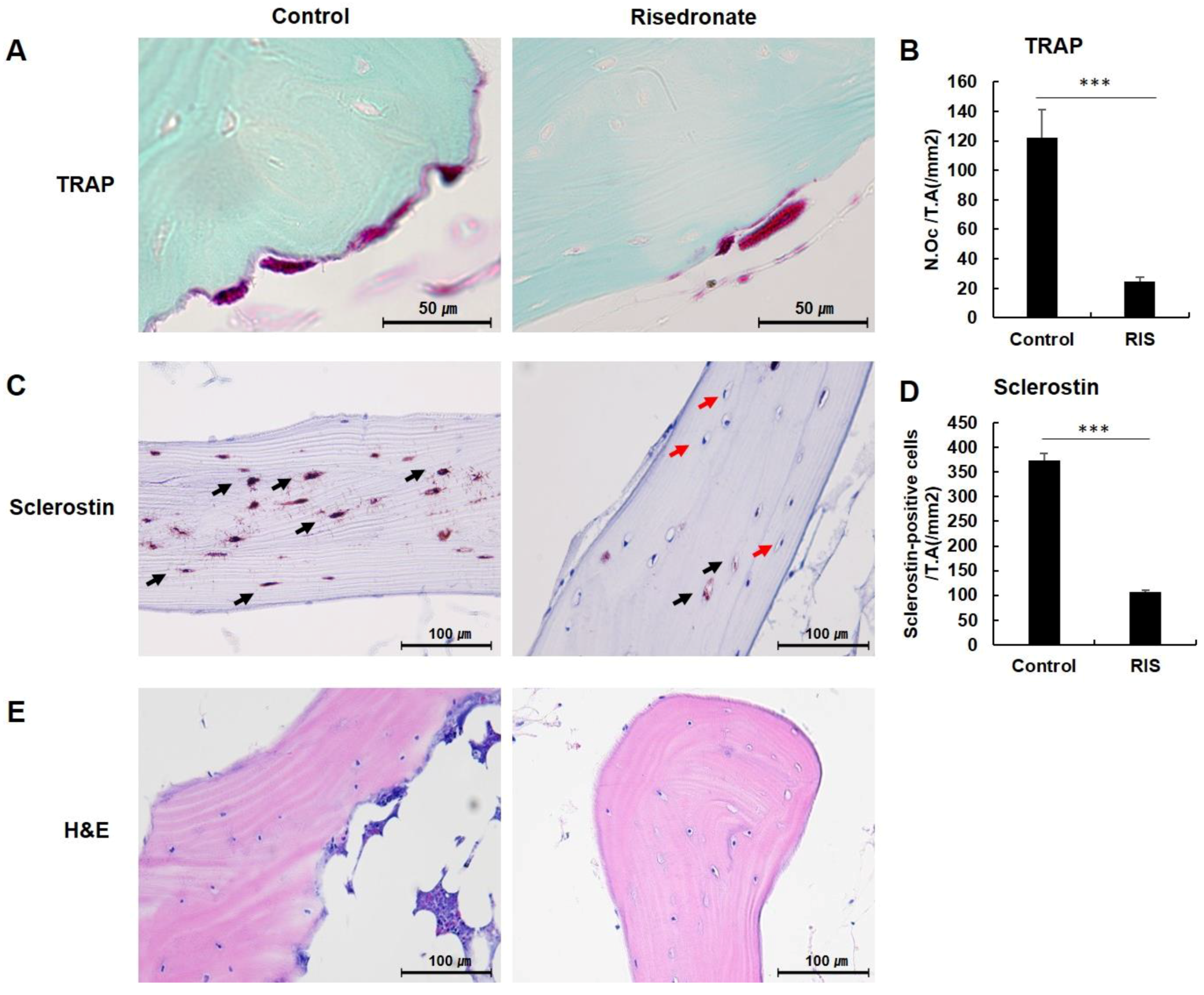
3.2. TRAP Staining
3.3. Sclerostin Immunohistochemistry
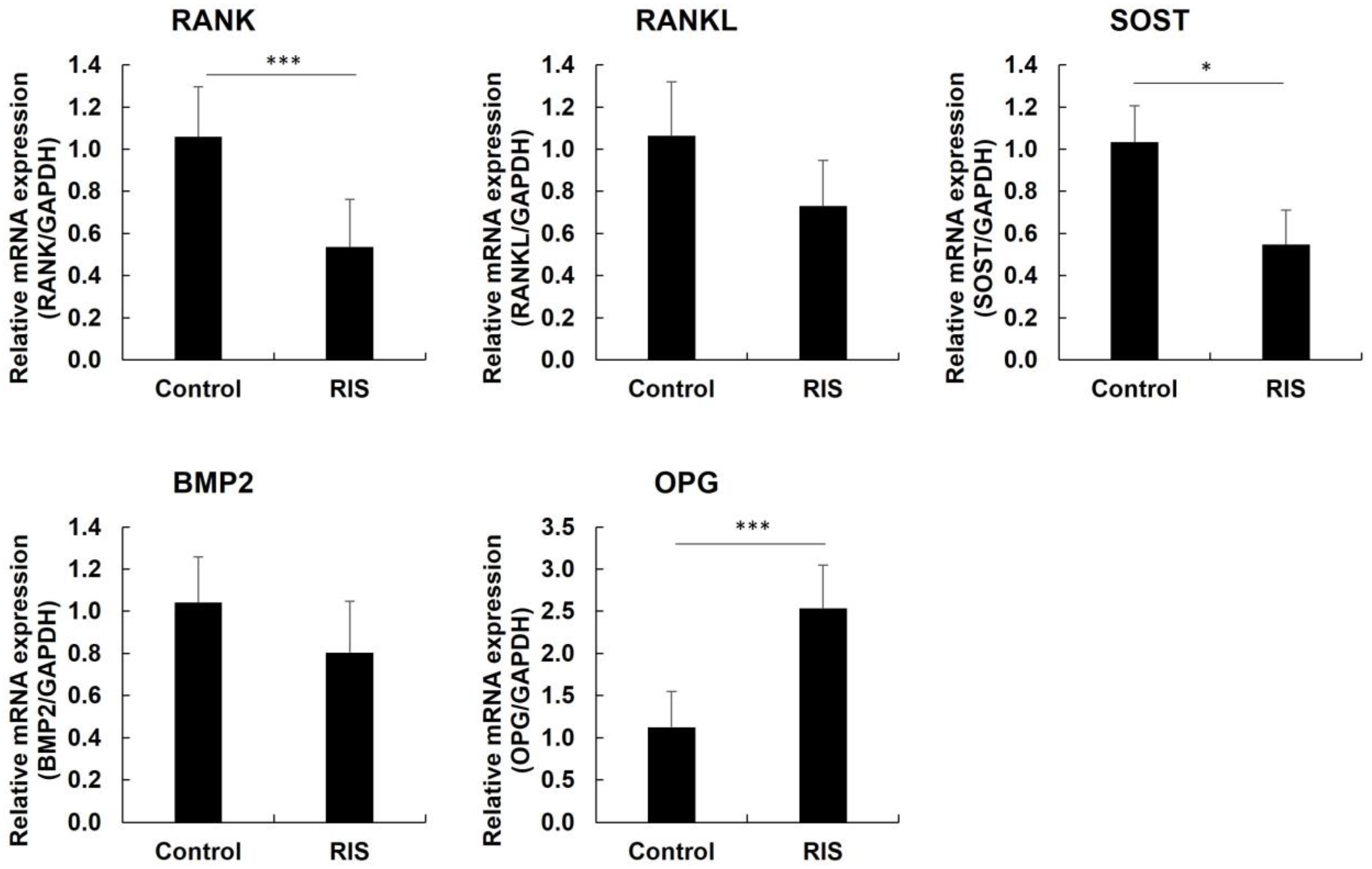
3.4. Western Blotting and Quantitative Real-Time PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClung, M.R.; O’Donoghue, M.L.; Papapoulos, S.E.; Bone, H.; Langdahl, B.; Saag, K.G.; Reid, I.R.; Kiel, D.P.; Cavallari, I.; Bonaca, M.P.; et al. Odanacatib for the treatment of postmenopausal osteoporosis: Results of the LOFT multicentre, randomised, double-blind, placebo-controlled trial and LOFT Extension study. Lancet Diabetes Endocrinol. 2019, 7, 899–911. [Google Scholar] [CrossRef]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Fukunaga, M.; Nakano, T.; Kishimoto, H.; Ito, M.; Hagino, H.; Sone, T.; Taguchi, A.; Tanaka, S.; Ohashi, M.; et al. Efficacy and safety of once-yearly zoledronic acid in Japanese patients with primary osteoporosis: Two-year results from a randomized placebo-controlled double-blind study (ZOledroNate treatment in Efficacy to osteoporosis; ZONE study). Osteoporos. Int. 2017, 28, 389–398. [Google Scholar] [CrossRef]
- Boivin, G.Y.; Chavassieux, P.M.; Santora, A.C.; Yates, J.; Meunier, P.J. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 2000, 27, 687–694. [Google Scholar] [CrossRef]
- Wells, G.A.; Hsieh, S.C.; Zheng, C.; Peterson, J.; Tugwell, P.; Liu, W. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst. Rev. 2022, 5, Cd004523. [Google Scholar] [CrossRef]
- Deardorff, W.J.; Cenzer, I.; Nguyen, B.; Lee, S.J. Time to Benefit of Bisphosphonate Therapy for the Prevention of Fractures Among Postmenopausal Women With Osteoporosis: A Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Nuti, R. Updates on mechanism of action and clinical efficacy of risedronate in osteoporosis. Clin. Cases Min. Bone Metab. 2014, 11, 208–214. [Google Scholar] [CrossRef]
- Dunn, C.J.; Goa, K.L. Risedronate: A review of its pharmacological properties and clinical use in resorptive bone disease. Drugs 2001, 61, 685–712. [Google Scholar] [CrossRef]
- Ke, C.H.; Li, H.Y.; Yang, D.; Ying, H.; Xu, J.; Wang, J.; Zhu, H.W.; Wang, L. Dynamic Effects of the Third Generation Bisphosphonate of Risedronate on Rat Osteoporotic Fractures for Clinical Usage Guidance. Orthop. Surg. 2021, 13, 2433–2441. [Google Scholar] [CrossRef]
- Yang, L. The efficiency of risedronate in reducing bone resorption after total hip arthroplasty: A meta-analysis of randomized control trials at a minimum of 6 months’ follow-up. J. Orthop. Surg. Res. 2018, 13, 88. [Google Scholar] [CrossRef]
- Viswanathan, M.; Reddy, S.; Berkman, N.; Cullen, K.; Middleton, J.C.; Nicholson, W.K.; Kahwati, L.C. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. In Screening to Prevent Osteoporotic Fractures: An Evidence Review for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018. [Google Scholar]
- Watts, N.B. Fundamentals and pitfalls of bone densitometry using dual-energy X-ray absorptiometry (DXA). Osteoporos Int. 2004, 15, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, H.H. DXA in vivo BMD methodology: An erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone 2007, 41, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Nakatoh, S. Effect of osteoporosis medication on changes in bone mineral density and bone turnover markers after 24-month administration of daily teriparatide: Comparison among minodronate, raloxifene, and eldecalcitol. J. Bone Miner. Metab. 2018, 36, 221–228. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Spiezia, F.; Tingart, M.; Maria, P.G.; Riccardo, G. Biomarkers as therapy monitoring for postmenopausal osteoporosis: A systematic review. J. Orthop. Surg. Res. 2021, 16, 318. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, K.K.; McGuigan, F.E.; Malmgren, L.; Gerdhem, P.; Johansson, H.; Kanis, J.A.; Akesson, K.E. Bone Turnover Marker Profiling and Fracture Risk in Older Women: Fracture Risk from Age 75 to 90. Calcif. Tissue Int. 2022, 111, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Gorter, E.A.; Reinders, C.R.; Krijnen, P.; Appelman-Dijkstra, N.M.; Schipper, I.B. Serum sclerostin levels in osteoporotic fracture patients. Eur. J. Trauma Emerg. Surg. 2022. [Google Scholar] [CrossRef]
- Ardawi, M.S.; Rouzi, A.A.; Al-Sibiani, S.A.; Al-Senani, N.S.; Qari, M.H.; Mousa, S.A. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: The Center of Excellence for Osteoporosis Research Study. J. Bone Miner. Res. 2012, 27, 2592–2602. [Google Scholar] [CrossRef]
- Ozaki, Y.; Koide, M.; Furuya, Y.; Ninomiya, T.; Yasuda, H.; Nakamura, M.; Kobayashi, Y.; Takahashi, N.; Yoshinari, N.; Udagawa, N. Treatment of OPG-deficient mice with WP9QY, a RANKL-binding peptide, recovers alveolar bone loss by suppressing osteoclastogenesis and enhancing osteoblastogenesis. PLoS ONE 2017, 12, e0184904. [Google Scholar] [CrossRef]
- Inacio, M.C.; Chen, Y.; Paxton, E.W.; Namba, R.S.; Kurtz, S.M.; Cafri, G. Statistics in Brief: An Introduction to the Use of Propensity Scores. Clin. Orthop. Relat. Res. 2015, 473, 2722–2726. [Google Scholar] [CrossRef]
- Balemans, W.; Ebeling, M.; Patel, N.; Van Hul, E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; Van Den Ende, J.; Willems, P.; et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef]
- Moester, M.J.; Papapoulos, S.E.; Löwik, C.W.; van Bezooijen, R.L. Sclerostin: Current knowledge and future perspectives. Calcif. Tissue Int. 2010, 87, 99–107. [Google Scholar] [CrossRef]
- Winkler, D.G.; Sutherland, M.K.; Geoghegan, J.C.; Yu, C.; Hayes, T.; Skonier, J.E.; Shpektor, D.; Jonas, M.; Kovacevich, B.R.; Staehling-Hampton, K.; et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003, 22, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Semënov, M.; Tamai, K.; He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005, 280, 26770–26775. [Google Scholar] [CrossRef] [PubMed]
- Morales-Santana, S.; Díez-Pérez, A.; Olmos, J.M.; Nogués, X.; Sosa, M.; Díaz-Curiel, M.; Pérez-Castrillón, J.L.; Pérez-Cano, R.; Torrijos, A.; Jodar, E.; et al. Circulating sclerostin and estradiol levels are associated with inadequate response to bisphosphonates in postmenopausal women with osteoporosis. Maturitas 2015, 82, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Bratengeier, C.; Woloszczuk, W.; Papatheodorou, A.; Terpos, E. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women–the six-month effect of risedronate and teriparatide. Osteoporos. Int. 2012, 23, 1171–1176. [Google Scholar] [CrossRef]
- Boltenstål, H.; Qureshi, A.R.; Behets, G.J.; Lindholm, B.; Stenvinkel, P.; D’Haese, P.C.; Haarhaus, M. Association of Serum Sclerostin with Bone Sclerostin in Chronic Kidney Disease is Lost in Glucocorticoid Treated Patients. Calcif. Tissue Int. 2019, 104, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Chekroun, A.; Pujo-Menjouet, L.; Falcoz, S.; Tsuen, K.; Yueh-Hsun Yang, K.; Berteau, J.P. Theoretical evidence of osteoblast self-inhibition after activation of the genetic regulatory network controlling mineralization. J. Theor. Biol. 2022, 537, 111005. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, s.; Supriyono, T.; Kurdi, O.; Tauviqirrahman, M.; Winarni, T.; Jamari, J. Tresca stress study of CoCrMo-on-CoCrMo bearings based on body mass index using 2D computational model. J. Tribol. 2022, 33, 31–38. [Google Scholar]
- Pant, A.; Paul, E.; Niebur, G.L.; Vahdati, A. Integration of mechanics and biology in computer simulation of bone remodeling. Prog. Biophys. Mol. Biol. 2021, 164, 33–45. [Google Scholar] [CrossRef]
- Boaretti, D.; Wehrle, E.; Bansod, Y.D.; Tourolle Né Betts, D.C.; Müller, R. Perspectives on in silico bone mechanobiology: Computational modelling of multicellular systems. Eur. Cells Mater. 2022, 44, 56–73. [Google Scholar] [CrossRef] [PubMed]

| Primer | Direction | Sequence |
|---|---|---|
| GAPDH GAPDH RANK RANK RANKL RANKL SOST SOST BMP2 BMP2 OPG OPG | Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse | CCTGCCAAATATGATGACATCAAG GTGGTCGTTGAGGGCAATG ACGTGGACCCTTGCCCCAGT ACTGGCCACCAGGGGAGCTT CACCATCAGCTGAAGATAGT CCAAGATCTCTAACATGACG AGACCAAAGACGTGTCCGAG GGGATGCAGCGGAAGTC ATGGATTCGTGGTGGAAGTG GTGGAGTTCAGATGATCAGC GTAGGTGCCAGGAGCCATT CAATGAACAAGTGGCTGTGC |
| Control Group (n = 10) | Risedronate Group (n = 10) | p-Value | |
|---|---|---|---|
| Age (years) BMI (kg/m2) Hip BMD ASA score | 82.2 ± 4.1 23.19 ± 4.03 −3.19 ± 0.84 2.0 ± 0.82 | 81.1 ± 5.9 22.74 ± 2.58 −3.06 ± 0.82 1.9 ± 0.74 | 0.471 0.214 0.965 0.789 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-H.; Choi, E.-Y.; Jun, H.-S.; Kim, Y.-Y. Osteoclast and Sclerostin Expression in Osteocytes in the Femoral Head with Risedronate Therapy in Patients with Hip Fractures: A Retrospective Comparative Study. Medicina 2022, 58, 1566. https://doi.org/10.3390/medicina58111566
Lee H-H, Choi E-Y, Jun H-S, Kim Y-Y. Osteoclast and Sclerostin Expression in Osteocytes in the Femoral Head with Risedronate Therapy in Patients with Hip Fractures: A Retrospective Comparative Study. Medicina. 2022; 58(11):1566. https://doi.org/10.3390/medicina58111566
Chicago/Turabian StyleLee, Hwan-Hee, Eun-Yong Choi, Hyun-Sik Jun, and Young-Yul Kim. 2022. "Osteoclast and Sclerostin Expression in Osteocytes in the Femoral Head with Risedronate Therapy in Patients with Hip Fractures: A Retrospective Comparative Study" Medicina 58, no. 11: 1566. https://doi.org/10.3390/medicina58111566
APA StyleLee, H.-H., Choi, E.-Y., Jun, H.-S., & Kim, Y.-Y. (2022). Osteoclast and Sclerostin Expression in Osteocytes in the Femoral Head with Risedronate Therapy in Patients with Hip Fractures: A Retrospective Comparative Study. Medicina, 58(11), 1566. https://doi.org/10.3390/medicina58111566







